Corrosion Resistance, Interfacial Contact Resistance, and Hydrophobicity of 316L Stainless Steel Bipolar Plates Coated with TiN/Amorphous Carbon Double Layer under Different Carbon Target Currents
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Samples Characterization
2.3. Electrochemical Corrosion and Interfacial Contact Resistance
3. Results
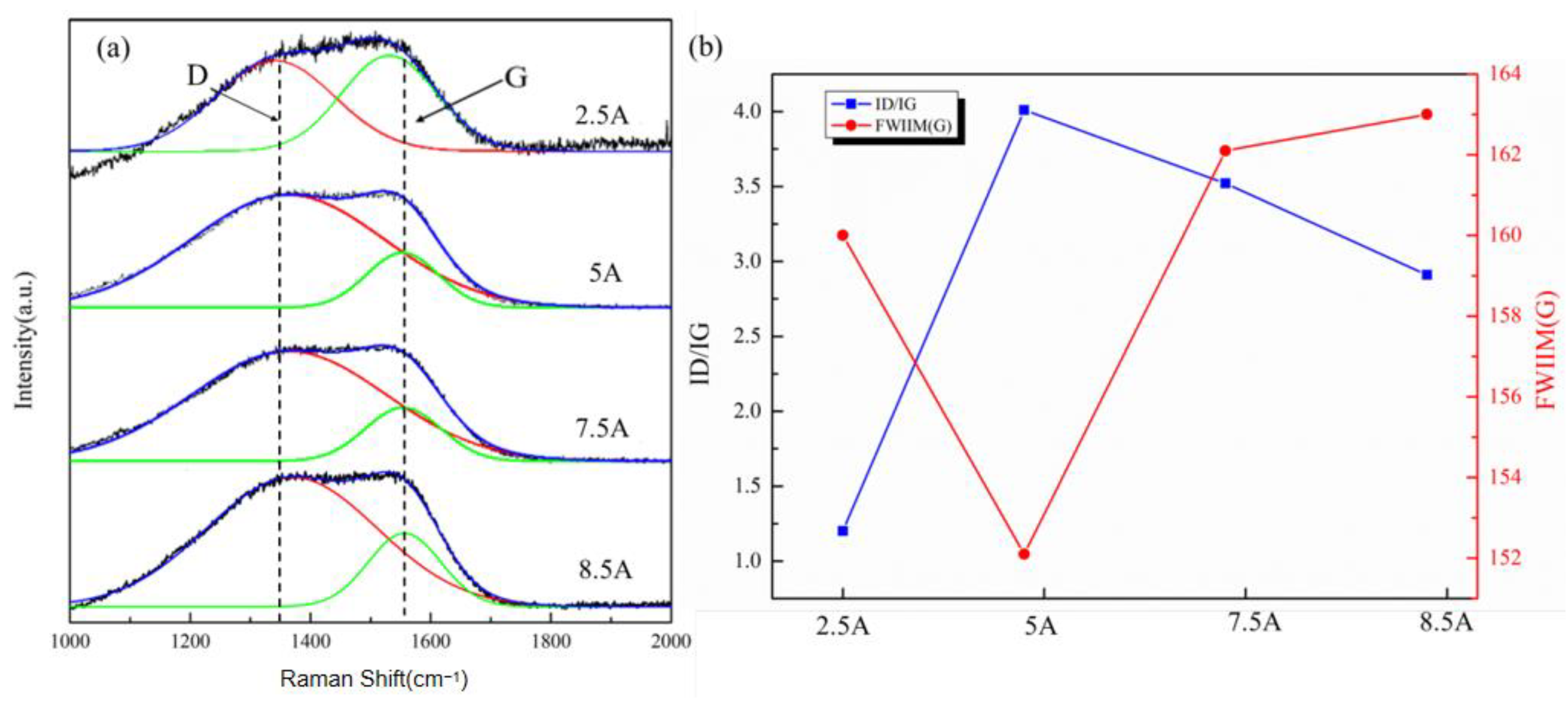
3.1. Morphology and Structure
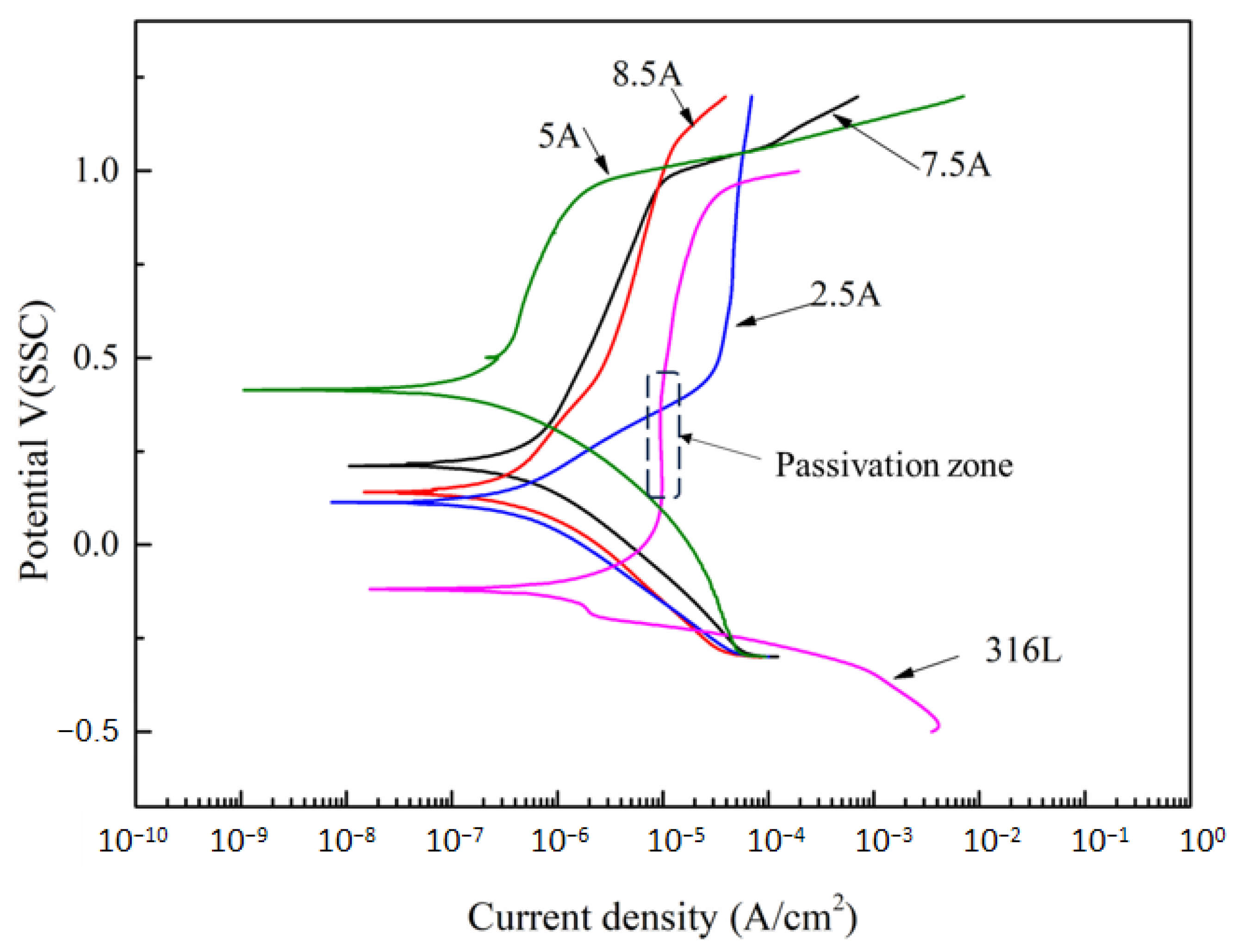
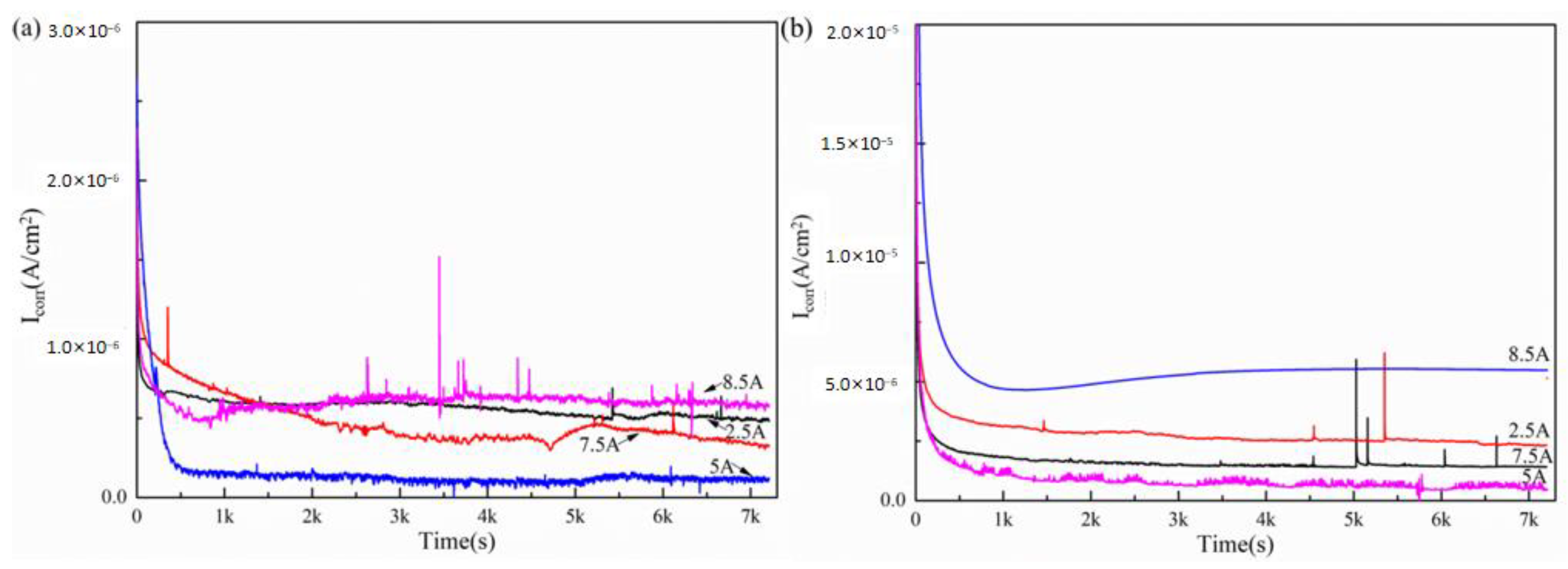
3.2. The Electrochemical Corrosion Behavior
3.3. Interfacial Contact Resistance (ICR)
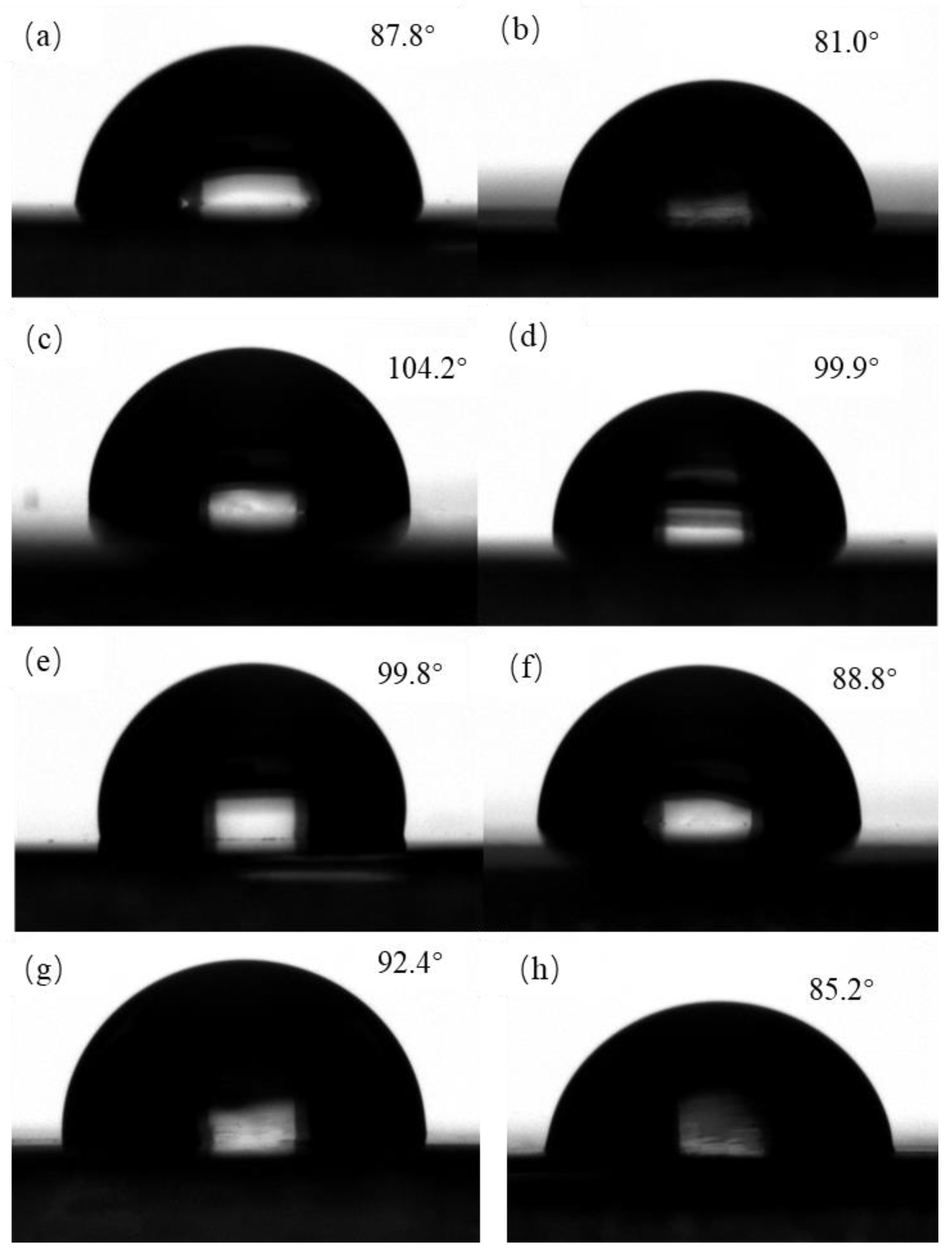
3.4. Contact Angle
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Li, X. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Zhang, T.; Pei, P. The reactant starvation of the proton exchange membrane fuel cells for vehicular applications: A review. Energy Convers. Manag. 2019, 182, 282–298. [Google Scholar] [CrossRef]
- Peng, L.; Yi, P.; Lai, X. Design and manufacturing of stainless steel bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2014, 39, 21127–21153. [Google Scholar] [CrossRef]
- Tang, Y.; Yuan, W.; Pan, M.; Wan, Z. Feasibility study of porous copper fiber sintered felt: A novel porous flow field in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 9661–9677. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, D.; Qiu, D.; Peng, L.; Lai, X. Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 6813–6843. [Google Scholar] [CrossRef]
- Lee, J.-B.; Oh, I.H. Electrochemical characteristics and interfacial contact resistance of multi-layered Ti/TiN coating for metallic bipolar-plate of polymer electrolyte membrane fuel cells. Met. Mater. Int. 2016, 20, 629–639. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.-Y.; Han, M.; Yong, R.-Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Wang, H.; Turner, J.A.; Li, X.; Bhattacharya, R. SnO2:F coated austenite stainless steels for PEM fuel cell bipolar plates. J. Power Sources 2007, 171, 567–574. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Tao, Y.; Cao, R.; Kou, X.; Tian, X. Investigation of corrosion properties with Ni–P/TiNO coating on aluminum alloy bipolar plates in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2022, 47, 22165–22179. [Google Scholar] [CrossRef]
- Yan, P.; Cao, S.; Ying, T.; Yang, Y.; Cao, F.; Zeng, X. Novel Graphite Paper/Cu-Coated Magnesium as Bipolar Plates for Ultra-lightweight Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2023, 170, 054510. [Google Scholar] [CrossRef]
- Zhang, P.C.; Han, Y.T.; Shi, J.F.; Li, T.; Wang, H.Y.; Wang, X.Y.; Sun, J.C. ZrC Coating Modified Ti Bipolar Plate for Proton Exchange Membrane Fuel Cell. Fuel Cells 2020, 20, 540–546. [Google Scholar] [CrossRef]
- Mi, B.; Chen, Z.; Wang, Q.; Li, Y.; Qin, Z.; Wang, H. Properties of C-doped CrTiN films on the 316L stainless steel bipolar plate for PEMFC. Int. J. Hydrogen Energy 2021, 46, 32645–32654. [Google Scholar] [CrossRef]
- Mi, B.; Wang, H.; Wang, Q.; Cai, J.; Qin, Z.; Chen, Z. Corrosion resistance and contact resistance properties of Cr-doped amorphous carbon films deposited under different carbon target current on the 316L stainless steel bipolar plate for PEMFC. Vacuum 2022, 203, 111263. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, X.-W.; Liu, S.; Wang, X.-Z.; Fu, X.-Z.; Luo, J.-L. High corrosion resistance of reduced graphene oxide coated 316L stainless steel bipolar plate for proton exchange membrane fuel cell prepared by a facile method. Mater. Chem. Phys. 2022, 290, 126663. [Google Scholar] [CrossRef]
- Steinhorst, M.; Auinger, M.; Roch, T.; Leyens, C. Modelling and corrosion of coated stainless steel substrates for bipolar plates at different temperatures. J. Appl. Electrochem. 2023, 53, 1491–1503. [Google Scholar] [CrossRef]
- Hu, Z.; Yuan, L.; Luo, H.; Jin, Y.; Chen, Z. Effects of Nitrogen Flow Rate on the Performances of Cathodic Arc Deposited TiN Films on 316L Stainless Steels as Bipolar Plates for the Proton--Exchange Membrane Fuel Cell. Steel Res. Int. 2023, 94, 2300089. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Wang, Y.-L. Performance of TiC coating prepared by in situ conversion of organic carbon film on 316L bipolar plate. J. Iron Steel Res. Int. 2023, 30, 1562–1573. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Zhang, B.; Lv, J.; Zhang, J. Nano-Cr2N dominated films with high conductivity and strong corrosion resistance for Ti bipolar plates. Mater. Des. 2022, 224, 111305. [Google Scholar] [CrossRef]
- Chen, M.; Ding, J.C.; Kwon, S.-H.; Wang, Q.; Zhang, S. Corrosion resistance and conductivity of NbN-coated 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Corros. Sci. 2022, 196, 110042. [Google Scholar] [CrossRef]
- Li, H.; Xin, Y.; Komatsu, K.; Guo, P.; Ma, G.; Ke, P.; Lee, K.-R.; Saito, H.; Wang, A. Controlling the compactness and sp2 clusters to reduce interfacial damage of amorphous carbon/316L bipolar plates in PEMFCs. Int. J. Hydrogen Energy 2022, 47, 11622–11632. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Chen, L.; Luo, J.; Pang, P.; Zhang, X.; Liao, B.; Ying, M. Corrosion behavior and interfacial conductivity of amorphous hydrogenated carbon and titanium carbide composite (a-C: H/TiC) films prepared on titanium bipolar plates in PEMFCs. Diam. Relat. Mater. 2021, 120, 108628. [Google Scholar] [CrossRef]
- Taherian, R.; Mohammadi, M.; Samiei, Z. Investigation of graphite conductive adhesive coated on SS316L used for bipolar plates of proton-exchange membrane fuel cell. J. Adhes. Sci. Technol. 2021, 36, 1094–1111. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Wang, Y.; Li, H.; Li, Z. Evaluation of vacuum heat-treated α-C films for surface protection of metal bipolar plates used in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 22983–22997. [Google Scholar] [CrossRef]
- Hou, K.; Yi, P.; Li, X.; Peng, L.; Lai, X. The effect of Cr doped in amorphous carbon films on electrical conductivity: Characterization and mechanism. Int. J. Hydrogen Energy 2021, 46, 30841–30852. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, W.; Bi, F.; Peng, L.; Lai, X. Microstructure and properties of a-C films deposited under different argon flow rate on stainless steel bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2019, 410–411, 188–195. [Google Scholar] [CrossRef]
- Bi, F.; Hou, K.; Yi, P.; Peng, L.; Lai, X. Mechanisms of growth, properties and degradation of amorphous carbon films by closed field unbalanced magnetron sputtering on stainless steel bipolar plates for PEMFCs. Appl. Surf. Sci. 2017, 422, 921–931. [Google Scholar] [CrossRef]
- Jia, Q.; Mu, Z.; Zhang, X.; Zhang, B.; Liu, R.; Gao, K.; Yu, Y.; Lai, Z.; Zhang, J. Electronic conductive and corrosion mechanisms of dual nanostructure CuCr-doped hydrogenated carbon films for SS316L bipolar plates. Mater. Today Chem. 2021, 21, 100521. [Google Scholar] [CrossRef]
- Gou, Y.; Jiang, G.; Geng, J.; Shao, Z. Properties of NbC/a-C:H films on titanium bipolar plates for proton exchange membrane fuel cells. Fuel Cells 2022, 23, 51–59. [Google Scholar] [CrossRef]
- Ouyang, C.; Xun, D. Carbon/Graphite Sheets/PTFE-Coated Porous Titanium as the Bipolar Plate by Hydrothermal Treatment. Coatings 2022, 12, 1649. [Google Scholar] [CrossRef]
- Gou, Y.; Jiang, G.; Hao, J.; Shao, Z.; Wei, Z. A corrosion mechanism of NbC/α-C:H films for metallic bipolar plates in proton exchange membrane fuel cell cathode based on percolation model. Surf. Coat. Technol. 2022, 445, 128711. [Google Scholar] [CrossRef]
- Meng, W.; Zhu, H.; Wang, X.; Li, G.; Fan, Y.; Sun, D.; Kong, F. Electrochemical Behavior and Surface Conductivity of C/TiC Nanocomposite Coating on Titanium for PEMFC Bipolar Plate. Metals 2022, 12, 771. [Google Scholar] [CrossRef]
- Mi, B.; Wang, Q.; Qi, T.; Qin, Z.; Chen, Z.; Wang, H. Performance and structure of Ti-doped amorphous carbon/CrN/Ti multilayer coating deposited on 316L stainless steel for use as bipolar plate in proton exchange membrane fuel cell. J. Alloys Compd. 2023, 943, 169080. [Google Scholar] [CrossRef]
- Papadias, D.D.; Ahluwalia, R.K.; Thomson, J.K.; Meyer, H.M.; Brady, M.P.; Wang, H.; Turner, J.A.; Mukundan, R.; Borup, R. Degradation of SS316L bipolar plates in simulated fuel cell environment: Corrosion rate, barrier film formation kinetics and contact resistance. J. Power Sources 2015, 273, 1237–1249. [Google Scholar] [CrossRef]
- Fukutsuka, T.; Yamaguchi, T.; Miyano, S.-I.; Matsuo, Y.; Sugie, Y.; Ogumi, Z. Carbon-coated stainless steel as PEFC bipolar plate material. J. Power Sources 2007, 174, 199–205. [Google Scholar] [CrossRef]
- Forouzanmehr, M.; Reza Kashyzadeh, K.; Borjali, A.; Ivanov, A.; Jafarnode, M.; Gan, T.H.; Wang, B.; Chizari, M. Detection and analysis of corrosion and contact resistance faults of TiN and CrN coatings on 410 stainless steel as bipolar plates in PEM fuel cells. Sensors 2022, 22, 750. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ke, P.; Moon, M.-W.; Lee, K.-R.; Wang, A. Investigation of the microstructure, mechanical properties and tribological behaviors of Ti-containing diamond-like carbon films fabricated by a hybrid ion beam method. Thin Solid Film. 2012, 520, 6057–6063. [Google Scholar] [CrossRef]
- Li, H.X.; Xu, T.; Chen, J.M.; Zhou, H.; Liu, H. The effect of applied dc bias voltage on the properties of a-C:H films prepared in a dual dc–rf plasma system. Appl. Surf. Sci. 2004, 227, 364–372. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, W.Z.; Shi, L.Q.; Zhang, B. Preparation of single-crystal TiC (111) by radio frequency magnetron sputtering at low temperature. Thin Solid Film. 2012, 520, 6882–6887. [Google Scholar] [CrossRef]
- Zendejas Medina, L.; da Costa, M.T.; Paschalidou, E.M.; Lindwall, G.; Riekehr, L.; Korvela, M.; Fritze, S.; Kolozsvári, S.; Gamstedt, E.K.; Nyholm, L.; et al. Enhancing corrosion resistance, hardness, and crack resistance in magnetron sputtered high entropy CoCrFeMnNi coatings by adding carbon. Mater. Des. 2021, 205, 109711. [Google Scholar] [CrossRef]
- Alishahi, M.; Mahboubi, F.; Mousavi Khoie, S.M.; Aparicio, M.; Hübner, R.; Soldera, F.; Gago, R. Electrochemical behavior of nanocrystalline Ta/TaN multilayer on 316L stainless steel: Novel bipolar plates for proton exchange membrane fuel-cells. J. Power Sources 2016, 322, 1–9. [Google Scholar] [CrossRef]




| Stainless Steel | Cr | Ni | Mo | Al | C | Mn | Si |
|---|---|---|---|---|---|---|---|
| Chemical composition | 12.24%–13.25% | 7.5%–8.5% | 2.0%–2.5% | 0.90%–1.35% | ≤0.030 | ≤2.00 | ≤1.00 |
| Sample | 316L | 2.5 A | 5 A | 7.5 A | 8.5 A |
|---|---|---|---|---|---|
| Icorr/(μA·cm−2) | 29 | 0.43 | 0.12 | 0.35 | 0.67 |
| Ecorr/mV(SSC) | −118.2 | 113.3 | 413.8 | 210.9 | 140.8 |
| Sample | 2.5 A | 5 A | 7.5 A | 8.5 A |
|---|---|---|---|---|
| +0.6 V vs. SCE (μA·cm−2) | 0.480 | 0.121 | 0.325 | 0.582 |
| +1.1 V vs. SHE (μA·cm−2) | 5.476 | 0.453 | 5.139 | 140.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y. Corrosion Resistance, Interfacial Contact Resistance, and Hydrophobicity of 316L Stainless Steel Bipolar Plates Coated with TiN/Amorphous Carbon Double Layer under Different Carbon Target Currents. Coatings 2023, 13, 1494. https://doi.org/10.3390/coatings13091494
Huang Y. Corrosion Resistance, Interfacial Contact Resistance, and Hydrophobicity of 316L Stainless Steel Bipolar Plates Coated with TiN/Amorphous Carbon Double Layer under Different Carbon Target Currents. Coatings. 2023; 13(9):1494. https://doi.org/10.3390/coatings13091494
Chicago/Turabian StyleHuang, Yifei. 2023. "Corrosion Resistance, Interfacial Contact Resistance, and Hydrophobicity of 316L Stainless Steel Bipolar Plates Coated with TiN/Amorphous Carbon Double Layer under Different Carbon Target Currents" Coatings 13, no. 9: 1494. https://doi.org/10.3390/coatings13091494




