Fabrication of pH-Responsive PDPAEMA Thin Film Using a One-Step Environmentally Friendly Plasma Enhanced Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin Film Synthesis by PECVD
2.3. Characterizations
3. Results and Discussion
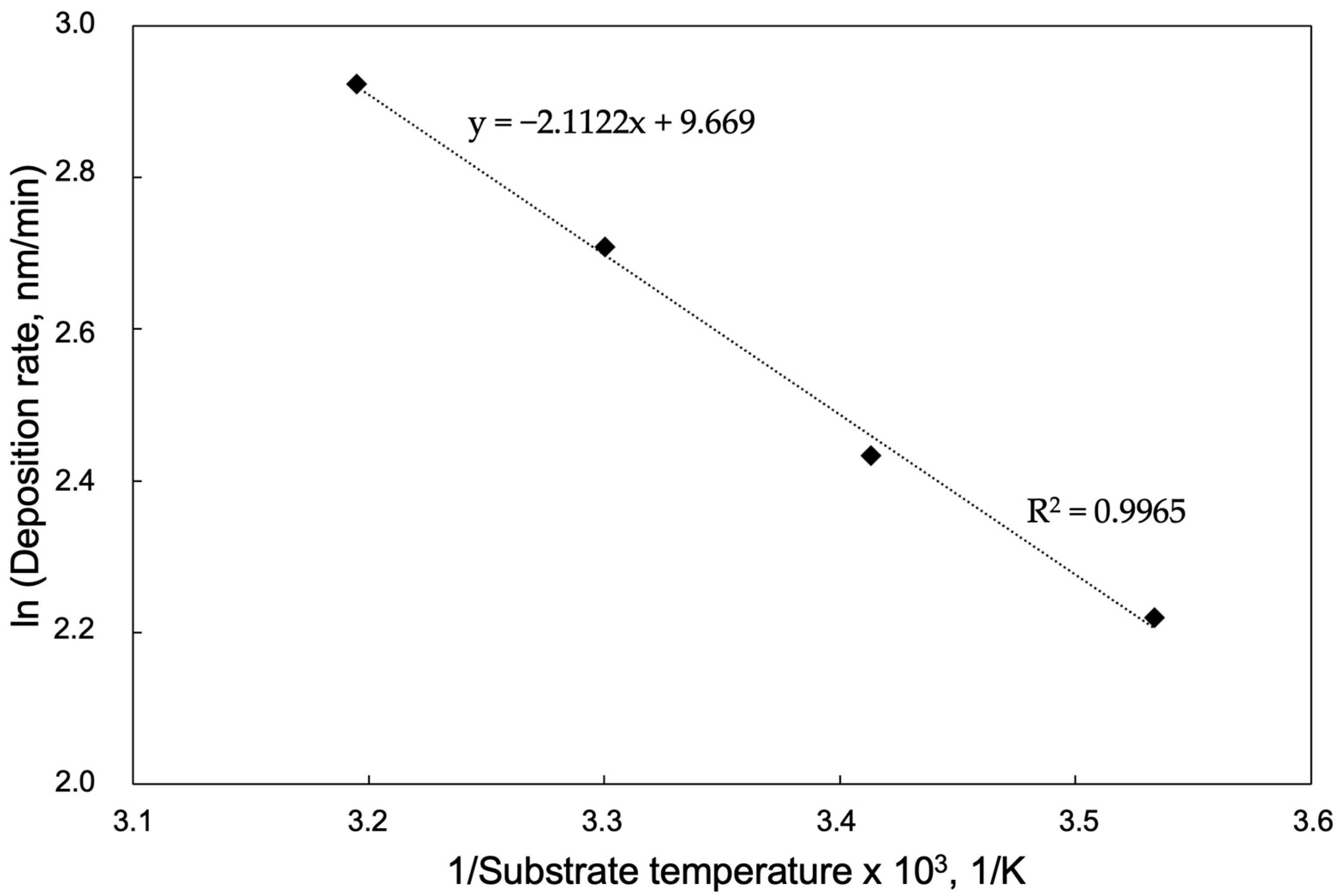
3.1. Deposition Rates
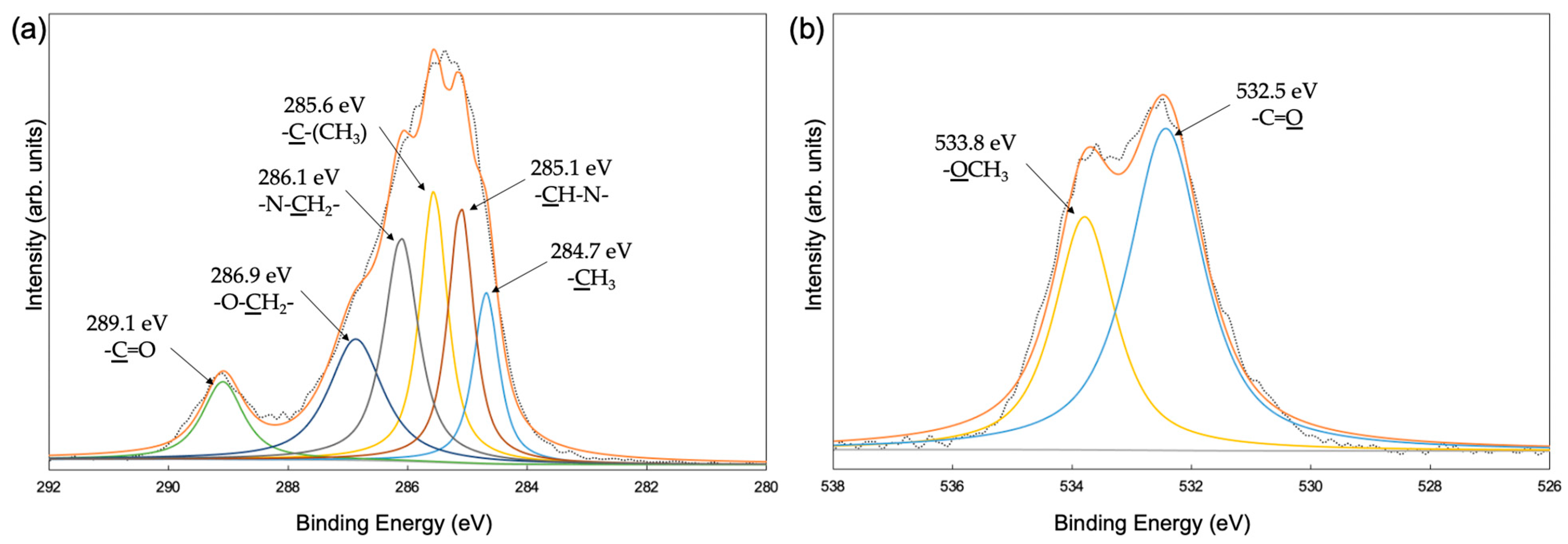
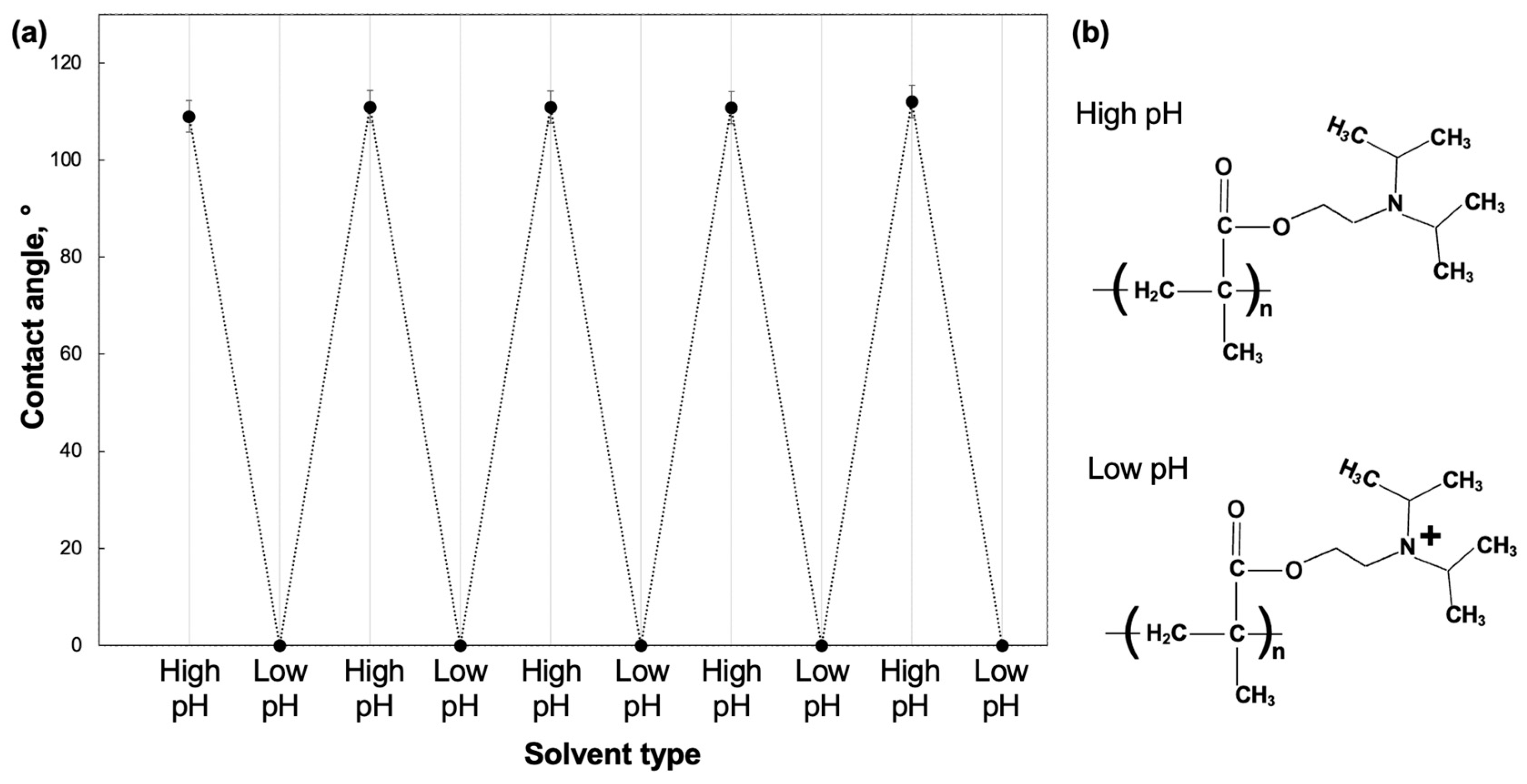
3.2. Film Structures
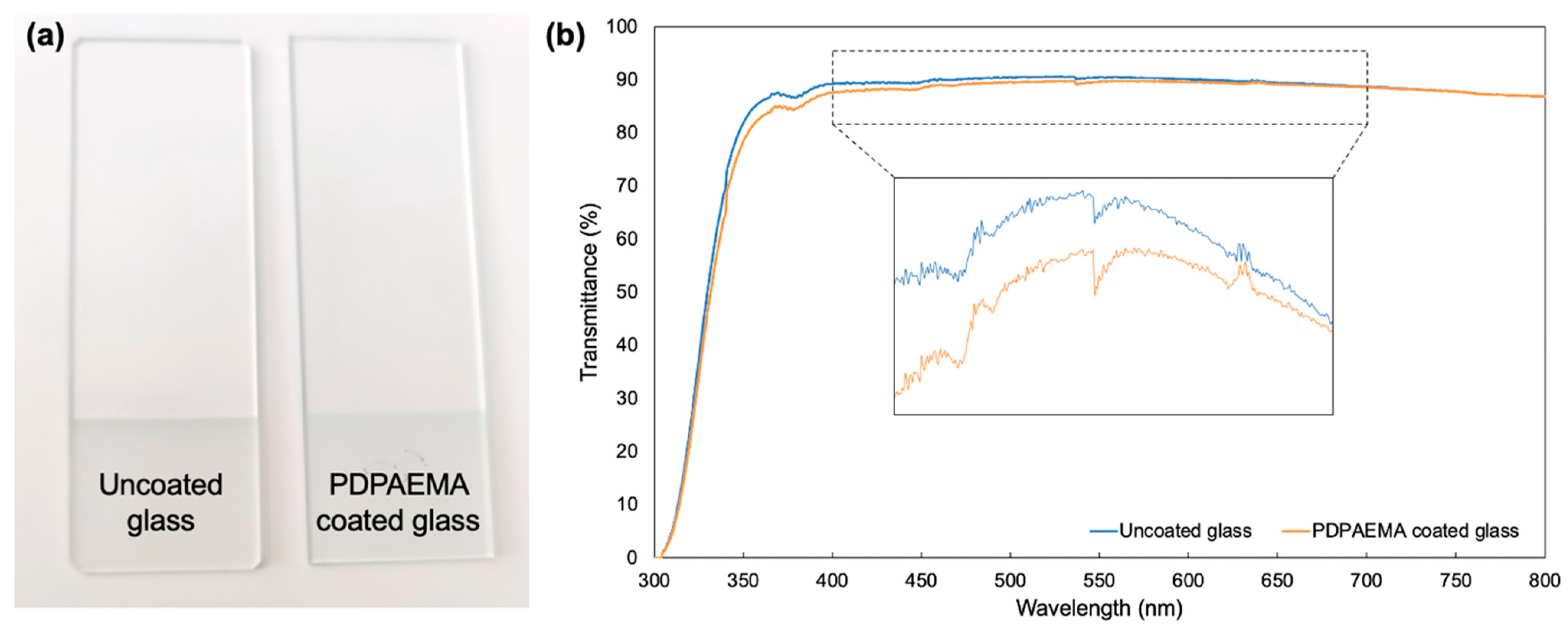
3.3. Large-Scale Deposition
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Png, Z.M.; Wang, C.-G.; Yeo, J.; Lee, J.J.C.; Surat’man, N.E.B.; Tan, Y.L.; Liu, H.; Wang, P.; Tan, M.B.H.; Xu, J.; et al. Stimuli-responsive Structure-Property Switchable Polymer Materials. Mol. Syst. Des. Eng. 2023, 8, 1097–1129. [Google Scholar] [CrossRef]
- Wurm, F.R.; Boyer, C.; Sumerlin, B.S. Progress on Stimuli-Responsive Polymers. Macromol. Rapid Commun. 2021, 42, 2100512. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, M.; Li, X.; Xu, W.; Ahiabu, A.; Perdiz, J.; Liu, Z.; Serpe, M.J. Stimuli-responsive polymers: Fundamental considerations and applications. Macromol. Res. 2017, 25, 513–527. [Google Scholar] [CrossRef]
- Siniscalco, D.; Pessoni, L.; Boussonnière, A.; Castanet, A.-S.; Billon, L.; Vignaud, G.; Delorme, N. Design of an Azopolymer for Photo-Switchable Adhesive Applications. Coatings 2024, 14, 275. [Google Scholar] [CrossRef]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Moros, M.; de la Fuente, J.M.; Vallet-Regi, M. Magnetic-responsive release controlled by hot spot effect. Langmuir 2015, 31, 12777–12782. [Google Scholar] [CrossRef] [PubMed]
- Stamou, A.; Iatrou, H.; Tsitsilianis, C. NIPAm-based modification of poly (L-lysine): A pH-dependent LCST-type thermo-responsive biodegradable polymer. Polymers 2022, 14, 802. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef] [PubMed]
- Yusa, S.-I. Development and application of pH-responsive polymers. Polym. J. 2022, 54, 235–242. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. pH-sensitive polymers: Classification and some fine potential applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Wang, L.; Ren, K.-F.; Wang, H.-B.; Wang, Y.; Ji, J. pH-sensitive controlled release of doxorubicin from polyelectrolyte multilayers. Colloids Surf. B Biointerfaces 2015, 125, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Smith, P.P.; Boyes, S.G. pH-responsive polymers for imaging acidic biological environments in tumors. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1062–1067. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, G.; Ge, Z.; Liu, S. Stimuli-responsive tertiary amine methacrylate-based block copolymers: Synthesis, supramolecular self-assembly and functional applications. Prog. Polym. Sci. 2014, 39, 1096–1143. [Google Scholar] [CrossRef]
- Gorji, M.; Zarbaf, D.; Mazinani, S.; Noushabadi, A.S.; Cella, M.A.; Sadeghianmaryan, A.; Ahmadi, A. Multi-responsive on-demand drug delivery PMMA-co-PDEAEMA platform based on CO2, electric potential, and pH switchable nanofibrous membranes. J. Biomater. Sci. Polym. Ed. 2023, 34, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Ulery, N.; Zharov, I. pH-Responsive Membranes from Self-Assembly of Poly (2-(dimethylamino) ethyl methacrylate) Brush Silica Nanoparticles. Langmuir 2023, 39, 15792–15798. [Google Scholar] [CrossRef] [PubMed]
- Kongkatigumjorn, N.; Smith, S.A.; Chen, M.; Fang, K.; Yang, S.; Gillies, E.R.; Johnston, A.P.; Such, G.K. Controlling endosomal escape using pH-responsive nanoparticles with tunable disassembly. ACS Appl. Nano Mater. 2018, 1, 3164–3173. [Google Scholar] [CrossRef]
- Echalier, C.; Jebors, S.; Laconde, G.; Brunel, L.; Verdié, P.; Causse, L.; Bethry, A.; Legrand, B.; Van Den Berghe, H.; Garric, X. Sol–gel synthesis of collagen-inspired peptide hydrogel. Mater. Today 2017, 20, 59–66. [Google Scholar] [CrossRef]
- Amano, M.; Uchiyama, M.; Satoh, K.; Kamigaito, M. Sulfur-Free Radical RAFT Polymerization of Methacrylates in Homogeneous Solution: Design of exo-Olefin Chain-Transfer Agents (R−CH2C(=CH2)Z). Angew. Chem. Int. Ed. 2022, 61, e202212633. [Google Scholar] [CrossRef]
- Wu, X.; Wyman, I.; Zhang, G.; Lin, J.; Liu, Z.; Wang, Y.; Hu, H. Preparation of superamphiphobic polymer-based coatings via spray-and dip-coating strategies. Prog. Org. Coat. 2016, 90, 463–471. [Google Scholar] [CrossRef]
- Yu, X.; Yang, W.; Yang, Y.; Wang, X.; Liu, X.; Zhou, F.; Zhao, Y. Subsurface-initiated atom transfer radical polymerization: Effect of graft layer thickness and surface morphology on antibiofouling properties against different foulants. J. Mater. Sci. 2020, 55, 14544–14557. [Google Scholar] [CrossRef]
- Gosar, Ž.; Kovač, J.; Mozetič, M.; Primc, G.; Vesel, A.; Zaplotnik, R. Deposition of SiOxCyHz protective coatings on polymer substrates in an industrial-scale PECVD reactor. Coatings 2019, 9, 234. [Google Scholar] [CrossRef]
- Gürsoy, M.; Kocadayıoğulları, B. Environmentally Friendly Approach for the Plasma Surface Modification of Fabrics for Improved Fog Harvesting Performance. Fibers Polym. 2023, 24, 3557–3567. [Google Scholar] [CrossRef]
- Karaman, M.; Gürsoy, M.; Aykül, F.; Tosun, Z.; Kars, M.D.; Yildiz, H.B. Hydrophobic coating of surfaces by plasma polymerization in an RF plasma reactor with an outer planar electrode: Synthesis, characterization and biocompatibility. Plasma Sci. Technol. 2017, 19, 085503. [Google Scholar] [CrossRef]
- Gürsoy, M.; Uçar, T.; Tosun, Z.; Karaman, M. Initiation of 2-hydroxyethyl methacrylate polymerization by tert-butyl peroxide in a planar PECVD system. Plasma Process. Polym. 2016, 13, 438–446. [Google Scholar] [CrossRef]
- Saenger, K.; Tong, H. Laser interferometry: A measurement technique for diffusion studies in thin polymer films. Polym. Eng. Sci. 1991, 31, 432–435. [Google Scholar] [CrossRef]
- Yasuda, H.; Wang, C. Plasma polymerization investigated by the substrate temperature dependence. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 87–106. [Google Scholar] [CrossRef]
- d’Agostino, R.; Cramarossa, F.; Fracassi, F.; Illuzzi, F. Plasma polymerization of fluorocarbons. Plasma Depos. Treat. Etch. Polym. 1990, 2, 95–162. [Google Scholar]
- Gürsoy, M.; Karaman, M. Improvement of wetting properties of expanded perlite particles by an organic conformal coating. Prog. Org. Coat. 2018, 120, 190–197. [Google Scholar] [CrossRef]
- Wuu, D.; Lo, W.; Chang, L.; Horng, R.-H. Properties of SiO2-like barrier layers on polyethersulfone substrates by low-temperature plasma-enhanced chemical vapor deposition. Thin Solid Films 2004, 468, 105–108. [Google Scholar] [CrossRef]
- Ozaydin-Ince, G.; Gleason, K.K. Transition between kinetic and mass transfer regimes in the initiated chemical vapor deposition from ethylene glycol diacrylate. J. Vac. Sci. Technol. A 2009, 27, 1135–1143. [Google Scholar] [CrossRef]
- Mercan, E.S.; Karaman, M. Coating of hydrophilic poly (hydroxypropyl methacrylate) thin films via pulsed-initiated chemical vapor deposition method. J. Coat. Technol. Res. 2021, 18, 1261–1268. [Google Scholar] [CrossRef]
- Karaman, M.; Gürsoy, M.; Kuş, M.; Özel, F.; Yenel, E.; Şahin, Ö.G.; Kivrak, H.D. Chemical and physical modification of surfaces. In Surface Treatments for Biological, Chemical, and Physical Applications; Wiley: Hoboken, NJ, USA, 2017; pp. 23–66. [Google Scholar]
- Mao, Y.; Gleason, K.K. Hot filament chemical vapor deposition of poly (glycidyl methacrylate) thin films using tert-butyl peroxide as an initiator. Langmuir 2004, 20, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.P.; Gleason, K.K. Combinatorial initiated CVD for polymeric thin films. Chem. Vap. Depos. 2006, 12, 685–691. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Günzler, H.; Gremlich, H.-U. IR Spectroscopy. An Introduction; IAEA: Vienna, Austria, 2002. [Google Scholar]
- Gürsoy, M. Fabrication of paper-based microfluidic devices using PECVD for selective separation. Macromol. Res. 2021, 29, 423–429. [Google Scholar] [CrossRef]
- Biederman, H.; Slavınská, D. Plasma polymer films and their future prospects. Surf. Coat. Technol. 2000, 125, 371–376. [Google Scholar] [CrossRef]
- Rupper, P.; Vandenbossche, M.; Bernard, L.; Hegemann, D.; Heuberger, M. Composition and stability of plasma polymer films exhibiting vertical chemical gradients. Langmuir 2017, 33, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Beamson, G. High Relution XPS of Organic Polymers. The Scienta ESCA 300 Database. ICIplc; Wiley: Chichester, UK, 1992. [Google Scholar]
- Karaman, M.; Çabuk, N. Initiated chemical vapor deposition of pH responsive poly (2-diisopropylamino) ethyl methacrylate thin films. Thin Solid Films 2012, 520, 6484–6488. [Google Scholar] [CrossRef]
- Fielding, L.A.; Edmondson, S.; Armes, S.P. Synthesis of pH-responsive tertiary amine methacrylate polymer brushes and their response to acidic vapour. J. Mater. Chem. 2011, 21, 11773–11780. [Google Scholar] [CrossRef]
- Giacomelli, F.C.; Stepánek, P.; Giacomelli, C.; Schmidt, V.; Jäger, E.; Jäger, A.; Ulbrich, K. pH-triggered block copolymer micelles based on a pH-responsive PDPA (poly [2-(diisopropylamino) ethyl methacrylate]) inner core and a PEO (poly (ethylene oxide)) outer shell as a potential tool for the cancer therapy. Soft Matter 2011, 7, 9316–9325. [Google Scholar] [CrossRef]
- Cheng, C.; Gupta, M. Roll-to-roll surface modification of cellulose paper via initiated chemical vapor deposition. Ind. Eng. Chem. Res. 2018, 57, 11675–11680. [Google Scholar] [CrossRef]
- Shrestha, S.; Parajuli, S.; Park, J.; Yang, H.; Cho, T.-Y.; Eom, J.-H.; Cho, S.-K.; Lim, J.; Cho, G.; Jung, Y. Improving Stability of Roll-to-Roll (R2R) Gravure-Printed Carbon Nanotube-Based Thin Film Transistors via R2R Plasma-Enhanced Chemical Vapor-Deposited Silicon Nitride. Nanomaterials 2023, 13, 559. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Value |
|---|---|
| Substrate temperature (°C) | 10, 20, 30, and 40 |
| Reactor pressure (mtorr) | 100, 300, and 500 |
| Plasma power (W) | 20, 40, 60, and 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gürsoy, M. Fabrication of pH-Responsive PDPAEMA Thin Film Using a One-Step Environmentally Friendly Plasma Enhanced Chemical Vapor Deposition. Coatings 2024, 14, 347. https://doi.org/10.3390/coatings14030347
Gürsoy M. Fabrication of pH-Responsive PDPAEMA Thin Film Using a One-Step Environmentally Friendly Plasma Enhanced Chemical Vapor Deposition. Coatings. 2024; 14(3):347. https://doi.org/10.3390/coatings14030347
Chicago/Turabian StyleGürsoy, Mehmet. 2024. "Fabrication of pH-Responsive PDPAEMA Thin Film Using a One-Step Environmentally Friendly Plasma Enhanced Chemical Vapor Deposition" Coatings 14, no. 3: 347. https://doi.org/10.3390/coatings14030347






