Hydrogen Production Using Modern Photocatalysts
Abstract
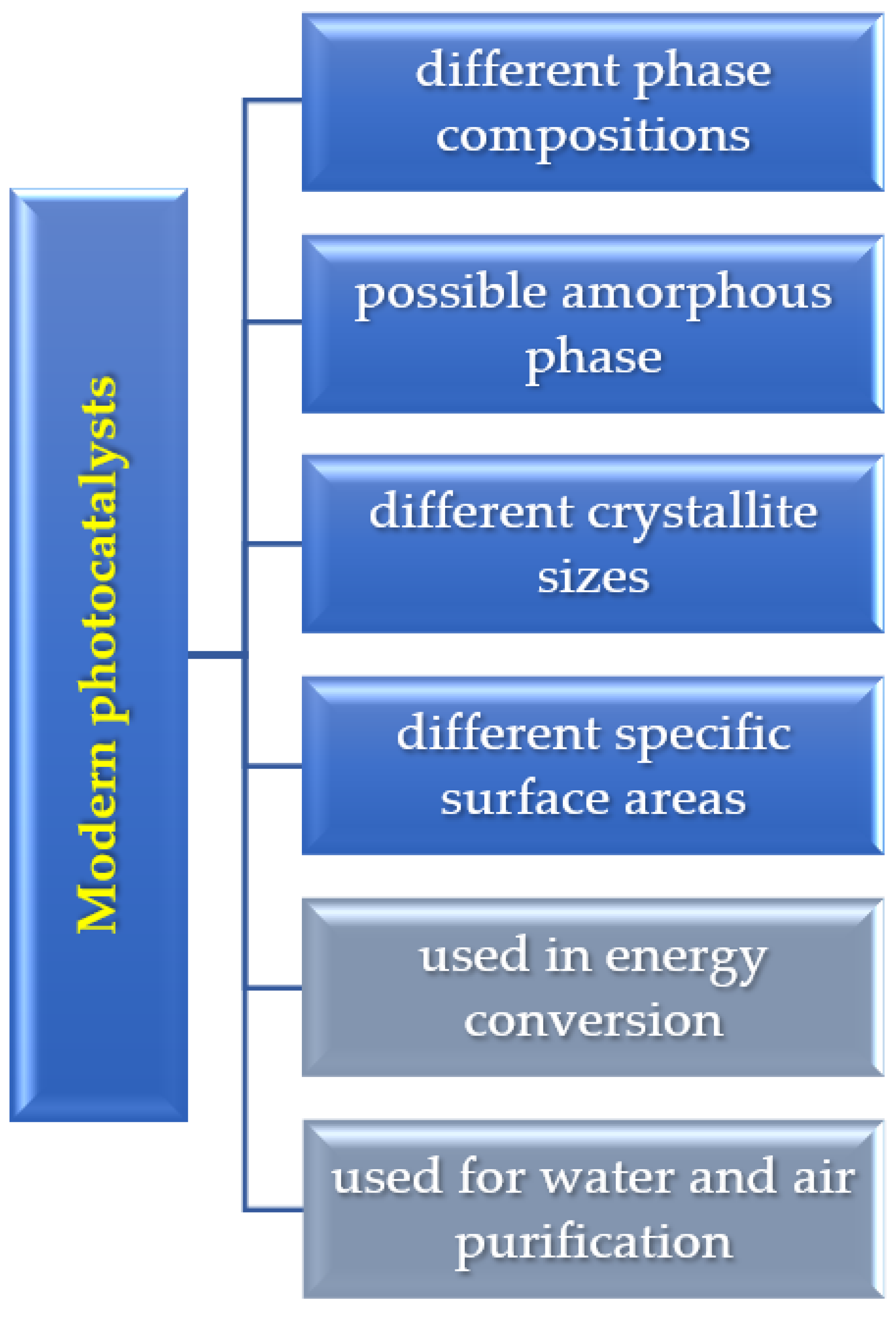
:1. Introduction
2. Photocatalysis as a Method for H2 Production
3. Hydrogen as the Fuel of the Future
- Fuel economics, which can make it difficult to replace diesel in some applications [43].
- Sustainability issues related to biofuels are one of the obstacles in this transition process, and the industry faces unique key challenges in the transition process for developing and deploying future fuels [46].
- The economic export of clean energy—many countries do not have sufficient domestic energy supplies, neither renewable nor other, and rely on imported energy sources [47].
- Keeping up with modern technologies—older technologies often do not have a chance to take root on the market due to the rapid introduction of new fuels [48].
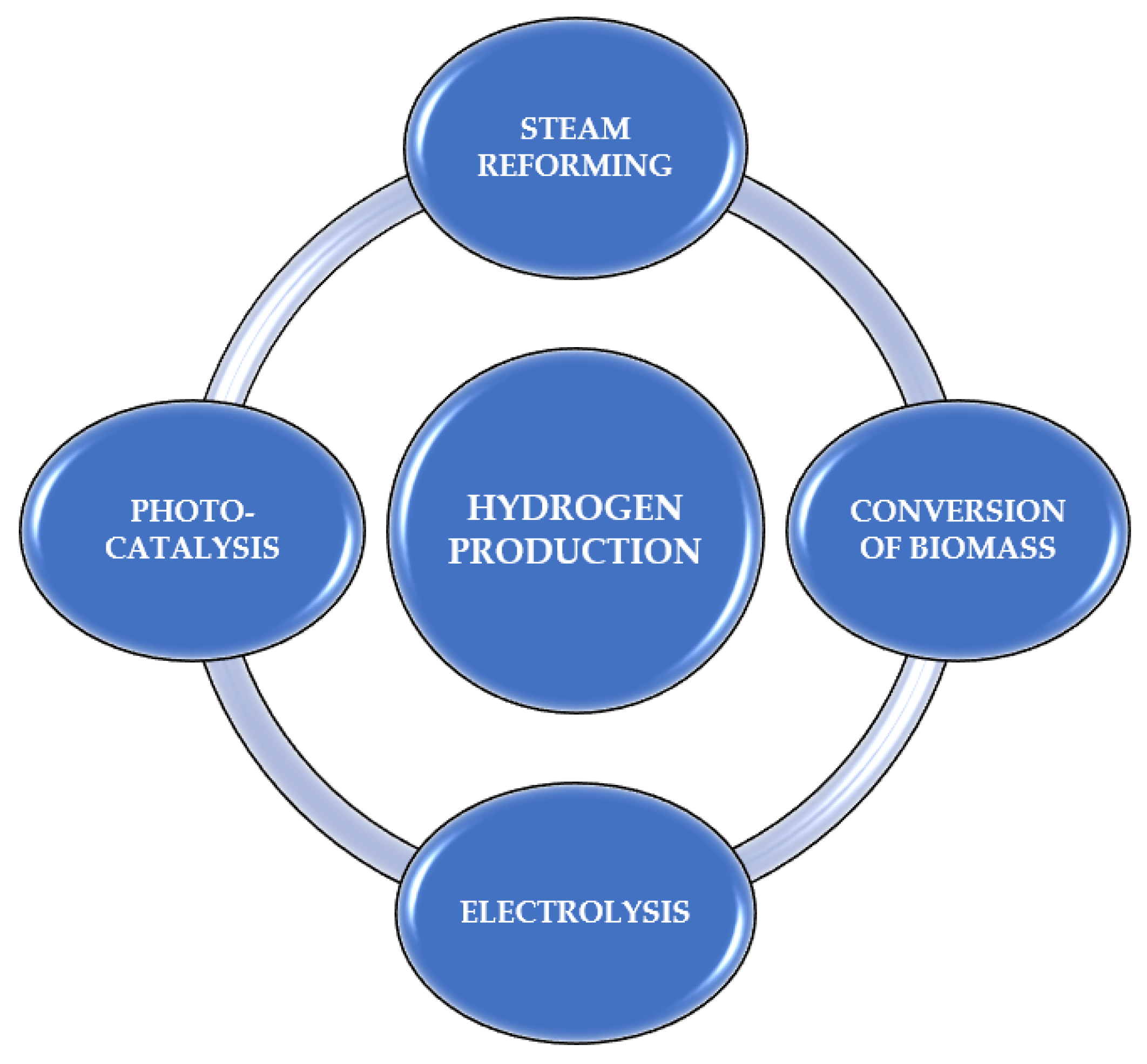
4. The Role of Photocatalysts in Hydrogen Production
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Centi, G.; Perathoner, S.; Genovese, C.; Arrigo, R. Advanced (Photo)Electrocatalytic Approaches to Substitute the Use of Fossil Fuels in Chemical Production. Chem. Commun. 2023, 6, 3005–3023. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Redesign Chemical Processes to Substitute the Use of Fossil Fuels: A Viewpoint of the Implications on Catalysis. Catal. Today 2022, 387, 216–223. [Google Scholar] [CrossRef]
- Stankiewicz, A.I.; Nigar, H. Beyond Electrolysis: Old Challenges and New Concepts of Electricity-Driven Chemical Reactors. React. Chem. Eng. 2020, 1, 1005–1016. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Centi, G.; Perathoner, S.; Lanzafame, P. Catalysis for E-Chemistry: Need and Gaps for a Future De-Fossilized Chemical Production, with Focus on the Role of Complex (Direct) Syntheses by Electrocatalysis. ACS Catal. 2022, 4, 2861–2876. [Google Scholar] [CrossRef] [PubMed]
- Centi, G.; Perathoner, S. Status and Gaps toward Fossil-Free Sustainable Chemical Production. Green Chem. 2022, 24, 7305–7331. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Ahmad, I.; Zou, Y.; Yan, J.; Liu, Y.; Shukrullah, S.; Naz, M.Y.; Hussain, H.; Khan, W.Q.; Khalid, N.R. Semiconductor Photocatalysts: A Critical Review Highlighting the Various Strategies to Boost the Photocatalytic Performances for Diverse Applications. Adv. Colloid Interface Sci. 2023, 311, 102830. [Google Scholar] [CrossRef]
- Wang, T.; Tian, B.; Han, B.; Ma, D.; Sun, M.; Hanif, A.; Xia, D.; Shang, J. Recent Advances on Porous Materials for Synergetic Adsorption and Photocatalysis. Energy Environ. Mater. 2022, 5, 711–730. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Bakbolat, B.; Baimenov, A.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Photocatalysts for a Sustainable Future: Innovations in Large-Scale Environmental and Energy Applications. Sci. Total Environ. 2023, 885, 163914. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31–85. [Google Scholar] [CrossRef]
- Wang, X.; Tang, W.; Jiang, L.; Feng, J.; Yang, J.; Zhou, S.; Li, W.; Yuan, X.; Wang, H.; Wang, J.; et al. Mechanism insights into visible light-induced crystalline carbon nitride activating periodate for highly efficient ciprofloxacin removal. J. Chem. Eng. 2023, 471, 144521. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Lee, J.S. Nanomaterials for Photocatalytic Hydrogen Production: From Theoretical Perspectives. RSC Adv. 2017, 7, 34875–34885. [Google Scholar] [CrossRef]
- Ain, N.; Amin, A.M.; Fatimah, H.; Zaid, M. A Review of Photocatalytic Water Splitting for Hydrogen Production Using Tandem Solar Cell. Preprints 2023, 2023092044. [Google Scholar] [CrossRef]
- Galbao, S.J.; Chandrappa, S.; Murthy, D.H.K. Recent Progress and Developments in Photocatalytic Overall Water Splitting. In Towards Hydrogen Infrastructure: Advances and Challenges in Preparing for the Hydrogen Economy; Jaiswal-Nagar, D., Dixit, V., Devasahayam, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 77–98. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Zhao, Y.; Han, X. Effect of Phase Composition, Morphology, and Specific Surface Area on the Photocatalytic Activity of TiO2 Nanomaterials. RSC Adv. 2014, 4, 47031–47038. [Google Scholar] [CrossRef]
- Rapti, I.; Kosma, C.; Albanis, T.; Konstantinou, I. Solar Photocatalytic Degradation of Inherent Pharmaceutical Residues in Real Hospital WWTP Effluents Using Titanium Dioxide on a CPC Pilot Scale Reactor. Catal. Today 2023, 423, 113884. [Google Scholar] [CrossRef]
- Abdullah, M.; Iqbal, J.; Ur Rehman, M.S.; Khalid, U.; Mateen, F.; Arshad, S.N.; Al-Sehemi, A.G.; Algarni, H.; Al-Hartomy, O.A.; Fazal, T. Removal of Ceftriaxone Sodium Antibiotic from Pharmaceutical Wastewater Using an Activated Carbon Based TiO2 Composite: Adsorption and Photocatalytic Degradation Evaluation. Chemosphere 2023, 317, 137834. [Google Scholar] [CrossRef]
- Mishra, S.; Sundaram, B. A Review of the Photocatalysis Process Used for Wastewater Treatment. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Jaison, A.; Mohan, A.; Lee, Y.C. Recent Developments in Photocatalytic Nanotechnology for Purifying Air Polluted with Volatile Organic Compounds: Effect of Operating Parameters and Catalyst Deactivation. Catalysts 2023, 13, 407. [Google Scholar] [CrossRef]
- Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Recent Progress on Semiconductor Heterogeneous Photocatalysts in Clean Energy Production and Environmental Remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Noureen, L.; Wang, Q.; Humayun, M.; Shah, W.A.; Xu, Q.; Wang, X. Recent Advances in Structural Engineering of Photocatalysts for Environmental Remediation. Environ. Res. 2023, 219, 115084. [Google Scholar] [CrossRef]
- Qureshi, W.A.; Haider, S.N.U.Z.; Naveed, A.; Ali, A.; Liu, Q.; Yang, J. Recent Progress in the Synthesis, Characterization and Photocatalytic Application of Energy Conversion over Single Metal Atoms Decorated Graphitic Carbon Nitride. Int. J. Hydrogen Energy 2023, 48, 19459–19485. [Google Scholar] [CrossRef]
- Chauhan, A.; Sudhaik, A.; Raizada, P.; Khan, A.A.P.; Singh, A.; Van Le, Q.; Nguyen, V.H.; Ahamad, T.; Thakur, S.; Singh, P.; et al. Enhancement Strategies for ZnSe Based Photocatalysts: Application to Environmental Remediation and Energy Conversion. Process Saf. Environ. 2023, 170, 415–435. [Google Scholar] [CrossRef]
- Chawla, A.; Sudhaik, A.; Sonu; Raizada, P.; Ahamad, T.; Van Le, Q.; Nguyen, V.H.; Thakur, S.; Mishra, A.K.; Selvasembian, R.; et al. Bi-Rich BixOyBrz-Based Photocatalysts for Energy Conversion and Environmental Remediation: A Review. Coord. Chem. Rev. 2023, 491, 215246. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. Recent Progress in Semiconductor/Graphene Photocatalysts: Synthesis, Photocatalytic Applications, and Challenges. RSC Adv. 2023, 13, 421–439. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pansambal, S.; Lin, K.Y.A.; Pore, D.; Oza, R. Recent Advances in Synthesis of CeVO4 Nanoparticles and Their Potential Scaffold for Photocatalytic Applications. Top. Catal. 2023, 66, 89–103. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Etim, I.I.N.; Okonkwo, B.O.; Olanrele, O.S.; Njoku, D.I.; Kolawole, S.K.; Emori, W.; Ikeuba, A.I.; Njoku, C.N.; Ekerenam, O.O.; et al. Recent Design Approaches, Adhesion Mechanisms, and Applications of Antibacterial Surfaces. Chem. Eng. J. Adv. 2023, 16, 100563. [Google Scholar] [CrossRef]
- Ferreira, M.F.S.; Brambilla, G.; Thévenaz, L.; Feng, X.; Zhang, L.; Sumetsky, M.; Jones, C.; Pedireddy, S.; Vollmer, F.; Dragic, P.D.; et al. Roadmap on Optical Sensors. J. Opt. 2024, 26, 013001–013037. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zou, J.P.; Zhao, L.N.; Zhou, G.; Luo, X.B.; Luo, S.L. Nanomaterial-Based Photocatalytic Hydrogen Production. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 59–82. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A Review of Intensification of Photocatalytic Processes. Chem. Eng. Process. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic Hydrogen Production. Mater. Sci. Semicond. Process 2013, 16, 561–575. [Google Scholar] [CrossRef]
- Li, L.; Guo, C.F.; Shen, J.L.; Ning, J.Q.; Zhong, Y.J.; Hu, Y. Construction of sugar-gourdshaped CdS/Co1-xS hollow heteronanostructure as an efficient Z-scheme photocatalyst for hydrogen generation. J. Chem. Eng. 2020, 400, 125925. [Google Scholar] [CrossRef]
- Megala, S.; Sathish, M.; Harish, S.; Navaneethan, M.; Sohila, S.; Liang, B.; Ramesh, R. Enhancement of photocatalytic H2 evolution from water splitting by construction of two dimensional gC3N4/NiAl layered double hydroxides. Appl. Surf. Sci. 2020, 509, 144656. [Google Scholar] [CrossRef]
- Ma, D.D.; Wang, Z.Y.; Shi, J.W.; Zhu, M.S.; Yu, H.; Zou, Y.J.; Lv, Y.X.; Sun, G.T.; Mao, S.M.; Cheng, Y.H. Cu-In2S3 nanorod induced the growth of Cu&In co-doped multi-arm CdS heterophase junction to promote photocatalytic H2 evolution. J. Chem. Eng. 2020, 399, 125785. [Google Scholar] [CrossRef]
- Sun, Z.J.; Chen, H.L.; Huang, Q.; Du, P.W. Enhanced photocatalytic hydrogen production in water under visible light using noble-metal-free ferrous phosphide as an active cocatalyst. Catal. Sci. Technol. 2015, 5, 4964–4967. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, J.J.; Ding, Y.P.; Zheng, D.L.; Lin, Y.J.; Chen, Y.W.; Gao, W.H. Rationally designed hierarchical hollow ZnCdS@MoS2 heterostructured cages with efficient separation of photogenerated carriers for photoelectrochemical aptasensing of lincomycin. Sens. Actuators B Chem. 2020, 306, 127552. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, C.; Cao, X.; Chen, T.; Kou, Y.; Zhang, L.; Wang, T.; Akram, N.; Wang, J. Photocatalytic performance and mechanism of hydrogen evolution from water over ZnCdS/Co@CoO in sacrificial agent-free system. Int. J. Hydrogen Energy 2022, 47, 25289–25299. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Krishna Chava, R.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Green Hydrogen: A Pathway to a Sustainable Energy Future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Stančin, H.; Mikulčić, H.; Wang, X.; Duić, N. A Review on Alternative Fuels in Future Energy System. Renew. Sustain. Energy Rev. 2020, 128, 109927. [Google Scholar] [CrossRef]
- Thiruselvi, D.; Kumar, P.S.; Kumar, M.A.; Lay, C.H.; Aathika, S.; Mani, Y.; Jagadiswary, D.; Dhanasekaran, A.; Shanmugam, P.; Sivanesan, S.; et al. A Critical Review on Global Trends in Biogas Scenario with Its Up-Gradation Techniques for Fuel Cell and Future Perspectives. Int. J. Hydrogen Energy 2021, 46, 16734–16750. [Google Scholar] [CrossRef]
- Bonenkamp, T.B.; Middelburg, L.M.; Hosli, M.O.; Wolffenbuttel, R.F. From Bioethanol Containing Fuels towards a Fuel Economy That Includes Methanol Derived from Renewable Sources and the Impact on European Union Decision-Making on Transition Pathways. Renew. Sustain. Energy Rev. 2020, 120, 109667. [Google Scholar] [CrossRef]
- Wang, Y.; Wright, L.A. A Comparative Review of Alternative Fuels for the Maritime Sector: Economic, Technology, and Policy Challenges for Clean Energy Implementation. World 2021, 2, 456–481. [Google Scholar] [CrossRef]
- Ansell, P.J. Review of Sustainable Energy Carriers for Aviation: Benefits, Challenges, and Future Viability. Prog. Aerosp. Sci. 2023, 141, 100919. [Google Scholar] [CrossRef]
- Elbehri, A.; Segerstedt, A.; Liu, P.; Food and Agriculture Organization of the United Nations. Trade and Markets Division. Biofuels and the Sustainability Challenge: A Global Assessment of Sustainability Issues, Trends and Policies for Biofuels and Related Feedstocks; FAO: Italy, Roma; Available online: https://www.fao.org/3/i3126e/i3126e.pdf (accessed on 23 January 2024).
- Sharma, G.D.; Mendy, J.; Shahzad, U. Editorial: Export Product Quality, Renewable Energy, and Sustainable Production. Front. Environ. Sci. 2022, 10, 1069041. [Google Scholar] [CrossRef]
- Dwivedi, Y.K.; Hughes, L.; Kar, A.K.; Baabdullah, A.M.; Grover, P.; Abbas, R.; Andreini, D.; Abumoghli, I.; Barlette, Y.; Bunker, D.; et al. Climate Change and COP26: Are Digital Technologies and Information Management Part of the Problem or the Solution? An Editorial Reflection and Call to Action. Int. J. Inf. Manag. 2022, 63, 102456. [Google Scholar] [CrossRef]
- Miller, I.; Warde, P.; Tanner, A.; McNeill, J.R.; Seow, V.; Valencius, C.B.; Lifset, R.D. Forum: The Environmental History of Energy Transitions. Environ. Hist. 2019, 24, 463–533. [Google Scholar] [CrossRef]
- Ntanos, S.; Skordoulis, M.; Kyriakopoulos, G.; Arabatzis, G.; Chalikias, M.; Galatsidas, S.; Batzios, A.; Katsarou, A. Renewable Energy and Economic Growth: Evidence from European Countries. Sustainability 2018, 10, 2626. [Google Scholar] [CrossRef]
- Hossain, E.; Faruque, H.M.R.; Sunny, M.S.H.; Mohammad, N.; Nawar, N. A Comprehensive Review on Energy Storage Systems: Types, Comparison, Current Scenario, Applications, Barriers, and Potential Solutions, Policies, and Future Prospects. Energies 2020, 13, 3651. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review. Carbon Capture Sci. Technol. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Qazi, U.Y. Future of Hydrogen as an Alternative Fuel for Next-Generation Industrial Applications; Challenges and Expected Opportunities. Energies 2022, 15, 4741. [Google Scholar] [CrossRef]
- Mufutau Opeyemi, B. Path to Sustainable Energy Consumption: The Possibility of Substituting Renewable Energy for Non-Renewable Energy. Energy 2021, 228, 120519. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Dogan, E.; Hodžić, S.; Šikić, T.F. Do Energy and Environmental Taxes Stimulate or Inhibit Renewable Energy Deployment in the European Union? Renew. Energy 2023, 202, 1138–1145. [Google Scholar] [CrossRef]
- Gyamfi, B.A.; Kwakwa, P.A.; Adebayo, T.S. Energy intensity among European Union countries: The role of renewable energy, income and trade. Int. J. Energy Sect. Manag. 2023, 17, 801–819. [Google Scholar] [CrossRef]
- Esposito, L.; Romagnoli, G. Overview of Policy and Market Dynamics for the Deployment of Renewable Energy Sources in Italy: Current Status and Future Prospects. Heliyon 2023, 9, e17406. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/eurostat/web/interactive-publications/energy-2023 (accessed on 23 January 2024).
- Rokicki, T.; Bórawski, P.; Szeberényi, A. The Impact of the 2020–2022 Crises on EU Countries’ Independence from Energy Imports, Particularly from Russia. Energies 2023, 16, 6629. [Google Scholar] [CrossRef]
- Watanabe, M.D.B.; Cherubini, F.; Tisserant, A.; Cavalett, O. Drop-in and Hydrogen-Based Biofuels for Maritime Transport: Country-Based Assessment of Climate Change Impacts in Europe up to 2050. Energy Conv. Manag. 2022, 273, 116403. [Google Scholar] [CrossRef]
- Vidović, T.; Šimunović, J.; Radica, G.; Penga, Ž. Systematic Overview of Newly Available Technologies in the Green Maritime Sector. Energies 2023, 16, 641. [Google Scholar] [CrossRef]
- Xing, H.; Stuart, C.; Spence, S.; Chen, H. Alternative Fuel Options for Low Carbon Maritime Transportation: Pathways to 2050. J. Clean. Prod. 2021, 297, 126651. [Google Scholar] [CrossRef]
- Prussi, M.; Lee, U.; Wang, M.; Malina, R.; Valin, H.; Taheripour, F.; Velarde, C.; Staples, M.D.; Lonza, L.; Hileman, J.I. CORSIA: The First Internationally Adopted Approach to Calculate Life-Cycle GHG Emissions for Aviation Fuels. Renew. Sustain. Energy Rev. 2021, 150, 111398. [Google Scholar] [CrossRef]
- Huang, J.; Fan, H.; Xu, X.; Deng, Z. Life Cycle Greenhouse Gas Emission Assessment for Using Alternative Marine Fuels: A Very Large Crude Carrier (VLCC) Case Study. J. Mar. Sci. Eng. 2022, 10, 1969. [Google Scholar] [CrossRef]
- Zhu, X.; Xiao, J.; Wang, C.; Zhu, L.; Wang, S. Global Warming Potential Analysis of Bio-Jet Fuel Based on Life Cycle Assessment. Carb. Neutrality 2022, 1, 25–38. [Google Scholar] [CrossRef]
- Aste, N.; Bocciolone, M.; Bogdanov, D.; Brost, E.; Breyer, C.; Burrows, V.; Calvo Ambel, C.; Colombo, E.; Del Pero, C.; Earl, T.; et al. Roadmap to 2050 a manual for nations to decarbonize by mid-century. In Roadmap to 2050 A Manual for Nations to Decarbonize by Mid-Century; Sustainable Development Solutions Network and Fondazione: Milano, Italy, 2019; pp. 1–144. [Google Scholar]
- IRENA. A Pathway to Decarbonise the Shipping Sector by 2050; International Renewable Energy Agency, 2021; Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2021/Oct/IRENA_Decarbonising_Shipping_2021.pdf?rev=b5dfda5f69e741a4970680a5ced1ac1e (accessed on 23 January 2024).
- Reggeti, S.A.; Kane, S.P.; Northrop, W.F. Hydrogen Production in Ammonia-Fueled Spark Ignition Engines. Appl. Energy Combust. Sci. 2023, 14, 100136. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F.; Ni, B.-J. Green Ammonia Production Technologies: A Review of Practical Progress. J. Environ. Manag. 2023, 342, 118348. [Google Scholar] [CrossRef]
- Adeli, K.; Nachtane, M.; Faik, A.; Saifaoui, D.; Boulezhar, A. How Green Hydrogen and Ammonia Are Revolutionizing the Future of Energy Production: A Comprehensive Review of the Latest Developments and Future Prospects. Appl. Sci. 2023, 13, 8711. [Google Scholar] [CrossRef]
- Linzenich, A.; Arning, K.; Bongartz, D.; Mitsos, A.; Ziefle, M. What Fuels the Adoption of Alternative Fuels? Examining Preferences of German Car Drivers for Fuel Innovations. Appl. Energy 2019, 249, 222–236. [Google Scholar] [CrossRef]
- Burchart-Korol, D.; Gazda-Grzywacz, M.; Zarȩbska, K. Research and Prospects for the Development of Alternative Fuels in the Transport Sector in Poland: A Review. Energies 2020, 13, 2988. [Google Scholar] [CrossRef]
- Sandaka, B.P.; Kumar, J. Alternative Vehicular Fuels for Environmental Decarbonization: A Critical Review of Challenges in Using Electricity, Hydrogen, and Biofuels as a Sustainable Vehicular Fuel. Chem. Eng. J. Adv. 2023, 14, 100442. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen Production, Storage, Utilisation and Environmental Impacts: A Review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Bäckstrand, K. Towards a Climate-Neutral Union by 2050? The European Green Deal, Climate Law, and Green Recovery. In Routes to a Resilient European Union; Bakardjieva Engelbrekt, A., Ekman, P., Michalski, A., Oxelheim, L., Eds.; Palgrave Macmillan: Cham, Switzerland, 2022; pp. 39–61. [Google Scholar] [CrossRef]
- Capros, P.; Zazias, G.; Evangelopoulou, S.; Kannavou, M.; Fotiou, T.; Siskos, P.; De Vita, A.; Sakellaris, K. Energy-system modelling of the EU strategy towards climate-neutrality. Energy Policy 2019, 134, 110960. [Google Scholar] [CrossRef]
- Bethoux, O. Hydrogen Fuel Cell Road Vehicles and Their Infrastructure: An Option towards an Environmentally Friendly Energy Transition. Energies 2020, 13, 6132. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Genovese, M.; Piraino, F.; Fragiacomo, P. 3E Analysis of a Virtual Hydrogen Valley Supported by Railway-Based H2 Delivery for Multi-Transportation Service. Renew. Sustain. Energy Rev. 2024, 191, 114070. [Google Scholar] [CrossRef]
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Baumann, M.; Domnik, T.; Haase, M.; Wulf, C.; Emmerich, P.; Rösch, C.; Zapp, P.; Naegler, T.; Weil, M. Comparative patent analysis for the identification of global research trends for the case of battery storage, hydrogen and bioenergy. Technol. Forecast. Soc. Chang. 2021, 165, 120505. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam Reforming of Methanol for Hydrogen Production: A Critical Analysis of Catalysis, Processes, and Scope. Ind. Eng. Chem. Res. 2021, 60, 89–113. [Google Scholar] [CrossRef]
- Pal, D.B.; Singh, A.; Bhatnagar, A. A Review on Biomass Based Hydrogen Production Technologies. Int. J. Hydrogen Energy 2022, 47, 1461–1480. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen Production by Water Electrolysis Technologies: A Review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Mansoor, S.; Tayyab, M.; Khan, M.; Akmal, Z.; Zhou, L.; Lei, J.; Anpo, M.; Zhang, J. Recent Advancements in Se- and Te-Enriched Cocatalysts for Boosting Photocatalytic Splitting of Water to Produce Hydrogen. Res. Chem. Intermed. 2023, 49, 3723–3745. [Google Scholar] [CrossRef]
- Megia, P.J.; Vizcaino, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A Comprehensive Review on Hydrogen Production from Coal Gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Azizan, M.T.; Aqsha, A.; Ameen, M.; Syuhada, A.; Klaus, H.; Sumaiya, Z.A.; Sher, F. Catalytic Reforming of Oxygenated Hydrocarbons for the Hydrogen Production: An Outlook. Biomass Convers. Biorefin. 2023, 13, 8441–8464. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.; Cheng, Y.W.; Ng, K.H.; Vo, D.V.N.; Lam, M.K.; Lim, J.W. Bio-Hydrogen Production from Steam Reforming of Liquid Biomass Wastes and Biomass-Derived Oxygenates: A Review. Fuel 2022, 311, 122623. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic Water Splitting Hydrogen Production via Environmental Benign Carbon Based Nanomaterials. Int. J. Hydrogen Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic Solar Hydrogen Production from Water on a 100-M2 Scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Rambhujun, N.; Salman, M.S.; Wang, T.; Pratthana, C.; Sapkota, P.; Costalin, M.; Lai, Q.; Aguey-Zinsou, K.F. Renewable Hydrogen for the Chemical Industry. MRS Energy Sustain. 2020, 7, 33–49. [Google Scholar] [CrossRef]
- Le, P.A.; Trung, V.D.; Nguyen, P.L.; Bac Phung, T.V.; Natsuki, J.; Natsuki, T. The Current Status of Hydrogen Energy: An Overview. RSC Adv. 2023, 40, 28262–28287. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Tahir, M.; Haq, M.; Mohamed, M.M.I.; Kamyab, H.; Nguyen, H.-H.T.; Vo, D.-V.N.; Ibrahim, H. Renewable Hydrogen Production via Biological and Thermochemical Routes: Nanomaterials, Economic Analysis and Challenges. Process Saf. Environ. 2023, 179, 68–88. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Melitos, G.; Voulkopoulos, X.; Zabaniotou, A. Waste to Sustainable Biohydrogen Production Via Photo-Fermentation and Biophotolysis—A Systematic Review. Renew. Energy Environ. Sustain. 2021, 6, 45–64. [Google Scholar] [CrossRef]
- Baroutaji, A.; Arjunan, A.; Robinson, J.; Abdelkareem, M.A.; Olabi, A.G. Additive Manufacturing for Proton Exchange Membrane (PEM) Hydrogen Technologies: Merits, Challenges, and Prospects. Int. J. Hydrogen Energy 2024, 52, 561–584. [Google Scholar] [CrossRef]
- Clean Hydrogen Monitor. Available online: https://hydrogeneurope.eu/wp-content/uploads/2023/10/Clean_Hydrogen_Monitor_11-2023_DIGITAL.pdf (accessed on 8 March 2024).
- Hydrogen. Net Zero Emissions Guide. Available online: https://www.iea.org/reports/hydrogen-2156#dashboard (accessed on 8 March 2024).
- Yaemsunthorn, K. Phase-Dependent Photocatalytic Activity of TiO2—The Role of Intrinsic and Extrinsic Factors. Ph.D. Thesis, Jagiellonian University, Kraków, Poland, 2023. Available online: https://ruj.uj.edu.pl/xmlui/bitstream/handle/item/317108/yaemsunthorn_phase-dependent_photocatalytic_activity_of_tio2_2023.pdf?sequence=1&isAllowed=y (accessed on 23 January 2024).
- Jiménez-Calvo, P. Synergy of visible-light responsive photocatalytic materials and device engineering for energy and environment: Minireview on hydrogen production and water decontamination. Mater. Today Cat. 2024, 4, 100040. [Google Scholar] [CrossRef]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.A.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current Understanding and Challenges of Solar-Driven Hydrogen Generation Using Polymeric Photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Du, H.; Liu, Y.N.; Shen, C.C.; Xu, A.W. Nanoheterostructured Photocatalysts for Improving Photocatalytic Hydrogen Production. Chin. J. Catal. 2017, 38, 1295–1306. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sánchez-Sánchez, M.C.; Alvarez-Galvan, M.C.; Del Valle, F.; Fierro, J.L.G. Hydrogen Production from Renewable Sources: Biomass and Photocatalytic Opportunities. Energy Environ. Sci. 2009, 2, 35–54. [Google Scholar] [CrossRef]
- Tahir, M.B.; Asiri, A.M.; Nawaz, T. A Perspective on the Fabrication of Heterogeneous Photocatalysts for Enhanced Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 24544–24557. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Wang, S.; Zhu, N.; Li, Z.; Zhao, L.; Wang, Y. Recent Advances of Semiconductor Photocatalysis for Water Pollutant Treatment: Mechanisms, Materials and Applications. Phys. Chem. Chem. Phys. 2023, 7, 25899–25924. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting-an Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of Photocatalytic Performance with the Use of Noble-Metal-Decorated TiO2 Nanocrystals as Highly Active Catalysts for Aerobic Oxidation under Visible-Light Irradiation. Appl. Catal. B 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, C.; Dragoe, D.; Beaunier, P.; Colbeau-Justin, C.; Remita, H. Highly Promoted Photocatalytic Hydrogen Generation by Multiple Electron Transfer Pathways. Appl. Catal. B 2021, 281, 119457. [Google Scholar] [CrossRef]
- Portugal, G.R.; Santos, S.F.; Arantes, J.T. NaTaO3 Cubic and Orthorhombic Surfaces: An Intrinsic Improvement of Photocatalytic Properties. Appl. Surf. Sci. 2020, 502, 144206. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Chiarello, G.L.; Pedroni, M.; Livraghi, S.; Giamello, E.; Selli, E. High Photocatalytic Hydrogen Production on Cu(II) Pre-Grafted Pt/TiO2. Appl. Catal. B 2017, 209, 417–428. [Google Scholar] [CrossRef]
- Yesupatham, M.S.; Augustin, A.; Agamendran, N.; Honnappa, B.; Shanmugam, M.; Sagayaraj, P.J.J.; Thennarasu, G.; Sagaya Selvam, N.C.; Sekar, K. Photocatalytic Seawater Splitting for Hydrogen Fuel Production: Impact of Seawater Components and Accelerating Reagents on the Overall Performance. Sustain. Energy Fuels 2023, 7, 4727–4757. [Google Scholar] [CrossRef]
- Ashfaq, Z.; Iqbal, T.; Ali, H.; Eldin, S.M.; Mahtab Alam, M.; Al-Harbi, F.F.; Arshad, M.; Galal, A.M. Review of different CdS/TiO2 and WO3/g-C3N4 composite based photocatalyst for hydrogen production. Arab. J. Chem. 2023, 16, 105024. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-Infrared Light Responsive TiO2 for Efficient Solar Energy Utilization. Adv. Funct. Mater. 2022, 32, 2108977. [Google Scholar] [CrossRef]
- Gomez-Cerezo, N.; Sayago-Carro, R.; Cortés-Bazo, A.; Fernández-García, M.; Kubacka, A. PdCu deposited alloys on TiO2 for hydrogen photo-production. Catal. Today 2023, 423, 114280. [Google Scholar] [CrossRef]
- Ma, J.; Dai, J.; Duan, Y.; Zhang, J.; Qiang, L.; Xue, J. Fabrication of PANI-TiO2/rGO hybrid composites for enhanced photocatalysis of pollutant removal and hydrogen production. Renew. Energy 2020, 156, 1008–1018. [Google Scholar] [CrossRef]
- Bharatvaj, J.; Preethi, V.; Kanmani, S. Hydrogen production from sulphide wastewater using Ce3+–TiO2 photocatalysis. Int. J. Hydrogen Energy 2018, 43, 3935–3945. [Google Scholar] [CrossRef]
- Perović, K.; Kovačić, M.; Roković, M.K.; Kušić, H.; Genorio, B.; Štangar, U.L.; Božić, A.L. The development of ternary-based TiO2-SnS2/GO-RGO composite material for photocatalytic H2 production under solar light irradiation. Mater. Res. Bull. 2023, 167, 112418. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, L.; Wang, H.; Yang, J.; Yuan, X.; Wang, H.; Liang, J.; Li, X.; Li, H.; Bu, Y. Oxygen Vacancies Modified TiO2/O-Terminated Ti3C2 Composites: Unravelling the Dual Effects between Oxygen Vacancy and High-Work-Function Titanium Carbide. Adv. Funct. Mater. 2023, 33, 2307702. [Google Scholar] [CrossRef]
- Li, H.; Lai, C.; Wei, Z.; Zhou, X.; Liu, S.; Qin, L.; Yi, H.; Fu, Y.; Li, L.; Zhang, M.; et al. Strategies for improving the stability of perovskite for photocatalysis: A review of recent progress. Chemosphere 2023, 344, 140395. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; He, Y.; Feng, H.; Zhang, Y.; Liu, C.; Wu, Z. 2D porphyrin-based MOFs with highly dispersed Pt nanoparticles via in-situ partial reduction strategy from porphyrin embedded with single-atom Pt for enhancing photocatalytic hydrogen production. Fuel 2023, 338, 127369. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Hu, T.; Li, L.; Liu, Y.; Gao, X.; Cao, Z.; Wang, L.; Cao, Y. 3DOM SrTiO3-TiO2 composite material modified by CQDs with up-conversion characteristics: Enhanced photocatalytic degradation and photolysis of water for hydrogen production. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131896. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Li, W.; Luo, N.; Hu, L.; Lin, Z.; Qie, Y.; Hu, W.; Yang, R.; Tang, B. Interface redox-induced synthesis of SrTiO3/α-Fe2O3 for much improved hydrogen production. J. Alloys Compd. 2023, 963, 171189. [Google Scholar] [CrossRef]
- Yavuz, C.; Ela, S.E. Fabrication of g-C3N4-reinforced CdS nanosphere-decorated TiO2 nanotablet composite material for photocatalytic hydrogen production and dye-sensitized solar cell application. J. Alloys Compd. 2023, 936, 168209. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, L.; Yang, J.; Zhou, S.; Yuan, X.; Liang, J.; Wang, H.; Wang, H.; Bu, Y.; Li, H. Synthesis and modification of ultrathin g-C3N4 for photocatalytic energy and environmental applications. Renew. Sustain. Energy Rev. 2023, 173, 113110. [Google Scholar] [CrossRef]
- Malekshah, R.E.; Moharramnejad, M.; Gharanli, S.; Shahi, M.; Ehsani, A.; Haribabu, J.; Ouachtak, H.; Mirtamizdoust, B.; Kamwilaisak, K.; Sillanpää, M.; et al. MOFs as Versatile Catalysts: Synthesis Strategies and Applications in Value-Added Compound Production. ACS Omega 2023, 8, 31600–31619. [Google Scholar] [CrossRef]
- Gao, J.; Huang, Q.; Wu, Y.; Lan, Y.Q.; Chen, B. Metal–Organic Frameworks for Photo/Electrocatalysis. Adv. Energy Sustain. Res. 2021, 2, 2100033. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Tan, Y.; Xu, B.; Cai, J.; Zhang, Y.; Wang, Q.; Wu, Q.; Yang, B.; Huang, J. Recent Advances in Metal-Organic Framework (MOF)-Based Photocatalysts: Design Strategies and Applications in Heavy Metal Control. Molecules 2023, 28, 6681. [Google Scholar] [CrossRef]
- Chi, L.X.; Clarisse, N.D.; Yang, S.; Bao, W.L.; Xiao, M.; Zhao, Y.P.; Wang, J.T.; Chen, Q.; Zhang, Z.H. Boosting photocatalytic hydrogen production based on amino acid derived Zn-MOF/CdS composite photocatalysts. J. Solid State Chem. 2023, 324, 124117. [Google Scholar] [CrossRef]
- Azhar, U.; Bashir, M.S.; Babar, M.; Arif, M.; Hassan, A.; Riaz, A.; Mujahid, R.; Sagir, M.; Suri, S.U.K.; Show, P.L.; et al. Template-Based Textural Modifications of Polymeric Graphitic Carbon Nitrides towards Waste Water Treatment. Chemosphere 2022, 302, 134792. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.N. G-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 121. [Google Scholar] [CrossRef]
- Doustkhah, E.; Kotb, A.; Tafazoli, S.; Balkan, T.; Kaya, S.; Hanaor, D.A.H.; Assadi, M.H.N. Templated Synthesis of Exfoliated Porous Carbon with Dominant Graphitic Nitrogen. ACS Mater. Au 2023, 3, 231–241. [Google Scholar] [CrossRef]
- Syazana Mohtar, S.; Aziz, F.; Fauzi Ismail, A.; Sambudi, N.S.; Abdullah, H.; Rosli, A.N.; Ohtani, B. Impact of Doping and Additive Applications on Photocatalyst Textural Properties in Removing Organic Pollutants: A Review. Catalysts 2021, 11, 1160. [Google Scholar] [CrossRef]
- Nam, N.N.; Bui, T.L.; Ho, N.T.; Son, S.J.; Joo, S.W. Controlling Photocatalytic Reactions and Hot Electron Transfer by Rationally Designing Pore Sizes and Encapsulated Plasmonic Nanoparticle Numbers. J. Phys. Chem. C 2019, 123, 23497–23504. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, K.; Tian, N.; Dong, F.; Zhang, T.; Du, X.; Zhang, Y. Template-Free Precursor-Surface-Etching Route to Porous, Thin g-C3N4 Nanosheets for Enhancing Photocatalytic Reduction and Oxidation Activity. J. Mater. Chem. A Mater. 2017, 5, 17452–17463. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Hu, Y.; Zhong, Y.; Wu, H.B.; Lou, X.W. Microwave-Assisted Synthesis of Porous Ag2S–Ag Hybrid Nanotubes with High Visible-Light Photocatalytic Activity. Angew. Chem. 2012, 124, 11669–11672. [Google Scholar] [CrossRef]
- Wang, X.J.; Yang, W.Y.; Li, F.T.; Xue, Y.B.; Liu, R.H.; Hao, Y.J. In Situ Microwave-Assisted Synthesis of Porous N-TiO2/g-C3N4 Heterojunctions with Enhanced Visible-Light Photocatalytic Properties. Ind. Eng. Chem. Res. 2013, 52, 17140–17150. [Google Scholar] [CrossRef]
- Dharmarajan, N.P.; Vidyasagar, D.; Yang, J.-H.; Talapaneni, S.N.; Lee, J.; Ramadass, K.; Singh, G.; Fawaz, M.; Kumar, P.; Vinu, A. Bio-Inspired Supramolecular Self-Assembled Carbon Nitride Nanostructures for Photocatalytic Water Splitting. Adv. Mater. 2024, 36, 2306895. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.-X.; Zhou, J.-W.; Lin, S.-W.; Lu, C.-Z. Pollen Carbon-Based Rare-Earth Composite Material for Highly Efficient Photocatalytic Hydrogen Production from Ethanol–Water Mixtures. ACS Omega 2022, 7, 30495–30503. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Li, Y.; Lu, A.; Ding, H.; Wong, P.K.; Sun, H.; Shi, J. Natural wolframite as a novel visible-light photocatalyst towards organics degradation and bacterial inactivation. Cat. Today 2020, 358, 177–183. [Google Scholar] [CrossRef]
- Cui, S.; Tian, L.-J.; Li, J.; Wang, X.-M.; Liu, H.-Q.; Fu, X.-Z.; He, R.-L.; Lam, P.K.S.; Huang, T.-Y.; Li, W.-W. Light-assisted fermentative hydrogen production in an intimately-coupled inorganic-bio hybrid with self-assembled nanoparticles. Chem. Eng. J. 2022, 428, 131254. [Google Scholar] [CrossRef]
- Almomani, F.; Al-Rababah, A.; Tawalbeh, M.; Al-Othman, A. A Comprehensive Review of Hydrogen Generation by Water Splitting Using 2D Nanomaterials: Photo vs Electro-Catalysis. Fuel 2023, 332, 125905. [Google Scholar] [CrossRef]
- Ajith Mohan, A.; Sandhyarani, N. Carbon Nanostructures for Energy Generation and Storage. In Applications of Multifunctional Nanomaterials; Thomas, S., Abraham, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–94. [Google Scholar] [CrossRef]
- Vozniuk, O.; Tanchoux, N.; Millet, J.M.; Albonetti, S.; Di Renzo, F.; Cavani, F. Spinel Mixed Oxides for Chemical-Loop Reforming: From Solid State to Potential Application. Stud. Surf. Sci. Catal. 2019, 178, 281–302. [Google Scholar] [CrossRef]
- Hao, X.; Shao, Y.; Xiang, D.; Jin, Z. Photocatalytic Overall Water Splitting Hydrogen Production over ZnCdS by Spatially-Separated WP and Co3O4 Cocatalysts. Sol. Energy Mater. Sol. Cells 2022, 248, 111970. [Google Scholar] [CrossRef]
- Cao, R.; Yang, H.; Zhang, S.; Xu, X. Engineering of Z-Scheme 2D/3D Architectures with Ni(OH)2 on 3D Porous g-C3N4 for Efficiently Photocatalytic H2 Evolution. Appl. Catal. B 2019, 258, 117997. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Li, L.; Zhao, R.; Han, J.; Wang, L. Hollow rod-shaped Cu-In-Zn-S@ZnCo2O4@In2O3 tandem heterojunction for efficient visible light-induced photocatalytic hydrogen production. Fuel 2024, 355, 129537. [Google Scholar] [CrossRef]
- Huang, S.; Bao, R.; Wang, J.; Yi, J.; Zhang, Z.; Liu, L.; Han, Y.; Li, Z.; Min, D.; Zhang, W.; et al. Synergistic effect of oxygen vacancy defects and TiO2/WO3 heterostructures in photocatalytic hydrogen pro-duction and dye degradation. J. Alloys Compd. 2023, 961, 170945. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Sun, W.; Lei, Y.; Wang, Q.; Li, A.; Chen, W.; Zhou, G.; Zhang, Z.; Wang, Y.; et al. Engineering the Atomic Interface with Single Platinum Atoms for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2020, 59, 1295–1301. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, S.; Qiu, M.; Wang, S.; Zhang, Y.; Zhang, J.; Xiong, W.; Lou, D. Direct Probing of Atomically Dispersed Ru Species over Multi-Edged TiO2 for Highly Efficient Photocatalytic Hydrogen Evolution. Sci. Adv. 2020, 6, eabb9823. [Google Scholar] [CrossRef]
- Yan, B.; Liu, D.; Feng, X.; Shao, M.; Zhang, Y. Ru Species Supported on MOF-Derived N-Doped TiO2/C Hybrids as Efficient Electrocatalytic/Photocatalytic Hydrogen Evolution Reaction Catalysts. Adv. Funct. Mater. 2020, 30, 2003007. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Barhoum, A.; Nada, A.A.; Moustafa, Y.M.; Seliman, S.M.; Youssef, A.M.; Bechelany, M. Synthesis of Mesoporous Core-Shell CdS@TiO2 (0D and 1D) Photocatalysts for Solar-Driven Hydrogen Fuel Production. J. Photochem. Photobiol. A Chem. 2018, 351, 261–270. [Google Scholar] [CrossRef]
- Montoya, A.T.; Gillan, E.G. Enhanced Photocatalytic Hydrogen Evolution from Transition-Metal Surface-Modified TiO2. ACS Omega 2018, 3, 2947–2955. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-Doped TiO2 Photocatalysts with Effective Charge Transfer for Highly Efficient Hydrogen Production through Water Splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Luo, S.; Nguyen-Phan, T.D.; Vovchok, D.; Waluyo, I.; Palomino, R.M.; Gamalski, A.D.; Barrio, L.; Xu, W.; Polyansky, D.E.; Rodriguez, J.A.; et al. Enhanced, Robust Light-Driven H2 Generation by Gallium-Doped Titania Nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 2104–2112. [Google Scholar] [CrossRef]
- Kim, N.Y.; Lee, H.K.; Moon, J.T.; Joo, J.B. Synthesis of Spherical TiO2 Particles with Disordered Rutile Surface for Photocatalytic Hydrogen Production. Catalysts 2019, 9, 491. [Google Scholar] [CrossRef]
- Cao, S.; Li, H.; Tong, T.; Chen, H.C.; Yu, A.; Yu, J.; Chen, H.M. Single-Atom Engineering of Directional Charge Transfer Channels and Active Sites for Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2018, 28, 1802169. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.; Luo, Q.; Yan, H.; Lin, Y.; Liu, W.; Cao, L.; Lu, J.; Yang, J.; Yao, T.; et al. Atomic-Level Insight into Optimizing the Hydrogen Evolution Pathway over a Co1-N4 Single-Site Photocatalyst. Angew. Chem. Int. Ed. 2017, 56, 12191–12196. [Google Scholar] [CrossRef]
- Liu, W.; Cao, L.; Cheng, W.; Cao, Y.; Liu, X.; Zhang, W.; Mou, X.; Jin, L.; Zheng, X.; Che, W.; et al. Single-Site Active Cobalt-Based Photocatalyst with a Long Carrier Lifetime for Spontaneous Overall Water Splitting. Angew. Chem. Int. Ed. 2017, 56, 9312–9317. [Google Scholar] [CrossRef]
- Shi, R.; Tian, C.; Zhu, X.; Peng, C.Y.; Mei, B.; He, L.; Du, X.L.; Jiang, Z.; Chen, Y.; Dai, S. Achieving an Exceptionally High Loading of Isolated Cobalt Single Atoms on a Porous Carbon Matrix for Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. Chem. Sci. 2019, 10, 2585–2591. [Google Scholar] [CrossRef]
- Cao, A.H.; Zhang, L.J.; Wang, Y.; Zhao, H.J.; Deng, H.J.; Liu, X.M.; Lin, Z.; Su, X.T.; Yue, F. 2D-2D hetero-structured UNiMOF/g-C3N4 for enhanced photocatalytic H2 production under visible-light irradiation. ACS Sustain. Chem. Eng. 2019, 7, 2492–2499. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A.; Basaleh, A.S.; Bawazir, H.A. Photodeposition of Ag Nanoparticles on Mesoporous LaNaTaO3 Nanocomposites for Promotion H2 Evolution. Mater. Res. Bull. 2020, 131, 110962. [Google Scholar] [CrossRef]
- Kalaiselvi, C.R.; Ravi, P.; Senthil, T.S.; Sathish, M.; Kang, M. Synthesis of Ag and N Doped Potassium Tantalate Perovskite Nanocubes for Enhanced Photocatalytic Hydrogen Evolution. Mater. Lett. 2020, 275, 128166. [Google Scholar] [CrossRef]
- Alhaddad, M.; Ismail, A.A. Comparative Study on Mesoporous M/LaNaTaO3-Based Photocatalysts (M = Ag, In, and Nd) for Hydrogen Generation. J. Taiwan Inst. Chem. Eng. 2020, 117, 144–155. [Google Scholar] [CrossRef]
- Parnicka, P.; Mikolajczyk, A.; Pinto, H.P.; Lisowski, W.; Klimczuk, T.; Trykowski, G.; Bajorowicz, B.; Zaleska-Medynska, A. Experimental and DFT Insights into an Eco-Friendly Photocatalytic System toward Environmental Remediation and Hydrogen Generation Based on AgInS2 Quantum Dots Embedded on Bi2WO6. Appl. Surf. Sci. 2020, 525, 146596. [Google Scholar] [CrossRef]
- Liu, H.; Yan, T.; Jin, Z.L.; Ma, Q.X. CoP nanoparticles as cocatalyst modified the CdS/NiWO4 p–n heterojunction to produce hydrogen efficiently. New J. Chem. 2020, 44, 1426–1438. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.W.; Li, K.; Li, T.; Liu, F.T. Conductive Ti3C2 and MOF-derived CoSx boosting the photocatalytic hydrogen production activity of TiO2. CrystEngComm 2019, 21, 2416–2421. [Google Scholar] [CrossRef]
- Li, T.; Cui, J.D.; Xu, M.L.; Li, R.; Gao, L.M.; Zhu, P.L.; Xie, H.Q.; Li, K. Engineering a hetero-MOF-derived TiO2–Co3O4 heterojunction decorated with nickel nanoparticles for enhanced photocatalytic activity even in pure water. CrystEngComm 2020, 22, 5620–5627. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Wang, D.; Reinhartc, B.J. Huang, Electron shuttle in the MOF derived TiO2/CuO heterojunction boosts light driven hydrogen evolution. J. Mater. Chem. A 2021, 9, 6180–6187. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, W.; Liu, H.; Chen, R.; Pan, Q.; Li, Z.; Zhao, Y. Facile construction of fully sp2-carbon conjugated two-dimensional covalent organic frameworks containing benzobisthiazole units. Nat. Commun. 2022, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, J.; Li, H.; Wu, K.; Wang, J.; Yang, Q. Covalent organic frameworks with high quantum efficiency in sacrificial photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 2357. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Zhou, T.; Weng, W.; Unruangsri, J.; Hu, K.; Yang, W.; Wang, C.; Zhang, K.A.I.; Guo, J. Covalent Organic Frameworks Enabling Site Isolation of Viologen-Derived Electron-Transfer Mediators for Stable Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2021, 60, 9642–9649. [Google Scholar] [CrossRef]
- Su, D.W.; Ran, J.; Zhuang, Z.W.; Chen, C.; Qiao, S.Z.; Li, Y.D.; Wang, G.X. Atomically Dispersed Ni in Cadmium-Zinc Sulfide Quantum Dots for High-Performance Visible-Light Photocatalytic Hydrogen Production. Sci. Adv. 2020, 6, eaaz8447. [Google Scholar] [CrossRef]
- Hezam, A.; Wang, J.; Drmosh, Q.A.; Karthik, P.; Abdullah Bajiri, M.; Namratha, K.; Zare, M.; Lakshmeesha, T.R.; Shivanna, S.; Cheng, C.; et al. Rational Construction of Plasmonic Z-Scheme Ag-ZnO-CeO2 Heterostructures for Highly Enhanced Solar Photocatalytic H2 Evolution. Appl. Surf. Sci. 2021, 541, 148457. [Google Scholar] [CrossRef]
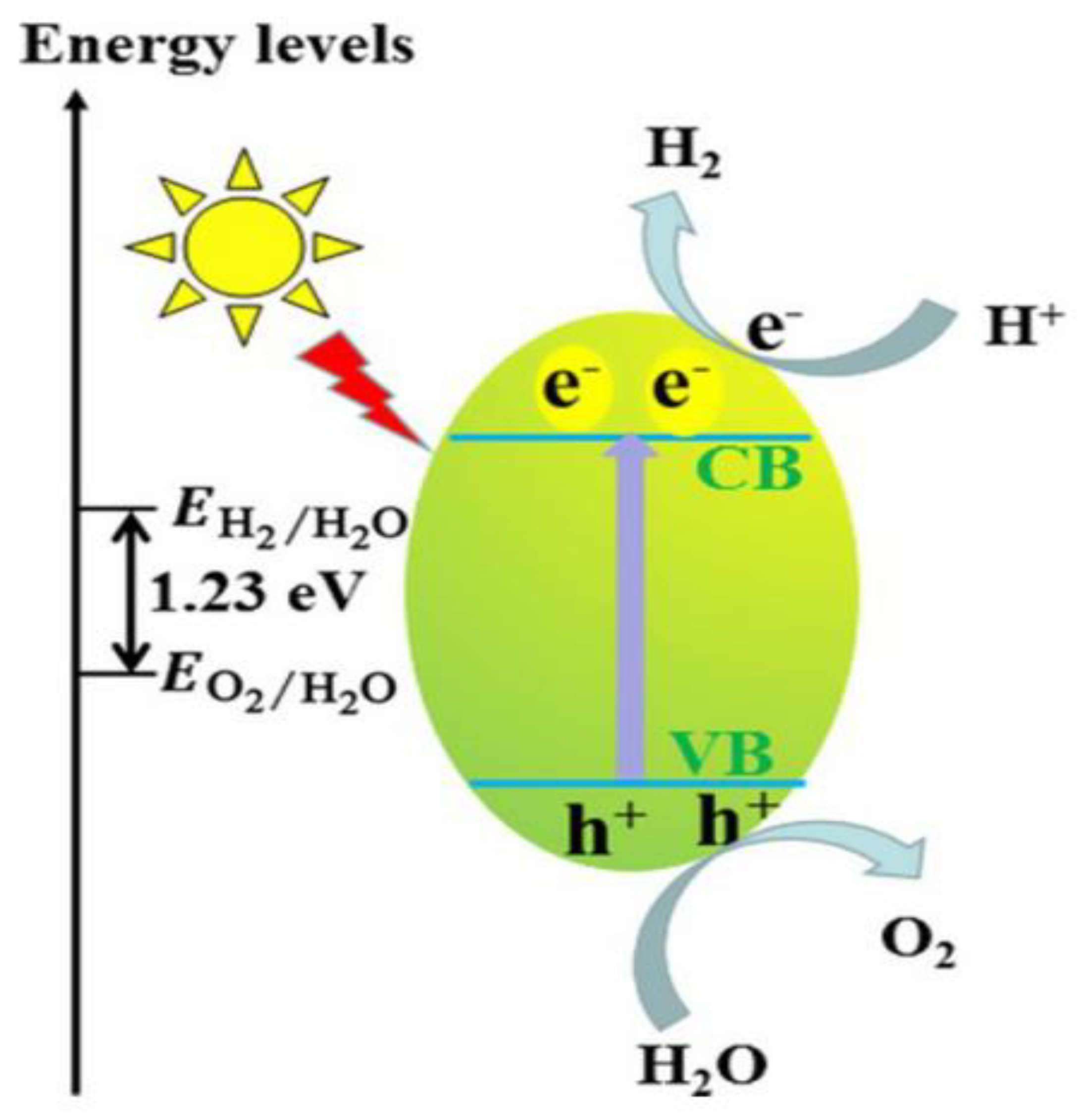
| Equation | E0 (V) | |
|---|---|---|
| 1. | e− + O2 → ∙O2− | −0.33 |
| 2. | h+ + OH− → ∙HO | +1.99 |
| 3. | h+ + H2O → ∙HO | +2.33 |
| 4. | ∙O2− (ads) + h+ → 1O2 | +0.65 |
| 5. | O2 + 2H+ + e− → H2O2 | +0.94 |
| 6. | ∙O2− + H+ + e− → ∙HO2 | −0.46 |
| 7. | ∙HO2 + H+ → H2O2 | +1.06 |
| 8. | H2O2 + e− → ∙OH + OH− | +0.32 |
| Sacrificial Agent | Expected Reaction Mechanism |
|---|---|
| Methanol | H2O + h+ → ·OH + H+ CH3OH + ·OH → ·CH2OH + H2O ·CH2OH → HCHO + H+ + e− 2H+ + 2e− → H2 HCHO + H2O → HCOOH + H2 HCOOH → CO2 + H2 Overall reaction: CH3OH + H2O → CO2 + 3H2 |
| Lactic acid | CH3-CH(OH)-COOH + H2O → CO2 + H2+ CH3-CO-COOH |
| Triethanolamine | C6H15NO3→ C6H15NO3+ + e− C6H15NO3+ →C6H14NO3· + H+ C6H14NO3· → C6H14NO3++ e− Overall reaction: C6H14NO3++ H2O → C4H11NO3 + CH3CHO + H+ |
| Sodium sulfide | Na2S + H2O → 2Na+ + S2− S2−+ H2O → HS− + OH− HS− + hν → HS−* HS−* + HS− → [(HS)2]−* → H2 + S22− |
| Sodium sulfite | Irradiation: SO32− → SO32−* Oxidation: SO32−* + 2OH− → SO42− + H2O + 2e− Reduction: 2 H2O + 2e− → H2 + 2OH− Oxidation: SO32− → S2H62− + 2e− Reduction: 2 H2O + 2e− → H2 + 2OH− |
| Raw Material | State of Concentration | Combustion Reaction | Description of Reaction | Presence of CO2 in Exhaust Gases |
|---|---|---|---|---|
| Hard coal | Solid | C + O2 → CO2 | In addition to CO2, harmful compounds (microdust) are produced. | YES |
| Gasoline | Liquid | C5H12 + 8O2 → 5CO2 + 6H2O | Expensive platinum catalysts are required for complete combustion. | YES |
| Natural gas | Gas | 2CH4 + 4O2 → 2CO2 + 4H2O | Gas combustion eliminates microdust formation and reduces CO2 emissions compared to coal. | YES |
| Hydrogen | Gas | 2H2+O2→2H2O | Emission-free fuel. | NO |
| Materials | H2 Production Rate | Reaction Conditions | Ref. |
|---|---|---|---|
| TiO2-based photocatalysts | |||
| Pt single atoms (SAs) on a defective TiO2 (Pt1/def-TiO2). | 52,720 μmol g−1 h−1 | CH3OH as sacrificial electron donor. | [152] |
| Ru on the polygonal TiO2 sphere. | 7.2 mmol g−1 h−1 | 300 W Xe lamp; CH3OH (aq.). | [153] |
| Ru single atoms (SAs) into N-doped TiO2/C carrier (Ru-SAs@N-TC) derived from a MOF of NH2-MIL-125. | 100.0 µmol g−1 h−1 | 300 W Xe lamp (λ = 320–780 nm); 20 mg of catalyst dispersed in 100 mL of H2O:MeOH solution (v/v = 4:1). | [154] |
| Mesoporous core−shell CdS@TiO2 with Pt. | 68,000 μmol g−1 h−1 | Sunlight irradiation. 10 mg photocatalyst dispersed in 50 mL of an aqueous solution of sacrificial reagent (0.1 M Na2S + 0.02 M Na2SO3). | [155] |
| Co-, Ni-, and Cu-doped TiO2. | 8470 μmol h−1g−1 (Cu) 3390 μmol h−1g−1 (Ni) | 450 W Hg lamp. 10 mg photocatalyst in solution with 50% MeOH as a sacrificial electron donor. | [156] |
| Ag/TiO2. | 470 μmol h−1g−1 | 254 nm wavelength of UV light catalyst concentration of 20 mg/L. 50 mL of solution without sacrificial agent. | [157] |
| Ga-doped TiO2. | 5722 μmol h−1g−1 | Side-irradiation by a 150 W xenon arc lamp equipped with an aqueous CuSO4 filter (310 nm < λ < 625 nm). 3 mg of the catalyst suspended in 3 mL of aqueous methanol solution (20 vol.%). | [158] |
| Spherical TiO2 particles. | 350 μmol h−1g−1 | ABET 150 W Xe lamp. 20 mg of catalyst dispersed in 50 mL of aqueous methanol solution (50%). | [159] |
| g-C3N4-based (graphitic carbon nitride) photocatalysts | |||
| Pd/g-CN. Pd single atoms in the space of adjacent g-CN layers and anchored Pd atoms on the surface of g-CN. | 6688 μmol g−1 h−1 | Solar simulator as a light source; 50 mg of the photocatalyst dispersed in 80 mL of water and triethanolamine solution (v/v = 9:1). | [160] |
| Co SAs on carbonitride, and creating an active single Co1–N4 site on g-C3N4. | 10.8 mmol h−1 | Simulated solar irradiation (λ ≥ 300 nm). Triethanolamine (TEOA) as the sacrificial electron donor. | [161] |
| Co1–P4 site confined on g-C3N4 nanosheets. | 410.3 mmol h−1g−1 | Simulated solar irradiation. 20 mg of the photocatalyst without sacrificial electron donor. | [162] |
| Co–N–C/g-C3N4. Isolated cobalt (Co) SAs synthesized and immobilized on a porous nitrogen-doped carbon support. | 1180 mmol h−1g−1 | LED light source (λ = 420 ± 10 nm). 2 mg of the catalyst suspended in an aqueous solution with 10 vol.% triethanolamine (TEOA) as the sacrificial electron donor. | [163] |
| NiAl-LDH/gC3N4. gC3N4 coupled NiAl layered double hydroxide (LDH) nanocomposite. | 3170 µmol h−1 g−1 | Stimulated light irradiation (300 mW/cm2). 15 mg of photocatalyst suspended in 50 mL of solution containing 45 mL H2O and 5 mL TEOA as a sacrificial reagent. | [] |
| UNiMOF/g-C3N4. Heterostructure with 2D nickel metal organic framework (UNiMOF) nanoflakes and 2D g-C3N4 nanoflakes. | 20.03 μmol h–1 | 300 W Xe lamp with a 420 nm filter. 50 mg of catalyst dispersed in 90 mL of H2O mixed with 10 mL of TEOA. | [164] |
| Perovskite-based photocatalysts | |||
| Ag/La0.02Na0.98TaO3. | 330 μmol h−1g−1 | UV illumination by a 500 W xenon lamp (λ > 320 nm). 50 mg of the photocatalyst dispersed in 200 mL of a glycerol solution (10 vol.%). | [165] |
| Ag/KTaO3. | 2072 μmol h−1g−1 | 450W Xe-Hg UV lamp. 10 mg of the photocatalyst dispersed in 38 mL water and 12 mL methanol as a sacrificial agent. | [166] |
| Ag/LaNaTaO3. | 329.5 μmol h−1g−1 | 500 W high-pressure xenon lamp (λ > 320 nm). 50 mg of catalyst suspended in 200 mL of 10 vol.% glycerol. | [167] |
| AgInS2 QDs/Bi2WO6 composite. | 611 μmol h−1g−1 | 1000 W xenon lamp. 0.1 g of the photocatalyst dispersed in an 80 mL aqueous solution containing 1 M NaOH, 0.1 M Na2S*9H2O, and 0.5 M Na2SO3. | [168] |
| CdS/NiWO4/CoP. Composite catalyst with CoP nanoparticles as a co-catalyst modifying the CdS/NiWO4 p–n heterojunction. | 47.7 mmol h−1 g−1 | Visible light irradiation (λ > 400 nm). 0.01 g of catalysts dispersed in 30 mL of 10% lactic acid solution. | [169] |
| MOFs-based (metal–organic frameworks) photocatalysts | |||
| TiO2–Ti3C2–CoSx. TiO2 nanocrystal photocatalyst confined by ZIF-67-templated porous CoSx, with conductive Ti3C2. | 9500 μmol h−1g−1 | UV–visible light irradiation methanol as the sacrificial agent. | [170] |
| TiO2/Co3O4/Ni. Highly porous ternary photocatalyst constructed from a heterometal–organic framework (H-MOF) template (ZIF-67@MIL-125). | 27,000 μmol h−1g−1 | Under UV–visible light methanol solution. | [171] |
| TiO2/CuO heterostructure derived from mixed-phase MOFs based on Ti and Cu metal nodes (MIL-125, Cu-BDC, MIL-125_xCu). | 19,036.2 μmol h−1g−1 | 450 nm LED lamp. 0.2 mg of metal oxide catalysts mixed with [Ru(bpy)3]Cl2·6H2O (2 mg), acetonitrile (3.8 mL), and TEOA (0.2 mL). | [172] |
| COFs-based (covalent organic frameworks) photocatalysts | |||
| Vinylene-linked 2D COFs containing benzobisthiazoles units. | 15.1 mmol h−1 g−1 | 300 W Xe lamp (λ > 420 nm). COFs materials suspended in 0.1 M ascorbic acid solution. | [173] |
| CYANO-COF—cyano-containing COF with ketene-cyano (D–A) pair. | 1217 μmol h−1g−1 | 300 W Xe lamp (λ > 420 nm). 20 mg catalyst suspended in 100 mL water, 1 wt.% Pt (co-catalyst), and 10 mmol ascorbic acid (sacrificial agent). | [174] |
| Tp-nC/BPy2+-COFs. Cyclic diquats (viologen-derived electron-transfer mediators) integrated into a 2,2′-bipyridine-based COF through a post-quaternization reaction. | 34,600 μmol h−1g−1 | Visible light irradiation (λ > 420 nm) in the presence of ascorbic acid (sacrificial donor) and Pt (co-catalyst). | [175] |
| Varia | |||
| CdS/Co1-xS HHNSs. Sugar-gourd-shaped hollow hetero-nanostructure, Co1-xS hollow polyhedrons skewered on CdS nanowires. | 13.48 mmol h−1 g−1 | 300 W Xe lamp (λ > 420 nm). 20 mg of photocatalysts dispersed in 100 mL of aqueous solution containing 20% lactic acid as the sacrificial agent. | [33] |
| CuIn-CdS. quantum dot level Cu and In co-doped CdS. | 105.44 mmol h−1 g−1 | 300 W Xe arc lamp as simulated solar light source (320–780 nm). 10 mg of photocatalyst dispersed in 25 mL of aqueous solution with 0.35 M of sodium sulfide and 0.25 M of sodium sulfite as sacrificial agent, and with 0.1 mL of H2PtCl6 solution (1 mg/mL) as co-catalyst. | [35] |
| ZCS QDs. Ni atomically dispersed in zinc sphalerite cadmium-zinc sulfide quantum dots. | 18.87 mmol h−1g−1 | Xe arc lamp (300 W) with a UV-cutoff filter (λ ≥ 420 nm). 10 mg of photocatalyst suspended in a mixed solution of water and TEOA (20 vol.%) as a sacrificial reagent. | [176] |
| Ag/ZnO/CeO2. | 18,345 μmol h−1g−1 | 300 W xenon lamp irradiation. 5 mg of the catalyst dispersed in 40 mL of water and 10 mL of glycerol. | [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyńczak, A.; Feliczak-Guzik, A. Hydrogen Production Using Modern Photocatalysts. Coatings 2024, 14, 366. https://doi.org/10.3390/coatings14030366
Wawrzyńczak A, Feliczak-Guzik A. Hydrogen Production Using Modern Photocatalysts. Coatings. 2024; 14(3):366. https://doi.org/10.3390/coatings14030366
Chicago/Turabian StyleWawrzyńczak, Agata, and Agnieszka Feliczak-Guzik. 2024. "Hydrogen Production Using Modern Photocatalysts" Coatings 14, no. 3: 366. https://doi.org/10.3390/coatings14030366






