A Comprehensive Assessment of Al-Si Coating Growth at Various Heating Rates, Soaking Temperatures, and Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
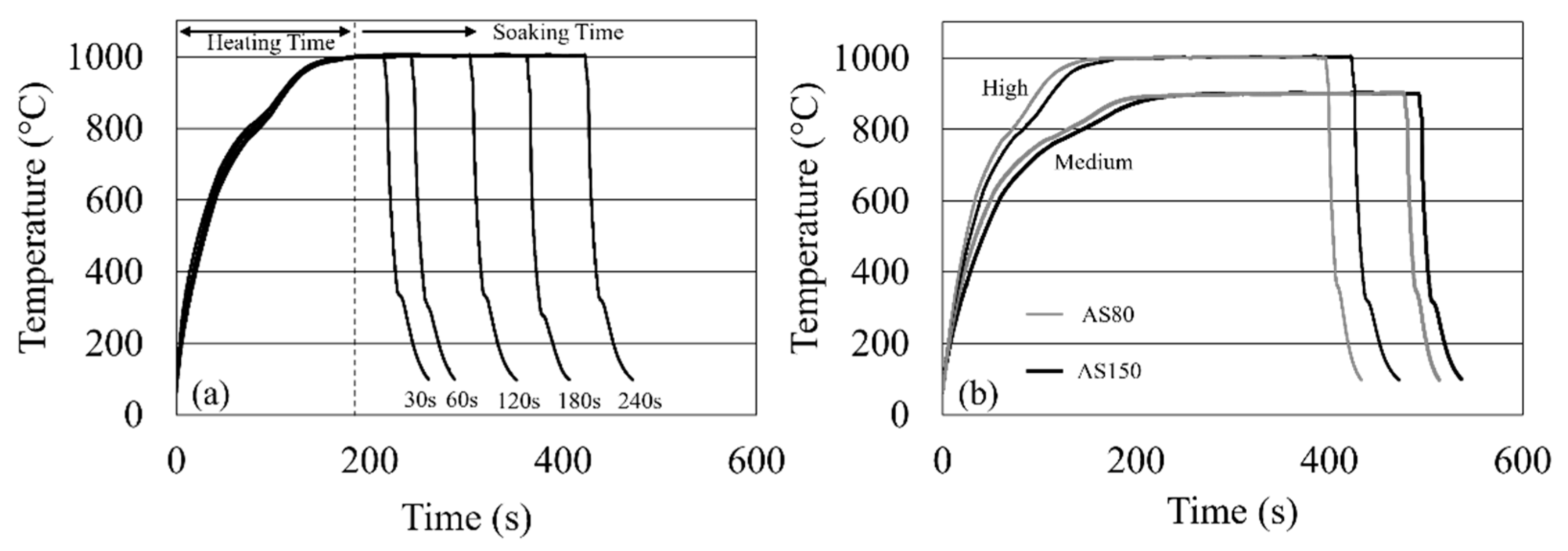
2.2. Heat Treatment in Chamber Furnace
2.3. SEM and EDS Analysis
3. Results and Discussion
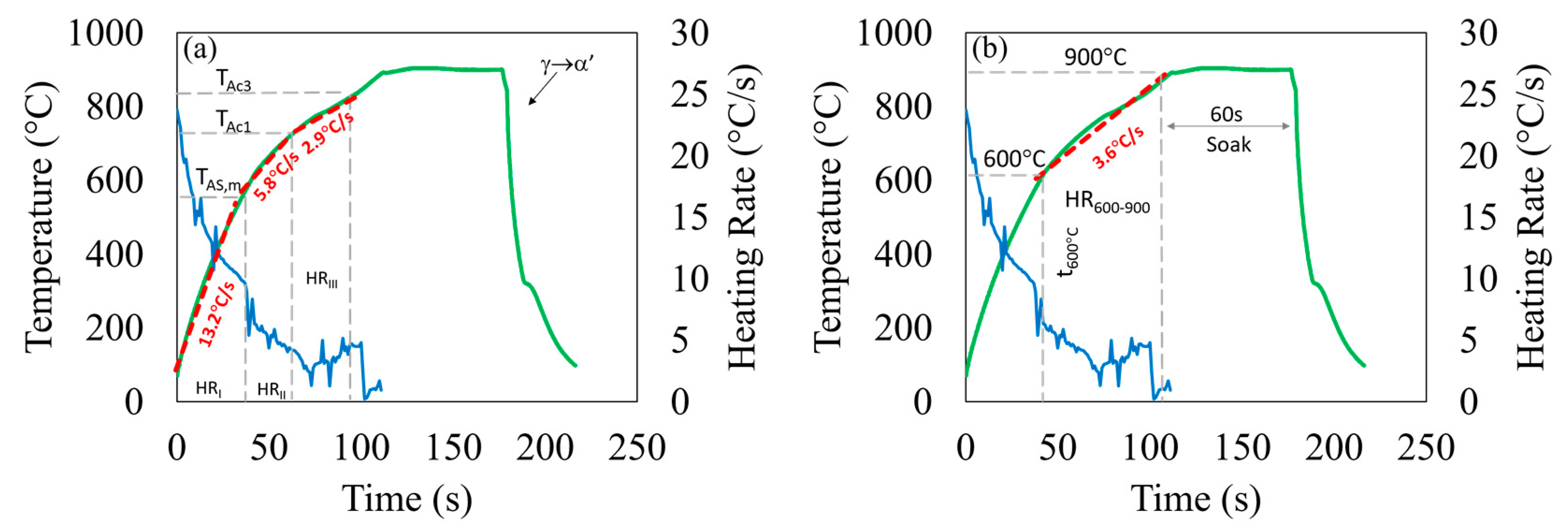
3.1. Heating Rate Determination
3.2. Intermetallic Phase Formation
3.2.1. AS150
3.2.2. AS80
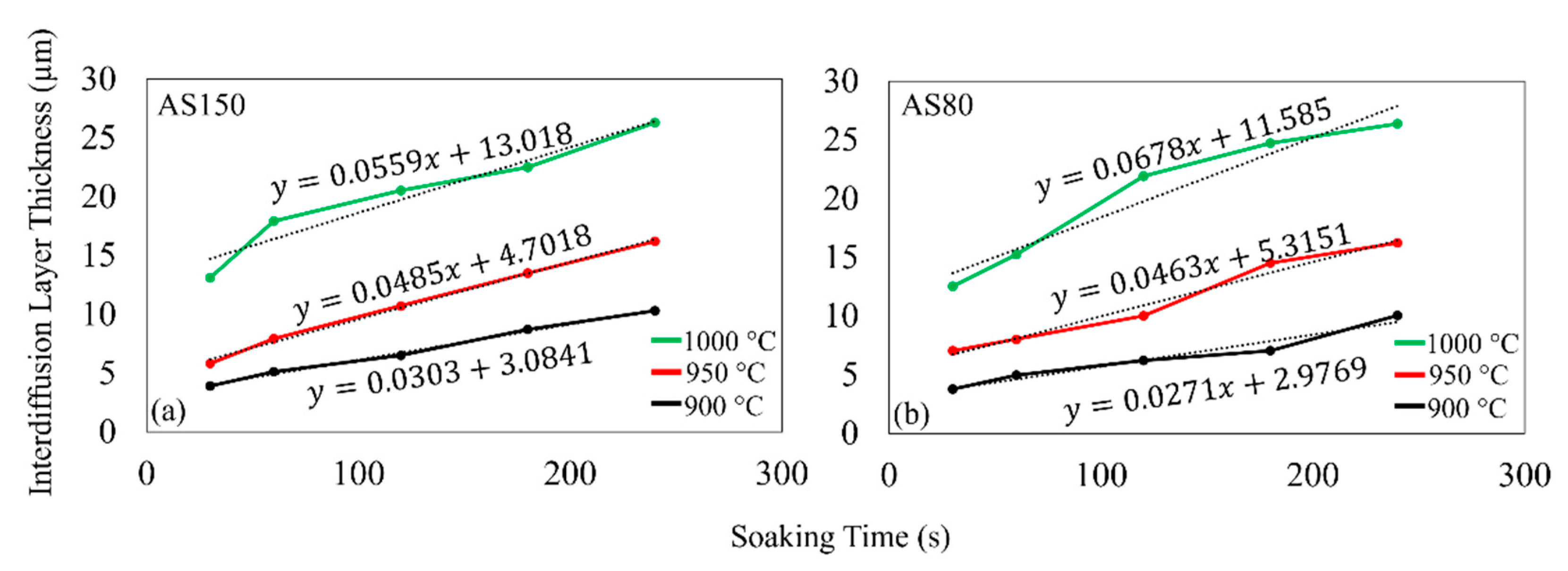
3.3. Interdiffusion Layer Growth
3.3.1. Effect of Soaking Temperature on the IDL
3.3.2. Effect of Heating Rate on the IDL
3.3.3. IDL Thickness Growth Model
4. Conclusions
- For the Medium-heating-rate soaking tests, coating materials of both weights had a banded and continuous Fe-Al-Si intermetallic phase morphology for all the soaking times, while the specimens soaked at 900 °C, but heated at the High heating rate, resulted in an island-type ternary microstructure for all of the soak times. This indicates that the heating rate had more of an effect on the ternary phase morphology than the other heat treatment parameters. For the Medium heating rate, the continuous Fe-Al-Si ternary intermetallic layers started from the interface and stop in the middle of the coating, regardless of the initial coating thickness.
- The intermetallic phase is the dominant layer at the beginning of the soaking stage, and the () phase is formed within the phase. gradually transforms into ( as the soaking time increases due to the interdiffusion of Fe and Al. The transformation of to occurred faster for higher soaking temperatures due to the greater diffusivity of Al and Fe.
- For both coating weights, the IDL consisted of and and the thickness of the IDL increased as the soaking time increased at a relatively constant growth rate. Higher soaking temperatures result in a thicker IDL for the same soaking time, which is a result of the higher diffusivity of Si and Al into the steel substrate at the higher soaking temperatures. The IDL growth of AS80 is generally the same as the growth quantified for AS150, which indicates that the coating thickness does not have a noticeable effect on the overall growth of the IDL.
- For both coating weights, the impact of heating rate had little effect on the IDL growth rate, but the Medium-heating-rate IDL was thicker than the High-heating-rate IDL, since the Medium heating rate tests required a longer time to reach the soaking temperature of 900 °C and therefore prolonged the diffusion time during heating.
- Utilizing the experimental results found in this work, an empirical IDL thickness model was successfully developed to predict IDL growth as a function of heating rate, soaking temperature, and soaking time.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karbasian, H.; Tekkaya, A.E. A Review on Hot Stamping. J. Mater. Process. Technol. 2010, 210, 2103–2118. [Google Scholar] [CrossRef]
- Åkerström, P. Modelling and Simulation of Hot Stamping. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2006. [Google Scholar]
- Bian, J.; Mohrbacher, H.; Zhang, J.S.; Zhao, Y.T.; Lu, H.Z.; Dong, H. Application Potential of High Performance Steels for Weight Reduction and Efficiency Increase in Commercial Vehicles. Adv. Manuf. 2015, 3, 27–36. [Google Scholar] [CrossRef]
- Merklein, M.; Lechler, J. Determination of Material and Process Characteristics for Hot Stamping Processes of Quenchenable Ultra High Strength Steels with Respect to a FE-Based Process Design. SAE Int. J. Mater. Manuf. 2008, 1, 411–426. [Google Scholar] [CrossRef]
- Jonasson, J.; Billur, E.; Ormaetxea, A. A Hot Stamping Line. In Hot Stamping of Ultra High-Strength Steels; Springer International Publishing: Cham, Switzerland, 2019; pp. 77–104. [Google Scholar]
- Bardelcik, A.; Salisbury, C.P.; Winkler, S.; Wells, M.A.; Worswick, M.J. Effect of Cooling Rate on the High Strain Rate Properties of Boron Steel. Int. J. Impact Eng. 2010, 37, 694–702. [Google Scholar] [CrossRef]
- Fan, D.W.; Kim, H.S.; De Cooman, B.C. A Review of the Physical Metallurgy Related to the Hot Press Forming of Advanced High Strength Steel. Steel Res. Int. 2009, 80, 241–248. [Google Scholar] [CrossRef]
- Maikranz-Valentin, M.; Weidig, U.; Schoof, U.; Becker, H.-H.; Steinhoff, K. Components with Optimised Properties due to Advanced Thermo-Mechanical Process Strategies in Hot Sheet Metal Forming. Steel Res. Int. 2008, 79, 92–97. [Google Scholar] [CrossRef]
- Merklein, M.; Lechler, J. Investigation of the Thermo-Mechanical Properties of Hot Stamping Steels. J. Mater. Process. Technol. 2006, 177, 452–455. [Google Scholar] [CrossRef]
- Fan, D.W.; De Cooman, B.C. State-of-the-Knowledge on Coating Systems for Hot Stamped Parts. Steel Res. Int. 2012, 83, 412–433. [Google Scholar] [CrossRef]
- Vander Voort, G.F. Understanfing and measuring decarbruization. Adv. Mater. Process. 2015, 173, 22–27. [Google Scholar]
- Fan, D.W.; Kim, H.S.; Oh, J.-K.; Chin, K.-G.; De Cooman, B.C. Coating Degradation in Hot Press Forming. ISIJ Int. 2010, 50, 561–568. [Google Scholar] [CrossRef]
- ArcelorMittal. Steels Coated with Alusi®, an Aluminium-Silicon Alloy. Available online: https://automotive.arcelormittal.com/products/flat/coatings/alusi (accessed on 12 June 2023).
- Shin, D.; Lee, J.-Y.; Heo, H.; Kang, C.-Y. TEM Microstructural Evolution and Formation Mechanism of Reaction Layer for 22MnB5 Steel Hot-Dipped in Al–10% Si. Coatings 2018, 8, 467. [Google Scholar] [CrossRef]
- Murray, J.L.; McAlister, A.J. The Al-Si (Aluminum-Silicon) System. Bull. Alloy Phase Diagr. 1984, 5, 74–84. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Tsaur, C.C.; Rock, J.C. Microstructure Studies of an Aluminide Coating on 9Cr-1Mo Steel during High Temperature Oxidation. Surf. Coat. Technol. 2006, 200, 6588–6593. [Google Scholar] [CrossRef]
- Windmann, M.; Röttger, A.; Theisen, W. Phase Formation at the Interface between a Boron Alloyed Steel Substrate and an Al-Rich Coating. Surf. Coat. Technol. 2013, 226, 130–139. [Google Scholar] [CrossRef]
- Jenner, F.; Walter, M.E.; Mohan Iyengar, R.; Hughes, R. Evolution of Phases, Microstructure, and Surface Roughness during Heat Treatment of Aluminized Low Carbon Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41, 1554–1563. [Google Scholar] [CrossRef]
- Grigorieva, R.; Drillet, P.; Mataigne, J.M.; Redjaïmia, A. Phase Transformations in the Al-Si Coating during the Austenitization Step. In Proceedings of the Solid State Phenomena; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; Volume 172–174, pp. 784–790. [Google Scholar]
- Cho, L.; Golem, L.; Seo, E.J.; Bhattacharya, D.; Speer, J.G.; Findley, K.O. Microstructural Characteristics and Mechanical Properties of the Al–Si Coating on Press Hardened 22MnB5 Steel. J. Alloys Compd. 2020, 846, 156349. [Google Scholar] [CrossRef]
- Liang, W.; Duan, J.; Wang, Q.; Dong, J.; Liu, Q.; Lin, C.; Zhang, Y. Influence of Multi-Step Heating Methods on Properties of al–Si Coating Boron Steel Sheet. Coatings 2021, 11, 164. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hazrati, J.; de Rooij, M.B.; van den Boogaard, A.H. Effect of Heating Temperatures on AlSi Coating Microstructure and Fracture during Hot-Tensile Tests. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1157, 012018. [Google Scholar] [CrossRef]
- Liang, W.K.; Tao, W.J.; Zhu, B.; Zhang, Y.S. Influence of Heating Parameters on Properties of the Al-Si Coating Applied to Hot Stamping. Sci. China Technol. Sci. 2017, 60, 1088–1102. [Google Scholar] [CrossRef]
- Klassen, C.M.; Daun, K.J. Investigating Coating Liquefaction and Solidification of Furnace-Heated Al-Si Coated 22MnB5 Steel Using Laser Reflectance. Surf. Coat. Technol. 2020, 393, 125795. [Google Scholar] [CrossRef]
- Cheng, W.J.; Wang, C.J. Study of Microstructure and Phase Evolution of Hot-Dipped Aluminide Mild Steel during High-Temperature Diffusion Using Electron Backscatter Diffraction. Appl. Surf. Sci. 2011, 257, 4663–4668. [Google Scholar] [CrossRef]
- Klassen, C.M.; Smith, R.D.L.; Daun, K.J. Characterizing the Al-Si Coating on 22MnB5 Steel Using Raman Spectroscopy. Mater. Charact. 2022, 189, 112002. [Google Scholar] [CrossRef]
- Windmann, M.; Röttger, A.; Theisen, W. Formation of Intermetallic Phases in Al-Coated Hot-Stamped 22MnB5 Sheets in Terms of Coating Thickness and Si Content. Surf. Coat. Technol. 2014, 246, 17–25. [Google Scholar] [CrossRef]
- Cheng, W.J.; Wang, C.J. Microstructural Evolution of Intermetallic Layer in Hot-Dipped Aluminide Mild Steel with Silicon Addition. Surf. Coat. Technol. 2011, 205, 4726–4731. [Google Scholar] [CrossRef]
- Sasaki, T.; Yakou, T. Features of Intermetallic Compounds in Aluminized Steels Formed Using Aluminum Foil. Surf. Coat. Technol. 2006, 201, 2131–2139. [Google Scholar] [CrossRef]
- Maitra, T.; Gupta, S.P. Intermetallic Compound Formation in Fe-Al-Si Ternary System: Part II. Mater. Charact. 2003, 49, 293–311. [Google Scholar] [CrossRef]
- Cui, G.; Meng, Y.; Ju, X.; Yan, C. Microstructure Characterization of Al–Si Coatings on Hot Stamping Steel under Different Heat Treatment Processes. ISIJ Int. 2023, 63, 719–726. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Shumakova, N.I. Effect of ‘Duplex’ Treatment on Changes of Physical and Mechanical Properties of Steel (0.3 wt% C) 1999. Available online: www.elsevier.nl/locate/surfcoat (accessed on 15 June 2023).
- Yakubtsov, I.; Sohmshetty, R. Evolution of Al-Si Coating Microstructure during Heat-Treatment of Usibor® 1500. IOP Conf. Ser. Mater. Sci. Eng. 2018, 418, 012015. [Google Scholar] [CrossRef]
- Bakker, H. Diffusion in Solid Metals and Alloys; Mehrer, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 26, ISBN 3-540-50886-4. [Google Scholar]
- Suehiro, M.; Maki, J.; Kusumi, K.; Ohgami, M.; Miyakoshi, T. Properties of Aluminum-Coated Steels for Hot-Forming; Nippon Steel Technical Report; Nippon Steel Corporation: Tokyo, Japan, 2003. [Google Scholar]
- Allély, C.; Dosdat, L.; Clauzeau, O.; Ogle, K.; Volovitch, P. Anticorrosion Mechanisms of Aluminized Steel for Hot Stamping. Surf. Coat. Technol. 2014, 238, 188–196. [Google Scholar] [CrossRef]
- Alley, C.; Petitjean, J.; Vietoris, T. Corrosion Resistance of Zinc Based and Aluminized Coatings on Press-Hardened Steels for Automotive. In Proceedings of the 3rd, International Conference on Hot Sheet Metal Forming of High Performance Steel, Kassel, Germany, 13–17 June 2011; pp. 153–160. [Google Scholar]
- Maki, J.; Kurosaki, M.; Kusumi, K.; Abe, M. Effect of Heating Condition and Hot Forming on Corrosion Resistance of Hot Stamped Aluminized Steels. In Proceedings of the 3rd International Conference on Hot Sheet Metal Forming of High-Performance Steel, Kassel, Germany, 13–17 June 2011; pp. 499–507. [Google Scholar]
- Grandhi, S.; Oh, M.S. Influence of calcium on the morphology and corrosion performance of hot-dip Al–Si coatings. Mater. Lett. 2023, 353, 135278. [Google Scholar] [CrossRef]
- Drillet, P.; Spehner, D.; Kefferstein, R. Coated Steel Strips, Methods of Making The Same, Methods of Using the Same, Stamping Blanks Prepared from the Same, Stamped Products Prepared from the Same, and Articles of Manufacture Which Contain Such a Stamped Product. French Patent WO08053273, 8 May 2008. [Google Scholar]
- Gui, Z.X.; Wang, K.; Zhang, Y.S.; Zhu, B. Cracking and Interfacial Debonding of the Al-Si Coating in Hot Stamping of Pre-Coated Boron Steel. Appl. Surf. Sci. 2014, 316, 595–603. [Google Scholar] [CrossRef]
- Gui, Z.X.; Liang, W.K.; Zhang, Y.S. Enhancing Ductility of the Al-Si Coating on Hot Stamping Steel by Controlling the Fe-Al Phase Transformation during Austenitization. Sci. China Technol. Sci. 2014, 57, 1785–1793. [Google Scholar] [CrossRef]
- Takagi, K.; Nakanishi, E.; Yoshida, T. Aluminium-Coated Structural Member and Production Method. European Patent EP1380666, 10 May 2004. [Google Scholar]
- Wu, S.; Bardelcik, A.; Chiriac, C.; Elsayed, A.; Shi, C. The Effect of Heating Rate and Coating Weight on the Intermetallic Growth of Al Si Coated Hot Stamping Steel. Surf. Coat. Technol. 2023, 471, 129913. [Google Scholar] [CrossRef]
- Jhajj, K.S.; Slezak, S.R.; Daun, K.J. Inferring the Specific Heat of an Ultra High Strength Steel during the Heating Stage of Hot Forming Die Quenching, through Inverse Analysis. Appl. Therm. Eng. 2015, 83, 98–107. [Google Scholar] [CrossRef]
- Klassen, C.M.; Emmert, J.; Daun, K.J. Effect of Coating Thickness on the In-Situ Reflectance and Surface Roughness of Al-Si Coated 22MnB5 Steel. Surf. Coat. Technol. 2021, 414, 127100. [Google Scholar] [CrossRef]
- Novák, P.; Michalcová, A.; Marek, I.; Mudrová, M.; Saksl, K.; Bednarčík, J.; Zikmund, P.; Vojtěch, D. On the Formation of Intermetallics in Fe-Al System—An in Situ XRD Study. Intermetallics 2013, 32, 127–136. [Google Scholar] [CrossRef]
- Gupta, S.P. Intermetallic Compound Formation in Fe-Al-Si Ternary System: Part I. Mater. Charact. 2003, 49, 269–291. [Google Scholar] [CrossRef]
- Rivlin, V.G.; Raynor, G.V. 4: Critical Evaluation of Constitution of Aluminium-Iron-Silicon System. Int. Met. Rev. 1981, 26, 133–151. [Google Scholar] [CrossRef]






| Heating Rate | Furnace Temperature (°C) | Soaking Temperature (°C) | Soaking Time (s) |
|---|---|---|---|
| Medium | 900 | 900 | 30, 60, 120, 180, 240 |
| High | 1000 | 900 | 30, 60, 120, 180, 240 |
| 950 | 30, 60, 120, 180, 240 | ||
| 1000 | 30, 60, 120, 180, 240 |
| HRI | HRII | HRIII | HR600–900 | |||||
|---|---|---|---|---|---|---|---|---|
| Heating Rate | AS80 | AS150 | AS80 | AS150 | AS80 | AS150 | AS80 | AS150 |
| Medium | 10 [0.98] | 8.8 [0.98] | 3.4 [0.98] | 3.16 [0.98] | 1.6 [0.99] | 1.4 [0.99] | 1.6 [0.95] | 1.6 [0.96] |
| High | 16 [0.98] | 13.2 [0.98] | 6.7 [0.99] | 5.8 [0.99] | 3.2 [0.99] | 2.9 [0.99] | 3.9 [0.97] | 3.6 [0.98] |
| Weight Percentage (%) | Phase | References | ||
|---|---|---|---|---|
| Al | Fe | Si | ||
| 30–36 | 53–59 | 9–13 | ) | [17,19,27,49] |
| 53–57 | 36–39 | 5–8 | [19,26] | |
| 49–53 | 45–50 | 2–5 | ) | [19,26,27,28] |
| 0–19 | 78–100 | 0–5 | [19,20] | |
| 27–33 | 62–70 | 7–10 | ) | [17,20] |
| AS80 | AS150 | |||||
|---|---|---|---|---|---|---|
| Constants | 1 | 2 | 3 | 1 | 2 | 3 |
| 4.6 × 10−7 | −0.000467 | 0.0748 | −2.16 × 10−6 | 0.00436 | −2.1441 | |
| 0.00078634 | −1.407965 | 633.21 | 0.0013397 | −2.446091 | 1119.409 | |
| −0.81183 | 6.143 | 2.9769 | −1.0993 | 7.0416 | 3.0841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Bardelcik, A.; Chiriac, C.; Shi, C. A Comprehensive Assessment of Al-Si Coating Growth at Various Heating Rates, Soaking Temperatures, and Times. Coatings 2024, 14, 399. https://doi.org/10.3390/coatings14040399
Wu S, Bardelcik A, Chiriac C, Shi C. A Comprehensive Assessment of Al-Si Coating Growth at Various Heating Rates, Soaking Temperatures, and Times. Coatings. 2024; 14(4):399. https://doi.org/10.3390/coatings14040399
Chicago/Turabian StyleWu, Siyu, Alexander Bardelcik, Constantin Chiriac, and Cangji Shi. 2024. "A Comprehensive Assessment of Al-Si Coating Growth at Various Heating Rates, Soaking Temperatures, and Times" Coatings 14, no. 4: 399. https://doi.org/10.3390/coatings14040399





