Surface-Enhanced Raman Scattering for Probe Detection via Gold Nanorods and AuNRs@SiO2 Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of AuNRs and AuNRs@SiO2
2.3. Characterization
3. Results and Discussion
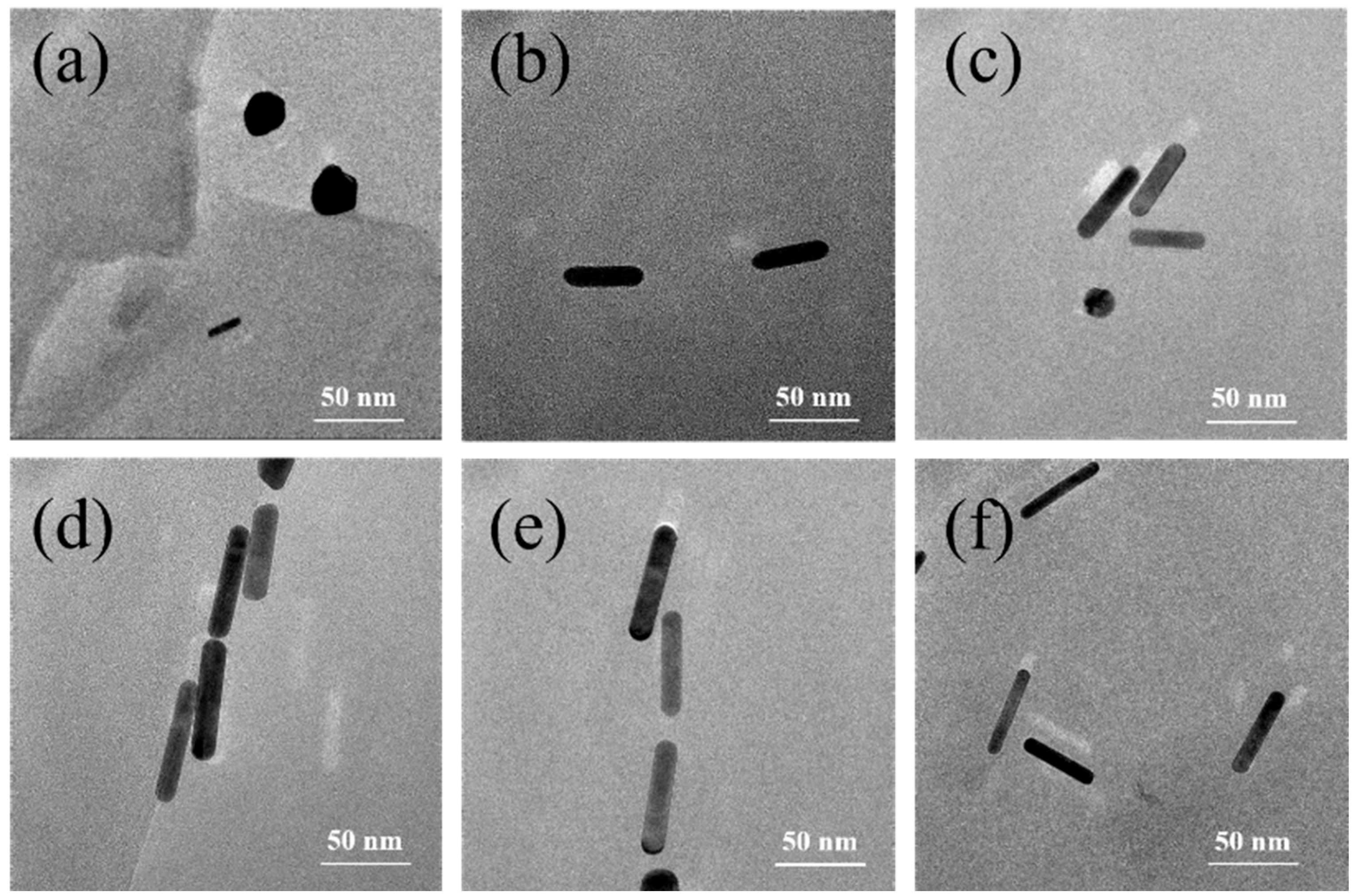
3.1. TEM Analysis of AuNRs and AuNRs@SiO2
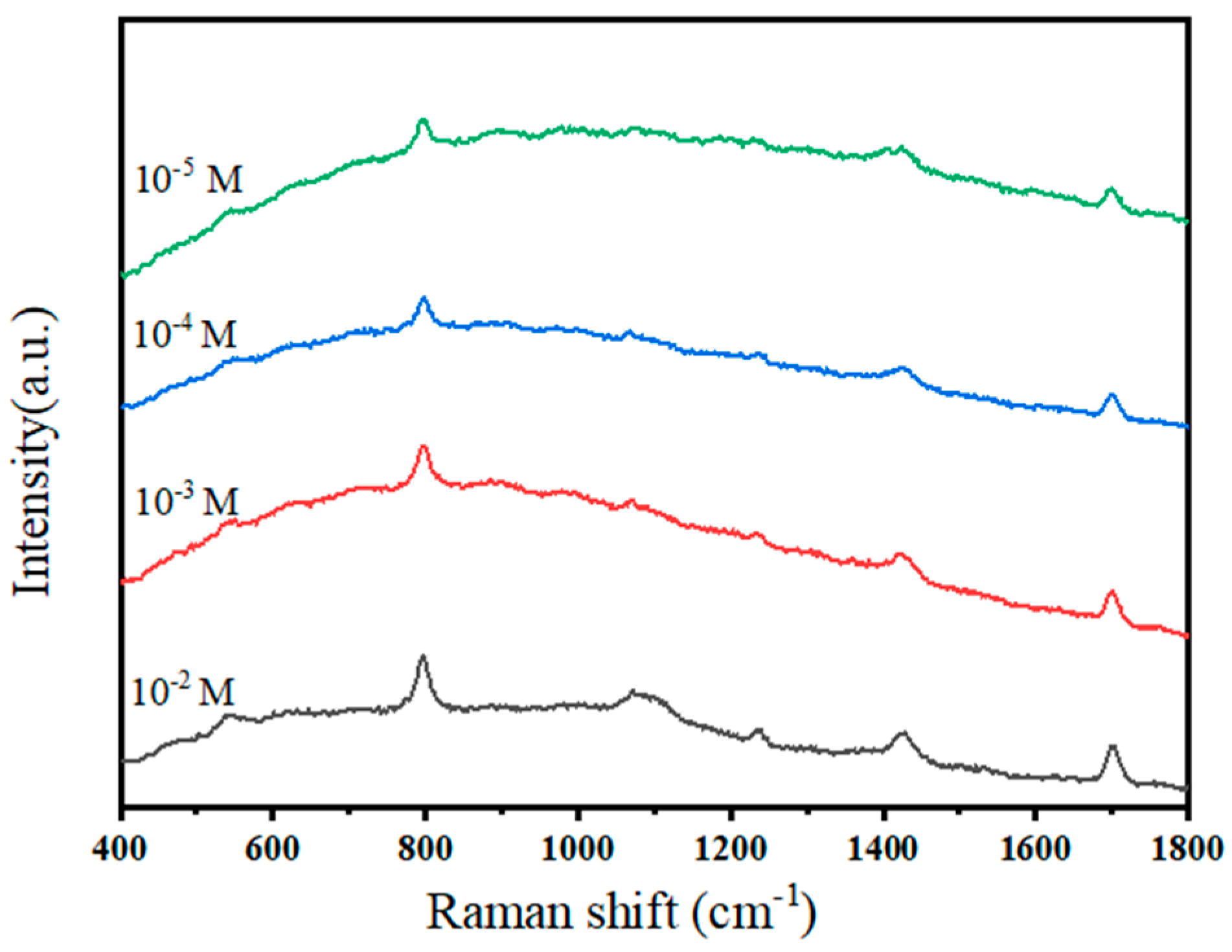
3.2. SERS Performance of AuNR and AuNRs@SiO2 Substrates
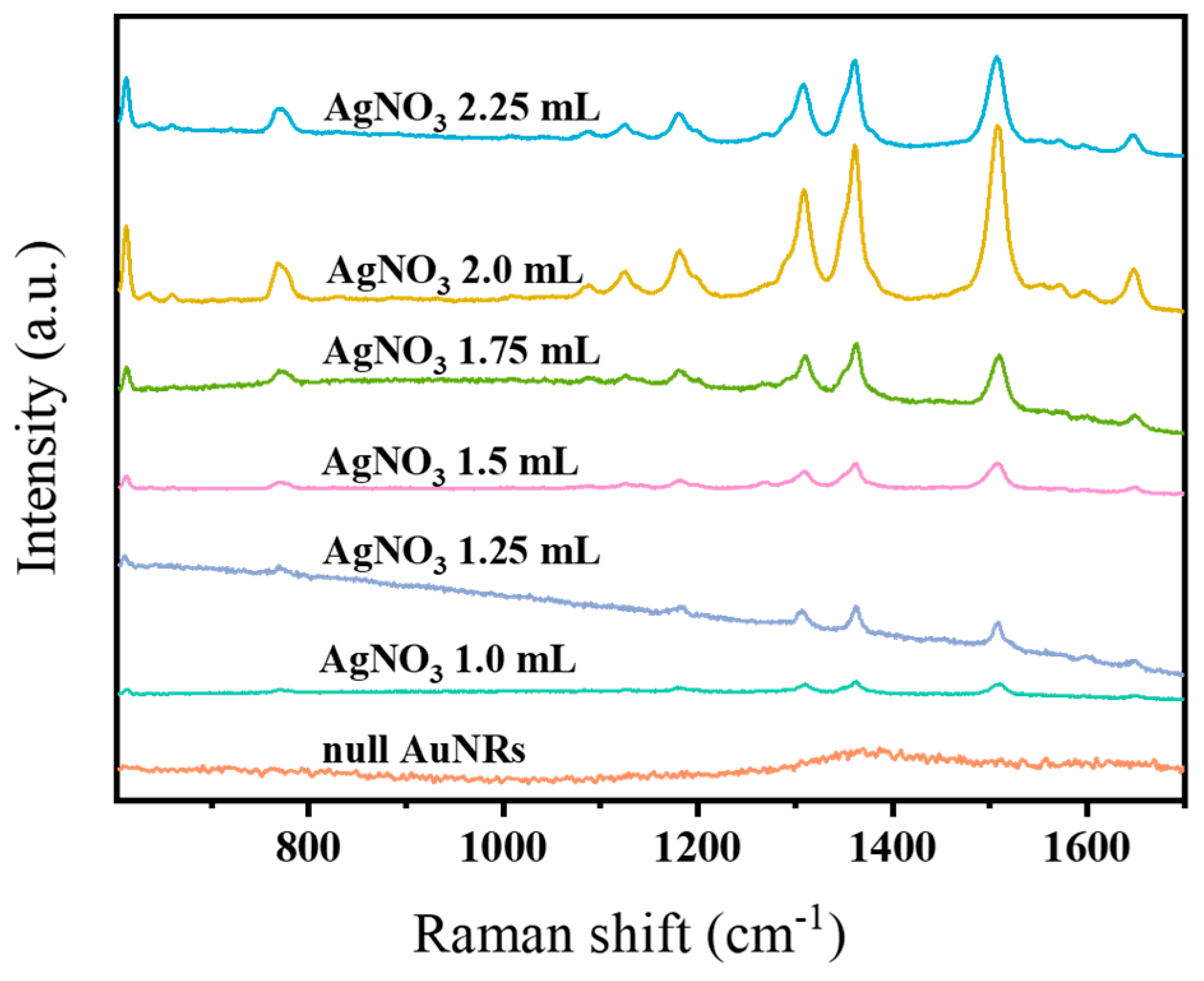
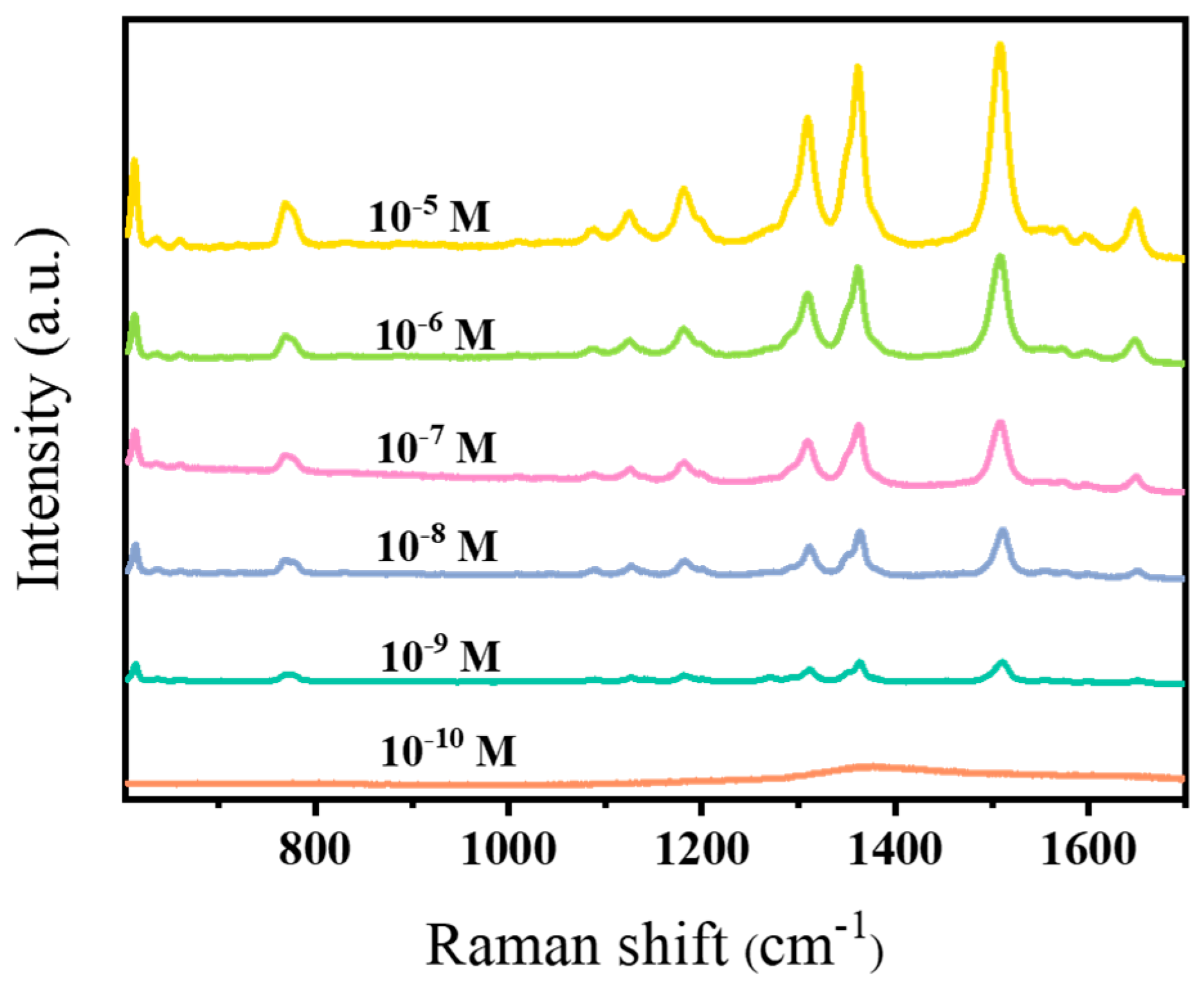
3.2.1. SERS Performance of AuNR Substrates
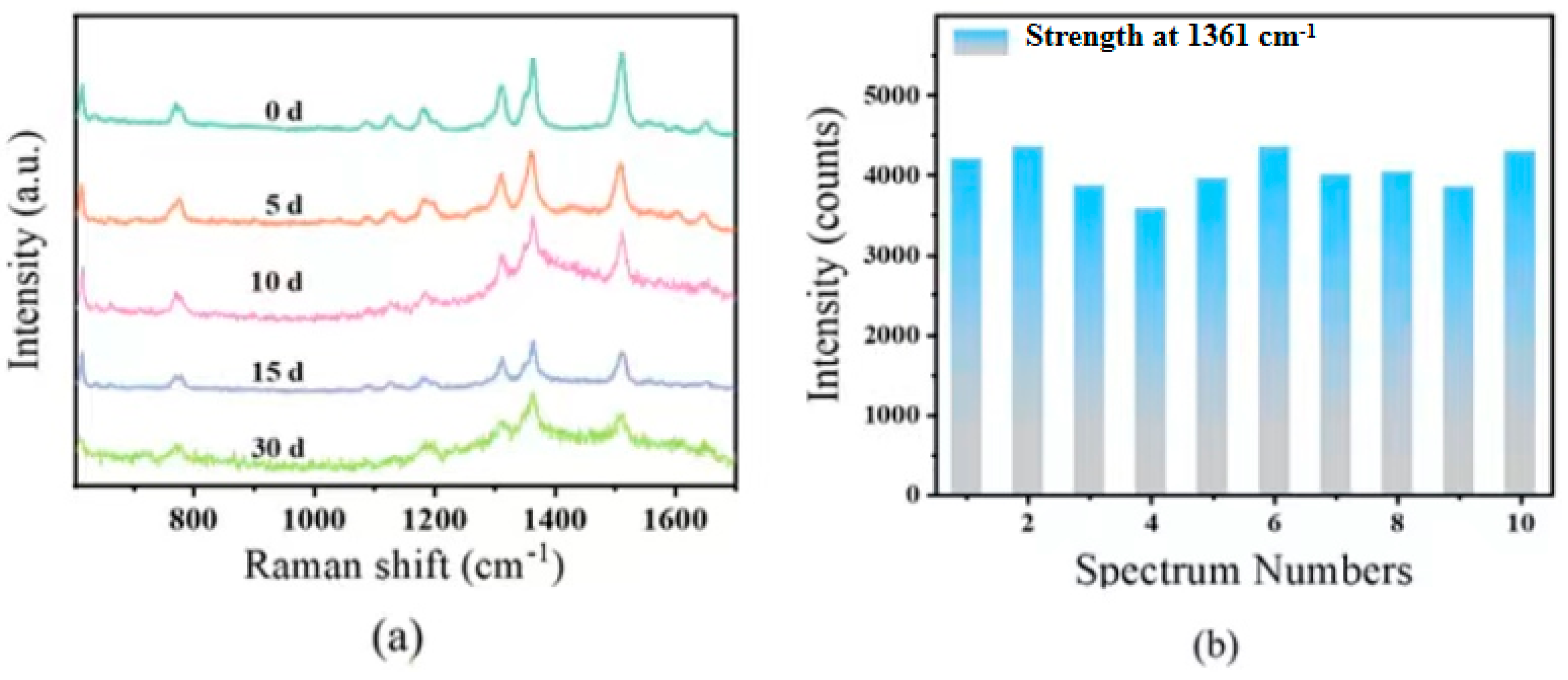
3.2.2. SERS Performance of AuNRs@SiO2 Substrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mokobia, K.E.; Ifijen, I.H.; Ikhuoria, E.U. ZnO-NPs-coated implants with osteogenic properties for enhanced osseointegration. In TMS Annual Meeting & Exhibition; Springer Nature: Cham, Switzerland, 2023; pp. 288–300. [Google Scholar]
- Rahman, A.; Rasid, H.; Ali, M.I.; Yeachin, N.; Alam, M.S.; Hossain, K.S.; Kafi, M.A. Facile Synthesis and Application of Ag-NPs for Controlling Antibiotic-Resistant Pseudomonas spp. and Bacillus spp. in a Poultry Farm Environment. J. Nanosci. Nanotechnol. 2023, 2023, 6260066. [Google Scholar] [CrossRef]
- Ziental, D.; Błaszkiewicz, P.; Długaszewska, J.; Güzel, E.; Dudkowiak, A.; Sobotta, L. Modified gold nanoparticles modulated fluorescence and singlet oxygen generation of pheophorbide a as an effective platform for photodynamic therapy against Staphylococcus aureus. Eur. J. Inorg. Chem. 2024, 27, e202300668. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, P.; Mao, L.; Wu, W.; Lin, H.; Xu, D.; Lu, X.; Shi, J. Piezocatalytic medicine: An emerging frontier using piezoelectric materials for biomedical applications. Adv. Mater. 2023, 35, 2208256. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ. Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Wen, C.; Guo, X.; Gao, C.; Zhu, Z.; Meng, N.; Shen, X.C.; Liang, H. NIR-II-responsive AuNRs@ SiO2–RB@MnO2 nanotheranostic for multimodal imaging-guided CDT/PTT synergistic cancer therapy. J. Mater. Chem. B 2022, 10, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, T.; Xu, X.; Xu, Y.; Li, D.; Song, W.; Zhan, T.; He, P.; Zhou, H.; Xu, J.J.; et al. Polyhedral Au Nanoparticle/MoOx Heterojunction-Enhanced Ultrasensitive Dual-Mode Biosensor for miRNA Detection Combined with a Nonenzymatic Cascade DNA Amplification Circuit. Anal. Chem. 2023, 95, 9271–9279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Wang, H.; Wang, S.; Liu, K.; Xu, T.; Jiang, J.; Chen, Y.C.; Liu, T. Ultra-sensitive DNAzyme-based optofluidic biosensor with liquid crystal-Au nanoparticle hybrid amplification for molecular detection. Sens. Actuators B Chem. 2022, 359, 131608. [Google Scholar] [CrossRef]
- Zheng, W.J.; Zhou, Z.L.; Lv, Q.Y.; Song, X.; Zhang, W.X.; Cui, H.F. Oxygen-Generated Hierarchical-Structured AuNRs@MnO2@SiO2 Nanocarrier for Enhanced NIR-and H2O2-Responsive Mild-Hyperthermia Photodynamic/Photothermal Combined Tumor Therapy. Adv. Ther. 2022, 5, 2200108. [Google Scholar] [CrossRef]
- Gupta, N.; Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef]
- Han, H.H.; Kim, S.J.; Kim, J.; Park, W.; Kim, C.; Kim, H.; Hahn, S.K. Bimetallic hyaluronate-modified Au@ Pt nanoparticles for noninvasive photoacoustic imaging and photothermal therapy of skin cancer. ACS Appl. Mater. Interfaces 2023, 15, 11609–11620. [Google Scholar] [CrossRef]
- Wang, C.; Nie, X.G.; Shi, Y.; Zhou, Y.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Direct Plasmon-Accelerated Electrochemical Reaction on Gold Nanoparticles. ACS Nano 2017, 11, 5897–5905. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.F.; Wang, M.M. Influencing Factors and Research Progress of Local Surface Plasmon Resonance. Adv. Anal. Chem. 2021, 11, 18. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yuan, G.F.; Ye, Z.H.; Yang, S.; Yang, Z.R. Polarization-Dependent Surface Enhanced Raman Scattig on Sliver Nanowires. Spectrosc. Spect. Anal. 2023, 43, 171–172. [Google Scholar]
- Xu, J.; Zhang, Y.; Yan, J.; Yang, Y.; Zhou, D.; Qiu, J.; Wang, Q.; Liu, C. Varying aspect ratios of Au nanorods with LSPR effect for efficient enhanced upconversion luminescence. Inorg. Chem. Commun. 2023, 156, 111134. [Google Scholar] [CrossRef]
- Chen, T.; Tong, F.; Enderlein, J.; Zheng, Z. Plasmon-driven modulation of reaction pathways of individual Pt-modified Au nanorods. Nano Lett. 2020, 20, 3326–3330. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Liu, J.C.; Ji, P.; Lin, Y.X.; Chen, L. Synthesis of high-density Au@Ag core-shell nanoparticles composite PA66 nanofibers and application in SERS detection of dyes. Mod. Chem. Ind. 2024, 44, 234–238+242. [Google Scholar]
- Zhang, W.X.; Zhou, Z.L.; Lv, Q.Y.; Song, X.; Chen, J.; Niu, C.B.; Cui, H.F. O2-Generation-Enhanced Responsive Starvation/Photothermal Synergistic Tumor Therapy Based on the AuNRs@MnO2@SiO2 Nanocarrier and Thermosensitive Biomimetic Camouflaging. ACS Appl. Bio Mater. 2023, 6, 4775–4790. [Google Scholar] [CrossRef]
- Ansah, I.B.; Lee, S.H.; Yang, J.Y.; Mun, C.; Jung, S.; Jung, H.S.; Lee, M.Y.; Kang, T.; Lee, S.; Kim, D.H.; et al. In-situ fabrication of 3D interior hotspots templated with a protein@ Au core–shell structure for label-free and on-site SERS detection of viral diseases. Biosens. Bioelectron. 2023, 220, 114930. [Google Scholar] [CrossRef]
- Guo, X.H.; Ma, J.Q. Fabrication of Magnetic Fe3O4@SiO2@TiO2-Au with Core-shell Structure and Its Photocatalytic Reduction Activity. Acta Mater. Compos. Sin. 2024, 42, 1–11. [Google Scholar]
- Zhang, J.; Teng, F.; Hu, B.; Liu, W.; Huang, Y.; Wu, J.; Wang, Y.; Su, H.; Yang, S.; Zhang, L.; et al. Early Diagnosis and Prognosis Prediction of Pancreatic Cancer Using Engineered Hybrid Core-Shells in Laser Desorption/Ionization Mass Spectrometry. Adv. Mater. 2024, 2311431. [Google Scholar] [CrossRef]
- Zhao, Y.; Sang, L.; Ren, Z. Decoupling the role of thermoplasmonics effect and hot electron injection of metallic nanoparticles in photoelectrochemical system. Sol. Energy Mater. Sol. Cells 2024, 267, 112728. [Google Scholar] [CrossRef]
- Wu, P.; Sun, X.; Hao, N.; Wang, L.; Huang, J.; Tang, J. Facile In-Situ photocatalytic reduction of AuNPs on multilayer Core-Shell Fe3O4@SiO2@PDA magnetic nanostructures and their SERS application. Spectrochim. Acta A 2023, 302, 123101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Zhou, N.; Li, M.; Huang, C.; Mao, H. Recyclable 3D SERS devices based on ZnO nanorod-grafted nanowire forests for biochemical sensing. Appl. Surf. Sci. 2022, 582, 152336. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, R.; Li, X.; Mao, X.; Xu, S.; Zhou, N.; Li, S.; Mao, H.; Huang, C. A SERS sensor based on 3D nanocone forests capable of intelligent classification of aquatic product dyes. J. Mater. Chem. C 2023, 11, 14237–14247. [Google Scholar] [CrossRef]
- Prasad, R.; Selvaraj, K. Effective Distribution of Gold Nanorods in Ordered Thick Mesoporous Silica: A Choice of Noninvasive Theranostics. ACS Appl. Mater. Interfaces 2023, 15, 47615–47627. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Prall, B.S.; Klimov, V.I. Hybrid gold/silica/nanocrystal-quantum-dot superstructures: Synthesis and analysis of semiconductor-metal interactions. J. Am. Chem. Soc. 2006, 128, 15362–15363. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Lu, D.; Ahn, S. Plasmon enhancement of luminescence upconversion. Chem. Soc. Rev. 2015, 44, 2940–2962. [Google Scholar] [CrossRef]
- Wu, D.M.; García-Etxarri, A.; Salleo, A. Plasmon-enhanced upconversion. J. Phys. Chem. Lett. 2014, 5, 4020–4031. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Tian, Y.; Yan, S.; Ren, L.; Ma, R.; Zhao, W.; Zhang, H.; Dou, S. Surface-Enhanced Raman Scattering for Probe Detection via Gold Nanorods and AuNRs@SiO2 Composites. Coatings 2024, 14, 530. https://doi.org/10.3390/coatings14050530
Li H, Tian Y, Yan S, Ren L, Ma R, Zhao W, Zhang H, Dou S. Surface-Enhanced Raman Scattering for Probe Detection via Gold Nanorods and AuNRs@SiO2 Composites. Coatings. 2024; 14(5):530. https://doi.org/10.3390/coatings14050530
Chicago/Turabian StyleLi, Huiqin, Yanyu Tian, Shaotian Yan, Lijun Ren, Rong Ma, Weiwei Zhao, Hongge Zhang, and Shumei Dou. 2024. "Surface-Enhanced Raman Scattering for Probe Detection via Gold Nanorods and AuNRs@SiO2 Composites" Coatings 14, no. 5: 530. https://doi.org/10.3390/coatings14050530



