Investigating the Suitability of Various Silver(I) Complexes for Use in a Cyanide-Free Silver Electrolyte
Abstract
1. Introduction
2. Experimentation
2.1. Methods and Materials
2.2. Mathematical Description
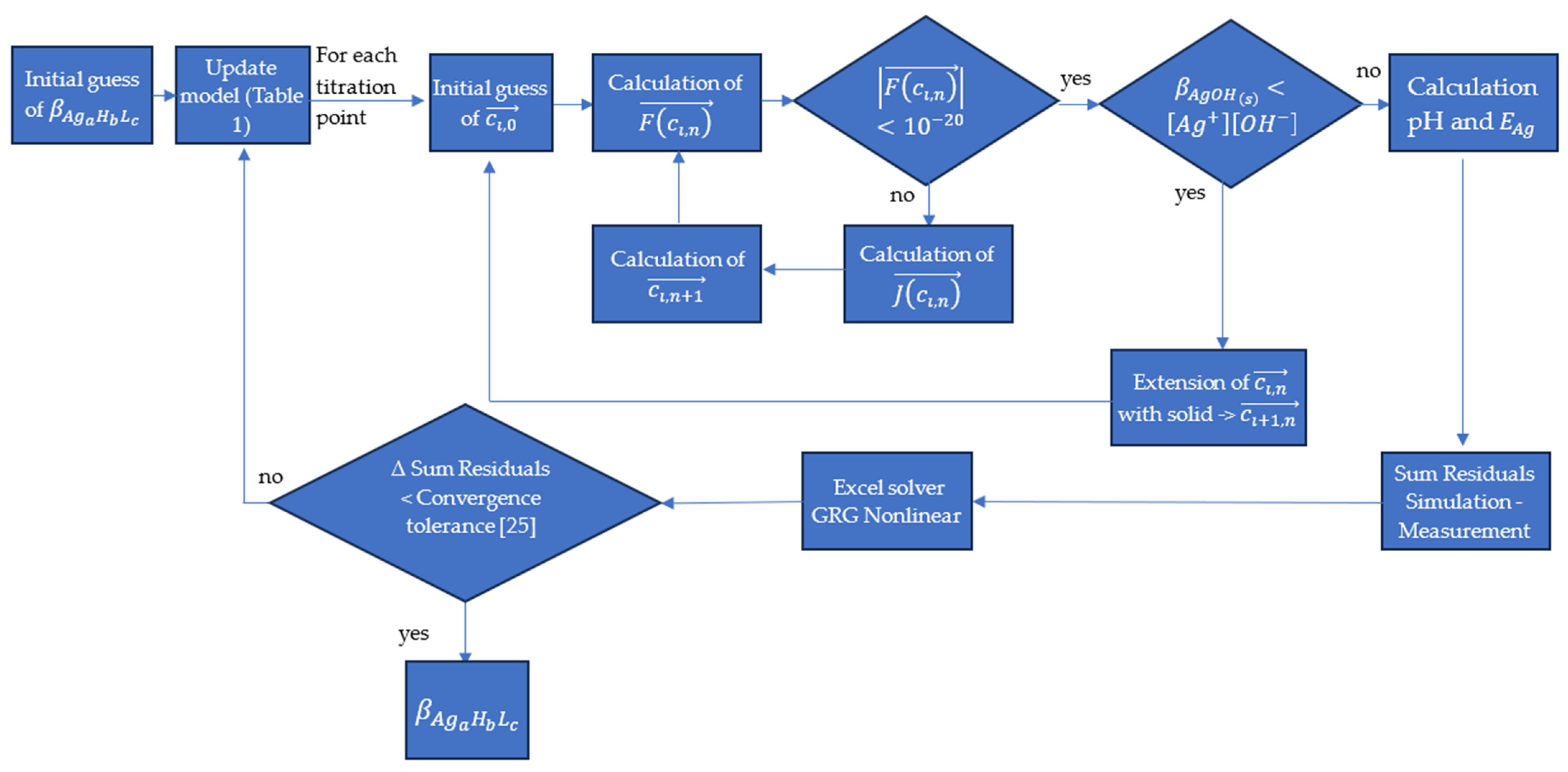
2.3. Evaluation of the Measurements
3. Results
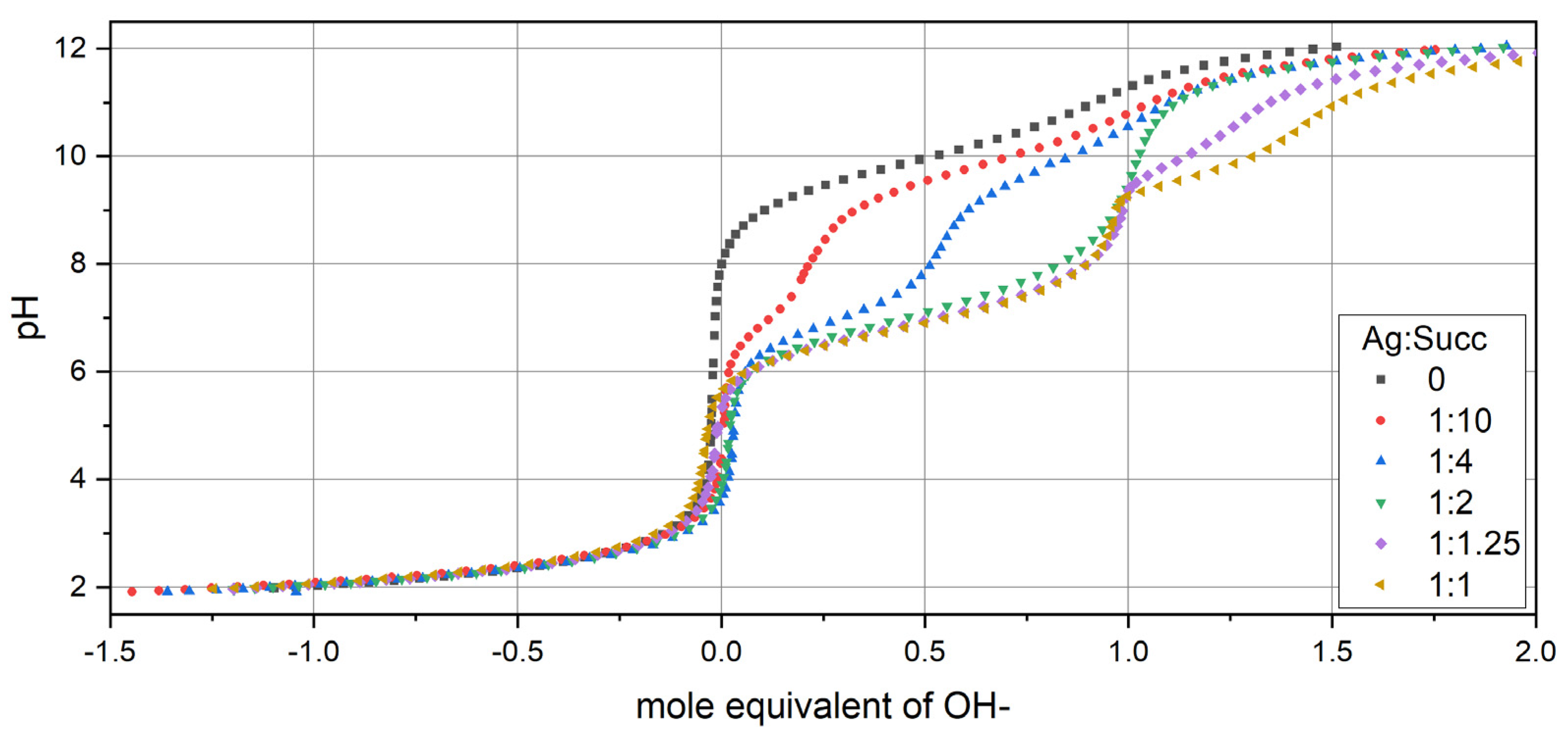
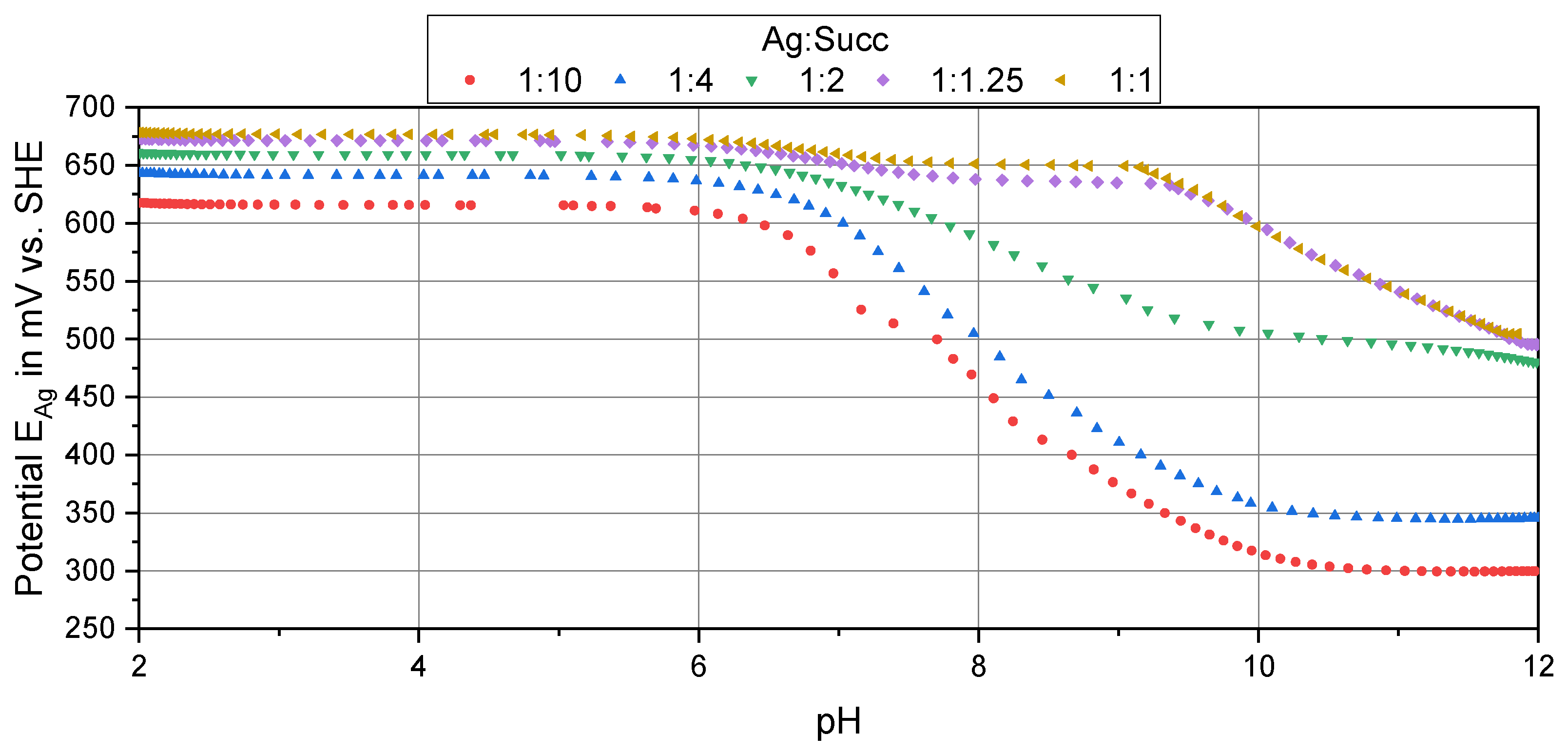
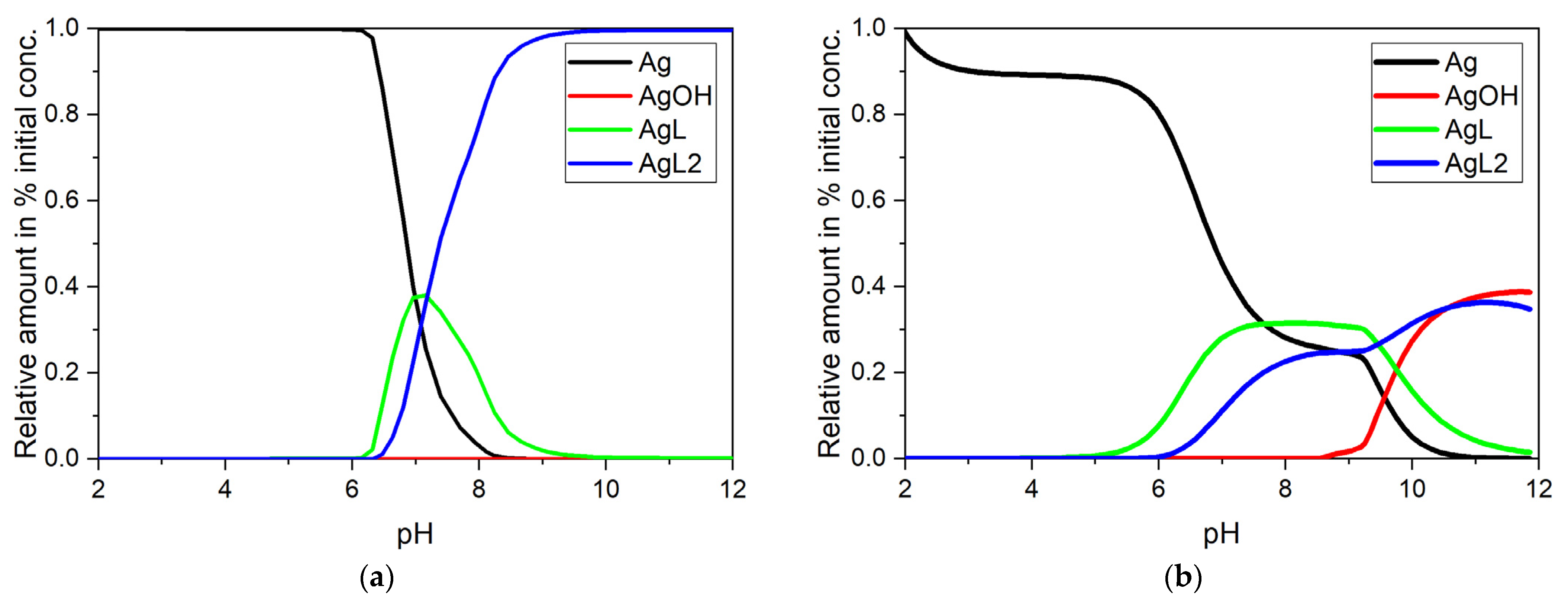
3.1. Potentiometric Titration of Succinimide (Succ)
3.2. Evaluation of the Studied Complexing Agents
3.2.1. Nitrogen Compounds
3.2.2. Sulfur Compounds
3.2.3. Oxygen Compounds
3.2.4. Phosphorous Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Substance | IUPAC Name | Supplier | Purity |
|---|---|---|---|
| Succinimide | Pyrrolidine-2,5-dione | VEB Jenapharm, Jena, Germany | 98% |
| Glycine | Aminoacetic acid | Reanal, Budapest, Hungary | 98%, <0.2% sulph. Ash <0.05% NH3 |
| 5,5-dimethylhydantoin | 4,4-Dimethyl-2,5-dioxoimidazolidin | Sigma-Aldrich, Merck KGaA, Darmstadt, Germany | 97% |
| Ethylenediamine tetra (methylene phosphonic acid) (ETDMP) | {Ethane-1,2-diylbis[nitrilobis(methylene)]}-tetrakis(phosphonic acid) | Zschimmer & Schwarz, Lahnstein, Germany | 94.7%, 0.03% Cl− |
| Etidronic acid (HEDP) | 1-Hydroxyethylidene-1,1-diphosphonic acid | Zschimmer & Schwarz, Lahnstein, Germany | 83.8% 0.06% H3PO3 0.01% Cl− |
| Iminodiacetic acid | 2,2′-Azanediyldiacetic acid | Fluka, Fisher Scientific GmbH, Schwerte, Germany | 90%, ~10% H2O |
| L-Histidine | L-2-Amino-3-(1H-imidazol-4-yl)propanoic acid | Reanal, Budapest, Hungary | 98.5% |
| L-Methionine | L-2-amino-4-(methylthio)butanoic acid | VEB-Berlin-Chemie, Berlin, Germany | 99% |
| Triethylenetetramine | N1,N1′-(Ethane-1,2-diyl)di(ethane-1,2-diamine) | Arcos Organics, Schwerte, Germany | 60% tech. |
| Thiodiglycolic acid | 2-(carboxymethylsulfanyl)acetic acid | Thermo-Fisher, Darmstadt, Germany | 98% |
| Uracil-1-acetic acid | 3,4-Dihydro-2,4-dioxo-1(2H)-pyrimidineacetic acid | Prepared [16] | 98% |
| Trisodium dicarboxymethyl alaninate (Trilon M) | Trisodium;2-[bis(carboxylatomethyl) amino]propanoate | BASF, Ludwigshafen, Germany | 40% |
| Iminodisuccinate (Baypure CX 100/34) | Tetrasodium N-(1,2-dicarboxylatoethyl)-ξ-aspartate(2−) | Lanxess, Köln, Germany | 34% |
| Sodium sulfite | Sodium sulfite | Reanal, Budapest, Hungary | p.A. |
| Thiourea | Thio urea | Binnenhandel Herzberg, Herzberg, Germany | p.A. |
| Sodium thiocyanate | Sodium thiocyanate | VEB Berlin-Chemie, Berlin, Germany | p.A. |
| 5-Sulfosalicylic acid | 2-Hydroxy-5-sulfobenzoic acid | VEB Berlin-Chemie, Berlin, Germany | p.A. |
| Phosphoric acid | Phosphoric acid | VEB Jenapharm, Jena, Germany | 99% |
| Phosphorous acid | Phosphonic acid | Riedel-de Haën, Seelze, Germany | >98% |
| Hypophosphorous acid | Phosphinic acid | Fluka, Fisher Scientific GmbH, Schwerte, Germany | 48.0–52.0% |
| Methanesulfonic acid | Methanesulfonic acid | Carl Roth, Karlsruhe, Germany | ≥99% |
| 2-Sulfobenzoic acid | 2-sulfobenzoic acid | VEB Laborchemie, Apolda, Germany | p.A. |
| 2-Pyrrolidone | Pyrrolidin-2-one | Sigma Aldrich, Merck KGaA, Darmstadt, Germany | ≥99% |
| Glutarimide | Piperidine-2,6-dione | Sigma Aldrich, Merck KGaA, Darmstadt, Germany | 98% |
| Thiosulfate | Thiosulfate | Reanal, Budapest, Hungary | p.A. |
| Hydroquinone | Benzene-1,4-diol | Reanal, Budapest, Hungary | p.A. |
| 1,2,4-Triazole | 1H-1,2,4-Triazole | Carl Roth, Karlsruhe, Germany | ≥98% |
| 4-Methyluracil | 6-methyl-1H-pyrimidine-2,4-dione | Reanal, Budapest, Hungary | p.A. |
| Ethylene dithiodiethanol (TIB Suract ETG) |
2-[2-(2-hydroxyethyldisulfanyl) ethyldisulfanyl]ethanol | TIB Chemicals, Mannheim, Germany | technical grade |
Appendix B
| Complexing Agent | Probable Donor Atom | pka | Number of Titrations | Complex | SD | |
|---|---|---|---|---|---|---|
| Succinimide | N | 9.7 | 6 | AgL AgL2 | 5 9.7 | 0.25 0.17 |
| Glycine | N | 2.1 9.4 | 9 | AgL AgL2 | 3.7 6.3 | 0.24 0.27 |
| 5,5-dimethylhydantoin | N | 8.9 | 9 | AgL AgL2 | 4.9 9.2 | 0.33 0.47 |
| Ethylenediamine tetra (methylene phosphonic acid) (ETDMP) | P | 2.3 3.5 5.5 6.5 8.0 9.9 | 9 | AgH3L AgH2L AgHL AgL | 27.7 22.2 13.8 6.9 | 0.20 0.32 0.59 0.63 |
| Etidronic acid (HEDP) | P | <2 2.9 7.5 11.5 | 9 | AgL AgL2 | 2.6 5.5 | 0.41 1.40 |
| Iminodiacetic acid | O | 2.7 9.5 | 9 | AgL | 3.7 | 0.05 |
| L-Histidine | N | 2.1 6.1 9.1 | 9 | AgHL Ag(HL)2 Ag2L2 | 13 23.8 16.4 | 0.33 0.88 0.43 |
| L-Methionine | N/S | 2.7 9.2 | 9 | AgH2L AgHL AgL AgL2 | 15.6 13.2 6.1 7.9 | 0.05 0.10 0.06 0.17 |
| Triethylenetetramine | N | 3.7 6.7 7.8 9.8 | 9 | AgL AgL2 | 7.4 9.8 | 0.12 0.06 |
| Thiodiglycolic acid | S | 4.2 7.5 | 4 | Insoluble precipitate | ||
| Uracil-1-acetic acid | N | 9.7 | 4 | AgL AgL2 | 5.0 9.2 | 0.27 0.38 |
| Trisodium dicarboxymethyl alaninate (Trilon M) | O | <2 2.6 10.2 | 4 | AgL | 5.1 | 0.16 |
| Iminodisuccinate (Baypure CX 100/34) | O | 3.3 3.5 4.7 10.3 | 4 | AgL | 4.5 | 0.30 |
| Sodium sulfite | O | 1.8 6.9 | 4 | No complexation detected | ||
| Thiourea | S | <2 | 4 | Insoluble precipitate | ||
| Thiosulfate | S | <2 | 4 | Insoluble precipitate | ||
| Sodium thiocyanate | S | <2 | 4 | Insoluble precipitate | ||
| 5-Sulfosalicylic acid | O | <2 | 4 | No complexation detected | ||
| Phosphoric acid | P | 2.1 6.8 12.3 | 2 | AgHL AgL | 15.7 5.5 | 0.28 0.21 |
| Phosphorous acid | P | 2.2 6.7 >12 | 2 | No complexation detected | ||
| Hypophosphorous acid | P | <2 | 2 | No complexation detected | ||
| Methanesulfonic acid | S | <2 | 4 | No complexation detected | ||
| 2-Sulfobenzoic acid | O | <2 | 4 | No complexation detected | ||
| Hydroquinone | O | 10.2 11.6 | 3 | Insoluble precipitate | ||
| 2-Pyrrolidone | N | >12 | 4 | No complexation detected | ||
| Glutarimide | N | 11.5 | 4 | AgL AgL2 | 5.1 10.5 | 0.12 0.50 |
| 1,2,4-Triazole | N | 2.5 | 4 | Insoluble precipitate | ||
| 4-Methyluracil | N | 9.5 | 4 | AgL AgL2 | 4.7 9.2 | 0.85 0.69 |
| Ethylene dithiodiethanol (TIB Suract ETG) | S | >12 | 4 | Ag(HL)2 | 38.2 | 3.29 |
References
- Yli-Pentti, A. 4.11—Electroplating and Electroless Plating. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; pp. 277–306. ISBN 978-0-08-096533-8. [Google Scholar]
- Satpathy, B.; Jena, S.; Das, S.; Das, K. A comprehensive review of various non-cyanide electroplating baths for the production of silver and gold coatings. Int. Mater. Rev. 2023, 68, 825–861. [Google Scholar] [CrossRef]
- Berger, S.; Hoffacker, G. Novel and innovative non-cyanide silver process: A report on commercial plating experiences. Plat. Surf. Finish. 2005, 92, 46–51. [Google Scholar]
- Gan, H.; Liu, G.; Huang, C.; Tang, R.; Liu, Y.; Shi, C. The influence of pH and current density for nano-silver electrodeposited in cyanide-free succinimide bath. Surf. Interfaces 2021, 27, 101487. [Google Scholar] [CrossRef]
- Dini, J.W.; Morrissey, R.J.; Pacheco, D.R. Materials characterization of a non-cyanide silver electrodeposit. Plat. Surf. Finish. 1997, 84, 62–67. [Google Scholar]
- Liu, A.; Ren, X.; Wang, B.; Zhang, J.; Yang, P.; Zhang, J.; An, M. Complexing agent study via computational chemistry for environmentally friendly silver electrodeposition and the application of a silver deposit. RSC Adv. 2014, 4, 40930–40940. [Google Scholar] [CrossRef]
- Liu, A.; Ren, X.; Wang, C.; Zhang, J.; Du, C.; Han, R.; An, M. DMH and NA–based cyanide-free silver electroplating bath: A promising alternative to cyanide ones in microelectronics. Ionics 2021, 27, 417–422. [Google Scholar] [CrossRef]
- Ren, F.; Yin, L.; Wang, S.; Volinsky, A.A.; Tian, B. Cyanide-free silver electroplating process in thiosulfate bath and microstructure analysis of Ag coatings. Trans. Nonferrous Met. Soc. China 2013, 23, 3822–3828. [Google Scholar] [CrossRef]
- Jasni, A.B.; Yoshihara, S.; Aiki, F.; Watanabe, H. Pyrimidine derivative-based cyanide-free silver electroplating bath. Trans. IMF 2022, 100, 193–199. [Google Scholar] [CrossRef]
- Xie, B.-G.; Sun, J.-J.; Lin, Z.-B.; Chen, G.-N. Electrodeposition of Mirror-Bright Silver in Cyanide-Free Bath Containing Uracil as Complexing Agent Without a Separate Strike Plating Process. J. Electrochem. Soc. 2009, 156, D79. [Google Scholar] [CrossRef]
- Cai, H.; Li, Y.; Wu, Y.; Zhang, H.; Zhang, Y.; Sun, Y.; Wang, G.; Song, J.; Ding, G. Non-Cyanide Thick Silver Electrodeposition Base on Instantaneous Nucleation for 3D Microstructures with High Performance. J. Electrochem. Soc. 2023, 170, 22504. [Google Scholar] [CrossRef]
- Fox, B.S.; Beyer, M.K.; Bondybey, V.E. Coordination chemistry of silver cations. J. Am. Chem. Soc. 2002, 124, 13613–13623. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, G.; Anderegg, G. Über die Stabilität großer Chelatringe. Z. Anorg. Allg. Chem. 1955, 282, 286–292. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Tran, D.P.; Truong, T.N. Computational Study on the Nature of Bonding between Silver Ions and Nitrogen Ligands. ACS Omega 2022, 7, 45231–45238. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, J. On the tendency of the metal ions toward complex formation. Chem. Rev. 1950, 46, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Shvachkin, Y.P.; Azarova, M.T. Potential antimetabolites. IV. Derivatives of 3-uracilylacetic acid. Zh. Obshch. Khim. 1963, 33, 590. [Google Scholar]
- de Bethune, A.J.; Licht, T.S.; Swendeman, N. The Temperature Coefficients of Electrode Potentials. J. Electrochem. Soc. 1959, 106, 616. [Google Scholar] [CrossRef]
- Haider, C. Electrodes in Potentiometry; Metrohm Ltd.: Herisau, Switzerland, 2004. [Google Scholar]
- Bottari, E.; D’Ambrosio, A.; Festa, M.R.; Iuliano, M.; Meschino, M.; de Tommaso, G. Stability and Structure of Silver–l-methionine Complexes. J. Solut. Chem. 2022, 51, 1393–1408. [Google Scholar] [CrossRef]
- Martell, A.E.; Motekaitis, R.J. The Determination and Use of Stability Constants, 2nd ed.; VCH: Weinheim, Germany, 1992; ISBN 3527895167. [Google Scholar]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. HySS Hyperquad Simulation and Speciation. Available online: http://www.hyperquad.co.uk/hyss.htm (accessed on 10 April 2024).
- Martell, A.E.; Smith, R.M.; Motekaitis, R.J. NIST Standard Reference Database 46 Version 8.0: NIST Critically Selected Stability Constants of Metal Complexes. 2004. Available online: https://www.nist.gov/srd/nist46 (accessed on 5 April 2024).
- Ingri, N.; Kakolowicz, W.; Sillén, L.G.; Warnqvist, B. High-speed computers as a supplement to graphical methods—V11Part IV—Arkiv Kemi, 1964, 23, 97; for commsnt on spelling “program” see Talanta, 1967, 14, 833.: Haltafall, a general program for calculating the composition of equilibrium mixtures. Talanta 1967, 14, 1261–1286. [Google Scholar] [CrossRef] [PubMed]
- Sebah, P.; Gourdon, X. Newton’s method and high order iterations. Numbers Comput. 2001, 1, 1–10. [Google Scholar]
- Mircosoft. SolverOptions Function. Available online: https://learn.microsoft.com/en-us/office/vba/excel/concepts/functions/solveroptions-function (accessed on 5 April 2024).
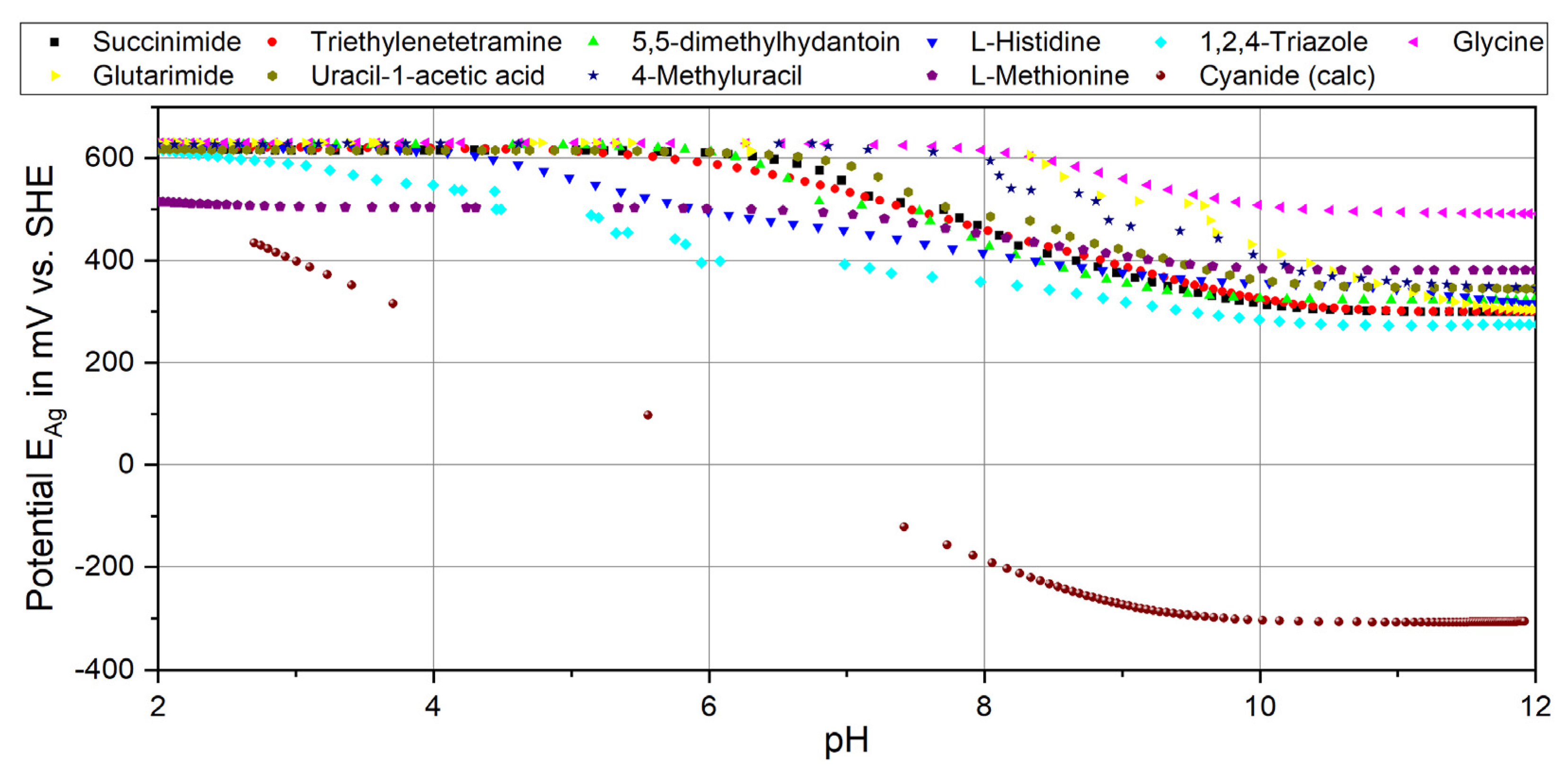
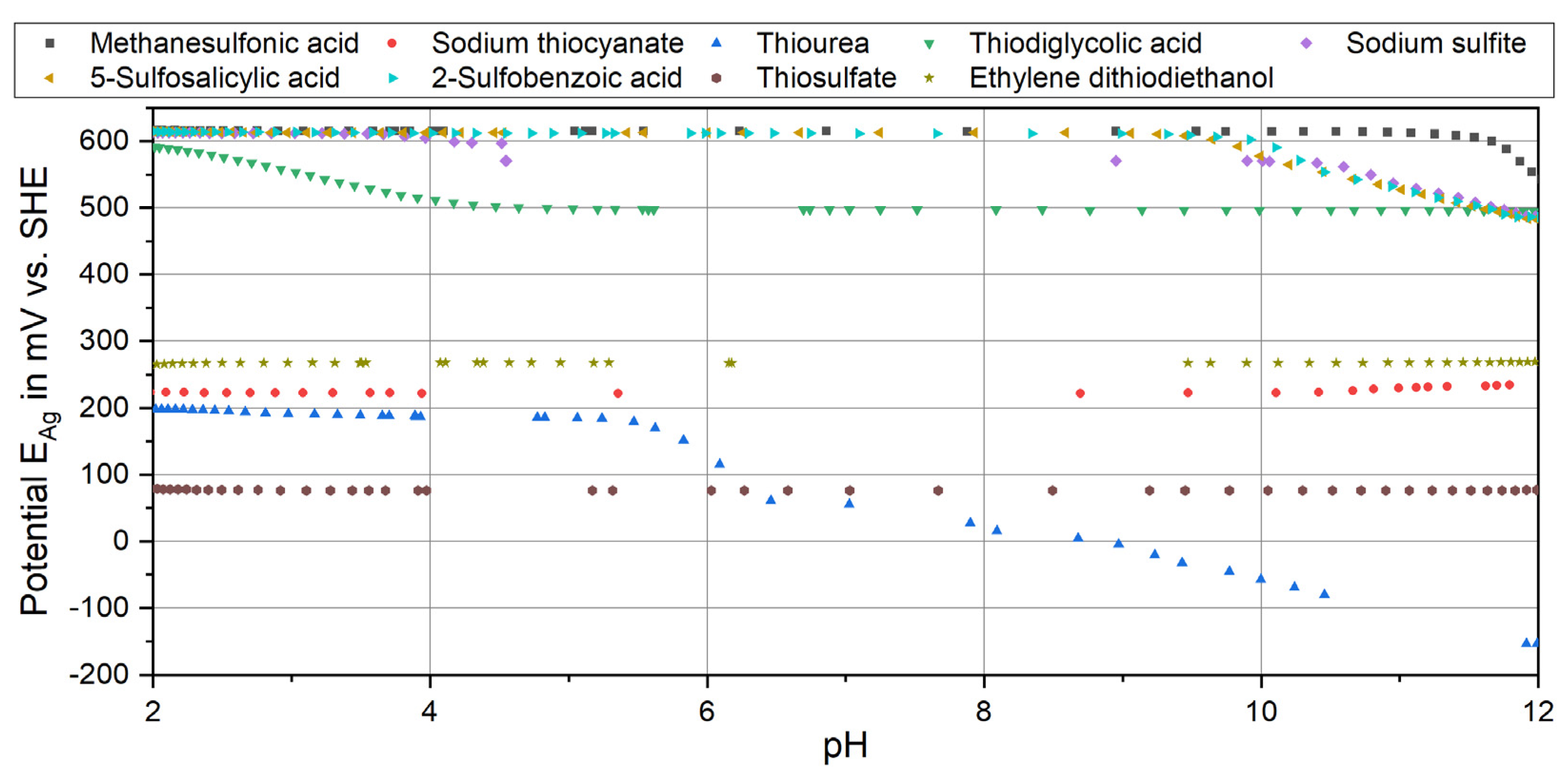


| Species Formula | Silver | Protons | Ligands | Log Constant (Equilibrium) | |
|---|---|---|---|---|---|
| a | b | c | |||
| Complexes | 1 | 0 | 1 | ||
| 1 | 0 | 2 | |||
| Protonated complexing agent | 0 | 1 | 1 | ||
| Solids/precipitations | 1 | −1 | 0 |
| Equilibrium Constant | Ratio of Silver to Succinimide (Ag/L) | Average | Standard Deviation | Published Values [23] | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1:10 | 1:4 | 1:2 | 1:1.25 | 1:1 | ||||
| 5.3 | 4.6 | 5.2 | 5.0 | 5.0 | 5.0 | 0.25 | 5.2 | ||
| 9.7 | 9.8 | 9.9 | 10.1 | 10.1 | 9.9 | 0.17 | 9.6 | ||
| 9.9 | 9.5 | 9.4 | 9.8 | 9.7 | 9.8 | 9.7 | 0.22 | 9.54 | |
| 7.5 | 7.3 | 7.3 | 7.4 | 0.08 | 7.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumer, C.; Schmidt, U.; Bund, A. Investigating the Suitability of Various Silver(I) Complexes for Use in a Cyanide-Free Silver Electrolyte. Coatings 2024, 14, 618. https://doi.org/10.3390/coatings14050618
Baumer C, Schmidt U, Bund A. Investigating the Suitability of Various Silver(I) Complexes for Use in a Cyanide-Free Silver Electrolyte. Coatings. 2024; 14(5):618. https://doi.org/10.3390/coatings14050618
Chicago/Turabian StyleBaumer, Christoph, Udo Schmidt, and Andreas Bund. 2024. "Investigating the Suitability of Various Silver(I) Complexes for Use in a Cyanide-Free Silver Electrolyte" Coatings 14, no. 5: 618. https://doi.org/10.3390/coatings14050618
APA StyleBaumer, C., Schmidt, U., & Bund, A. (2024). Investigating the Suitability of Various Silver(I) Complexes for Use in a Cyanide-Free Silver Electrolyte. Coatings, 14(5), 618. https://doi.org/10.3390/coatings14050618






