Surface Engineering for Bone Implants: A Trend from Passive to Active Surfaces
Abstract
:1. Introduction
2. Bone Implants
2.1. Implants: Interface between Living Tissue and Dead Matter
“Bone is deposited and reinforced at areas of greatest stress” [6].
2.2. Material Requirements for Load-Bearing Bone Implants
2.3. From Passive to Active Bone Implant Surfaces
2.4. Surface Modifications for Bone Implants
3. Surface Engineering: Coating Deposition
3.1. Biological Activity of Bone Implant Coatings
| Name | Description |
|---|---|
| OsseoSpeed (Astra Tech AB, Mölndal, Sweden) | Titanium oxide blasting followed by chemical modification of the surface by hydrofluoric acid treatment |
| SLActive (ITI; Institute Straumann, Waldenburg, Switzerland) | Coarse grit-blasting with 0.25–0.5 mm aluminum oxide grit at 5 bar followed by acid etching |
| TiUnite (Nobel Biocare Holding AG, Zürich, Switzerland) | Electrochemical anodization process |
| Nanotite (3i Implant Innovations, Palm Beach Gardens, FL, USA) | Sol-gel deposition |
| Friadent plus (Dentsply Friadent, Mannheim, Germany) | large grit blasting (354–500 μm) and acid etching in hydrochloric acid/sulfuric acid/hydrofluoric acid/oxalic acid |
| Ossean (intra-Lock, Boca-Raton, FL, USA) | is a grit-blasted/acid-etched/calcium phosphate impregnated surface |
3.2. Trends in Material for Inorganic Coatings on Bone Implants
3.3. Trends in Material for Organic Coatings on Bone Implants
3.4. Trends in Materials for Composite and Combined Coatings on Bone Implants
4. Coating Techniques
4.1. Dry Deposition Techniques
| Technique | Coating thickness | Advantage | Disadvantage | Precursor materials |
|---|---|---|---|---|
| Plasma spraying | 50–250 μm | High deposition rates | Non-uniform coating crystalinity; line of sight technique | HA [36,124,125,126,127,128], Si-HA [40,49] and antibacterial Ag- HA composite coatings [66,67,129] |
| RF magnetron sputtering | 0.5–5 μm | Uniform and dense coating; strong adhesion | Line of sight technique; time consuming; low deposition rates | HA [43], Si-HA [48,52], carbonated HA [32], and Zn, Mg, and Al-doped CaPs [130] |
| Plasma spraying | 50–250 μm | High deposition rates | Non-uniform coating crystalinity; line of sight technique | HA [36,124,125,126,127,128], Si-HA [40,49] and antibacterial Ag-HA composite coatings [66,67,129] |
| Pulsed laser deposition | 0.05–5 μm | Control over coating chemistry and morphology | Line of sight technique | HA resistant to dissolution in SBF [29], Ag-HA [131,132], HA [133,134,135,136,137,138,139,140] and fluorinated HA [60] alendronate-doped HA [57] |
| Ion beam dynamic mixing deposition | 0.05–1 μm | High adhesive strength | Line of sight technique; requires high sintering temperatures | CaP coatings [141,142,143,144,145,146,147] |
| Ion beam assisted deposition | 0.02–10 μm | increased tensile bond strength | Line of sight technique; | CaP [31,148,149,150] |
| Biomimetic deposition | <30 μm | Coating of complex geometries; co-deposition of biomolecules | Time consuming; requires controlled pH | osteocalcin [151], fibronectin [152] and poly(L-lysine) [153]. BMP-2 incorporated into biomimetic CaP coatings [154,155]. |
| Sol-gel deposition | <1 μm | Coating of complex geometries; low processing temperature | Requires controlled atmosphere processing; expensive raw materials | aluminosilicate [156], fluoridated hydroxyapatite, [157] Si-substituted hydroxyapatite [158], and bioglass [159,160,161] |
| Electrophoretic deposition | 0.1–2 mm | Uniform coating; coating of complex geometries; high deposition rates | Difficult to produce crack-free coatings; low adhesive strength | CaP-chitosan composite coatings successfully combined with CaSiO3, heparin, and silica [162,163,164] |
| Electrospray deposition | 0.1–5 μm | Co-deposition of biomolecules; control over coating composition and morphology | Low mechanical strength; Line of sight technique | HA [165,166],Nano HA [167], ALP [168], biomolecules-HA composite [88] collagen [169] |
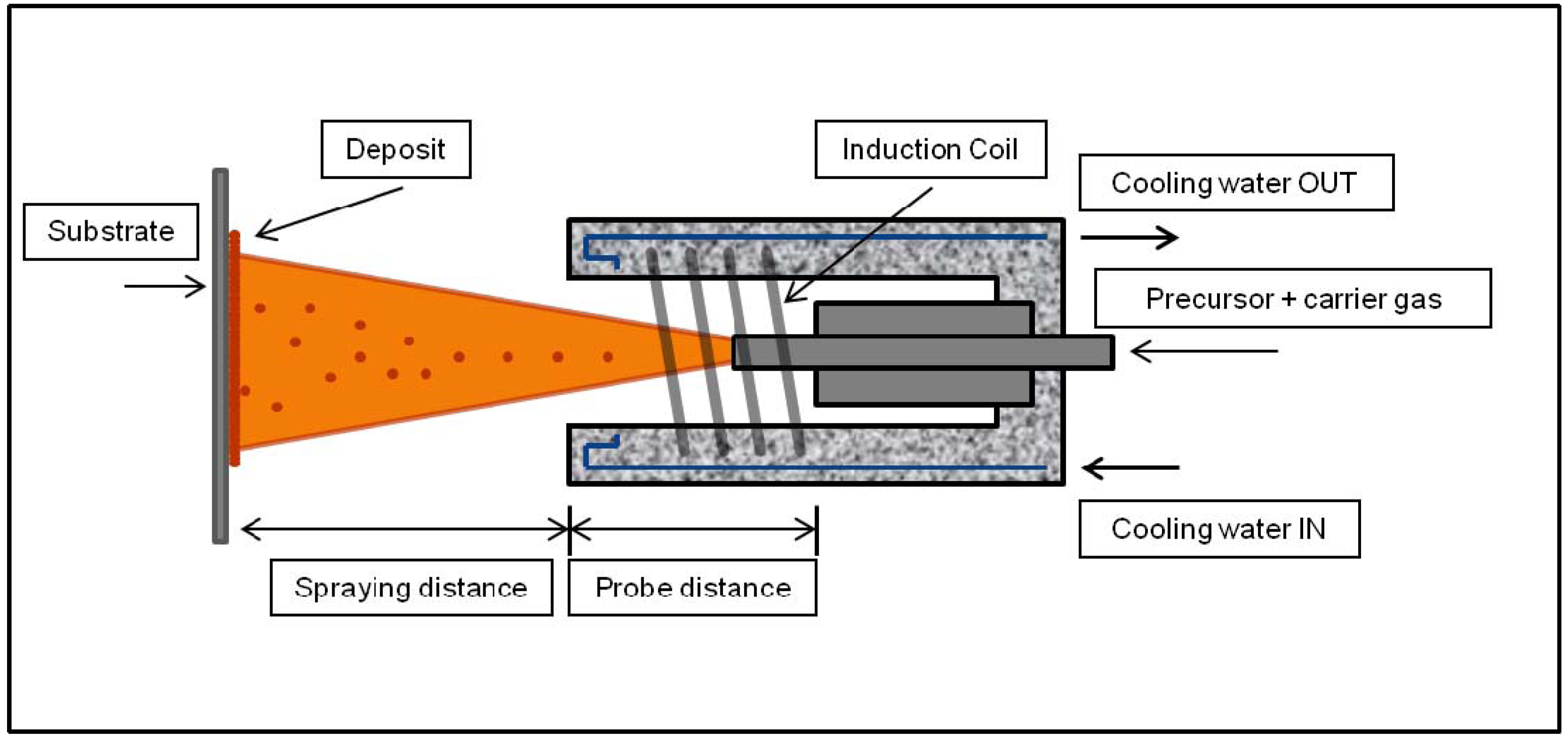

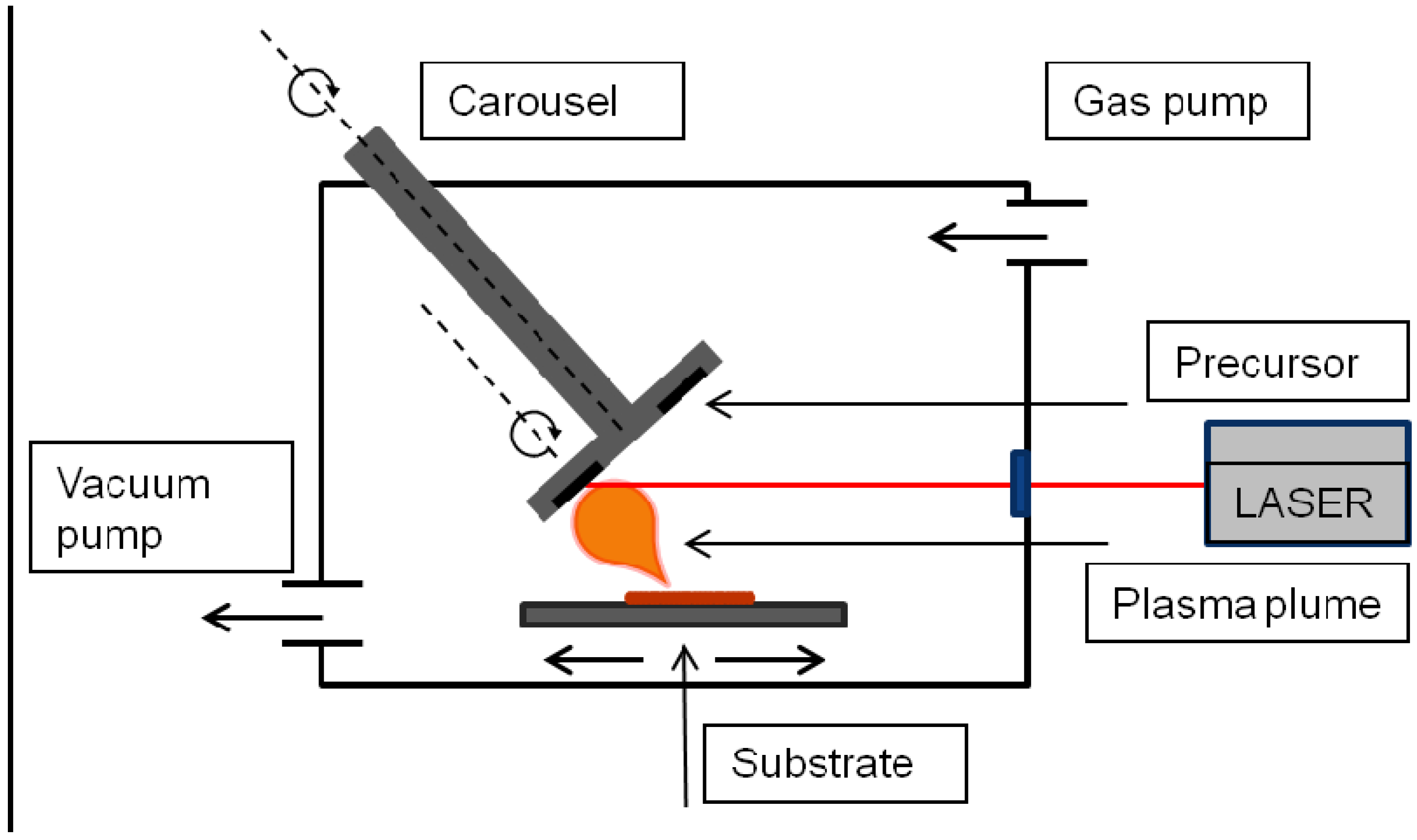
4.2. Wet Deposition Techniques
4.3. Electrochemical Deposition Techniques
4.4. Clinical Performance
5. Summary and Future Perspectives
Acknowledgments
References
- Zethraeus, N.; Borgström, F.; Ström, O.; Kanis, J.; Jönsson, B. Cost-effectiveness of the treatment and prevention of osteoporosis—A review of the literature and a reference model. Osteoporos. Int. 2007, 18, 9–23. [Google Scholar] [CrossRef]
- Barrère, F.; Mahmood, T.A.; de Groot, K.; van Blitterswijk, C.A. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Scholz, M.S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Gilbert, M.E.; Muflahi, S.A.; Pernice, M.F.; Rae, S.I.; et al. The use of composite materials in modern orthopaedic medicine and prosthetic devices: A review. Compos. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Flanagan, D.; Ilies, H.; McCullough, P.; McQuoid, S. Measurement of the fatigue life of mini dental implants: A pilot study. J. Oral Implantol. 2008, 34, 7–11. [Google Scholar] [CrossRef]
- Ahn, A.C.; Grodzinsky, A.J. Relevance of collagen piezoelectricity to “Wolff’s Law”: A critical review. Med. Eng. Phys. 2009, 31, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Turner, C.H.; Rho, J.; Takano, Y.; Tsui, T.Y.; Pharr, G.M. The elastic properties of trabecular and cortical bone tissues are similar: Results from two microscopic measurement techniques. J. Biomech. 1999, 32, 437–441. [Google Scholar] [CrossRef]
- Stephani, G.; Quadbeck, P.; Andersen, O. New multifunctional lightweight materials based on cellular metals—Manufacturing,properties and applications. In Proceedings of International Conference on Advanced Structural and Functional Materials Design, Osaka, Japan, 10-12 November 2008. [CrossRef]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Mitsuo, N. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Frost, H.M. The Utah paradigm of skeletal physiology: An overview of its insights for bone, cartilage and collagenous tissue organs. J. Bone Miner. Metab. 2000, 18, 305–316. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Tengvall, P.; Lunstrom, I. Physico-chemical considerations of titanium as a biomaterial. Clin. Mater. 1992, 9, 115–134. [Google Scholar] [CrossRef]
- John, C.W. Predicting clinical biological responses to dental materials. Dent. Mater. 2012, 28, 23–40. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Moura, C.C.G.; Souza, M.A.; Dechichi, P.; Zanetta-Barbosa, D.; Teixeira, C.C.; Coelho, P.G. The effect of a nanothickness coating on rough titanium substrate in the osteogenic properties of human bone cells. J. Biomed. Mater. Res. 2010, 94, 103–111. [Google Scholar]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.-S.; Sul, Y.-T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Aragço, F.J.L.; Cooper, L.F. Advancing dental implant surface technology—From micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef]
- Lemons, J.E. Biomaterials, biomechanics, tissue healing, and immediate-function dental implants. J. Oral Implantol. 2004, 30, 318–324. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological responses to materials. Ann. Rev. Mater. Sci. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Ong, J.L.; Chan, D.C. Hydroxyapatite and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 2000, 28, 667–707. [Google Scholar]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Wang, C.; Karlis, G.A.; Anderson, G.I.; Dunstan, C.R.; Carbone, A.; Berger, G.; Ploska, U.; Zreiqat, H. Bone growth is enhanced by novel bioceramic coatings on Ti alloy implants. J. Biomed. Mater. Res. Part A 2009, 90, 419–428. [Google Scholar]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interf. 2010, 7, S515–S527. [Google Scholar] [CrossRef]
- Dinda, G.P.; Shin, J.; Mazumder, J. Pulsed laser deposition of hydroxyapatite thin films on Ti-6Al-4V: Effect of heat treatment on structure and properties. Acta Biomater. 2009, 5, 1821–1830. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. 2010, 95, 1203–1214. [Google Scholar]
- Saithna, A. The influence of hydroxyapatite coating of external fixator pins on pin loosening and pin track infection: A systematic review. Injury 2010, 41, 128–132. [Google Scholar] [CrossRef]
- Barrere, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.; de Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. Appl. Biomater. 2003, 67, 655–665. [Google Scholar]
- Morris, H.F.; Ochi, S.; Spray, J.R.; Olson, J.W. Periodontal-type measurements associated with hydroxyapatite-coated and non-HA-coated implants: Uncovering to 36 months. Ann. Periodontol. 2000, 5, 56–67. [Google Scholar] [CrossRef]
- Dudek, A. Investigations of microstructure and properties in bioceramic coatings used in medicine. Arch. Metall. Mater. 2011, 56, 135–140. [Google Scholar] [CrossRef]
- Pichugin, V.F.; Surmenev, R.A.; Shesterikov, E.V.; Ryabtseva, M.A.; Eshenko, E.V.; Tverdokhlebov, S.I.; Prymak, O.; Epple, M. The preparation of calcium phosphate coatings on titanium and nickel-titanium by rf-magnetron-sputtered deposition: Composition, structure and micromechanical properties. Surf. Coatings Technol. 2008, 202, 3913–3920. [Google Scholar]
- Surmenev, R.A.; Ryabtseva, M.A.; Shesterikov, E.V.; Pichugin, V.F.; Peitsch, T.; Epple, M. The release of nickel from nickel-titanium (NiTi) is strongly reduced by a sub-micrometer thin layer of calcium phosphate deposited by rf-magnetron sputtering. J. Mater. Sci. Mater. Med. 2010, 21, 1233–1239. [Google Scholar] [CrossRef]
- Cao, N.; Dong, J.; Wang, Q.; Ma, Q.; Wang, F.; Chen, H.; Xue, C.; Li, M. Plasma-sprayed hydroxyapatite coating on carbon/carbon composite scaffolds for bone tissue engineering and related tests in vivo. J. Biomed. Mater. Res. 2010, 92, 1019–1027. [Google Scholar]
- Tang, Q.; Brooks, R.; Rushton, N.; Best, S. Production and characterization of HA and SiHA coatings. J. Mater. Sci. Mater. Med. 2010, 21, 173–181. [Google Scholar] [CrossRef]
- Cheng, G.J.; Ye, C. Experiment, thermal simulation, and characterizations on transmission laser coating of hydroxyapatite on metal implant. J. Biomed. Mater. Res. 2010, 92, 70–79. [Google Scholar] [CrossRef]
- Carrado, A. Structural, microstructural, and residual stress investigations of plasma-sprayed hydroxyapatite on Ti-6Al-4 V. ACS Appl. Mater. Interf. 2010, 2, 561–565. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Evdokimov, K.E.; Pichugin, V.F.; Peitsch, T.; Epple, M. The influence of the deposition parameters on the properties of an rf-magnetron-deposited nanostructured calcium phosphate coating and a possible growth mechanism. Surf. Coatings Technol. 2011, 205, 3600–3606. [Google Scholar]
- Wolke, J.G.C.; de Blieck-Hogervorst, J.M.A.; Dhert, W.J.A.; Klein, C.P.A.T.; de Groot, K. Studies on the thermal spraying of apatite bioceramics. J. Therm. Spray Technol. 1992, 1, 75–82. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Silicon-substituted hydroxyapatite thin films: Effect of annealing temperature on coating stability and bioactivity. J. Biomed. Mater. Res. 2006, 78, 121–128. [Google Scholar]
- Porter, A.E.; Rea, S.M.; Galtrey, M.; Best, S.M.; Barber, Z.H. Production of thin film silicon-doped hydroxyapatite via sputter deposition. J. Mater. Sci. 2004, 39, 1895–1898. [Google Scholar]
- Huang, T.; Xiao, Y.; Wang, S.; Huang, Y.; Liu, X.; Wu, F.; Gu, Z. Nanostructured Si, Mg, CO32− substituted hydroxyapatite coatings deposited by liquid precursor plasma spraying: Synthesis and characterization. J. Therm. Spray Technol. 2011, 20, 829–836. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Silicon-substituted hydroxyapatite: The next generation of bioactive coatings. Mater. Sci. Eng. 2007, 27, 251–256. [Google Scholar] [CrossRef]
- Xiao, F.J.; Peng, L.; Zhang, Y.; Yun, L.J. Silicon-substituted hydroxyapatite composite coating by using vacuum-plasma spraying and its interaction with human serum albumin. J. Mater. Sci. Mater. Med. 2009, 20, 1653–1658. [Google Scholar] [CrossRef]
- Gomes, P.S.; Botelho, C.; Lopes, M.A.; Santos, J.D.; Fernandes, M.H. Evaluation of human osteoblastic cell response to plasma-sprayed silicon-substituted hydroxyapatite coatings over titanium substrates. J. Biomed. Mater. Res. Appl. Biomater. 2010, 94, 337–346. [Google Scholar]
- Gomes, P.; Botelho, C.; Lopes, M.; Santos, J.; Fernandes, M. Effect of silicon-containing hydroxyapatite coating on human in vitro osteoblastic response. Bone 2009, 44, s267. [Google Scholar]
- Thian, E.S.; Best, S.M. Thin Calcium Phosphate Coatings for Medical Implants; Springer-Verlag: New York, NY, USA, 2009; p. 199. [Google Scholar]
- Vestermark, M.T. Strontium in the bone-implant interface. Dan Med. Bull. 2011, 58, B4286. [Google Scholar]
- Xue, W.; Hosick, H.L.; Bandyopadhyay, A.; Bose, S.; Ding, C.; Luk, K.D.K.; Cheung, K.M.C.; Lu, W.W. Preparation and cell-materials interactions of plasma sprayed strontium-containing hydroxyapatite coating. Surf. Coatings Technol. 2007, 201, 4685–4693. [Google Scholar]
- Mihailescu, I.N.; Ristoscu, C.; Bigi, A.; Mayer, I. Laser-Surface Interactions for New Materials Production. In Springer Series in Material Science; Springer: Berlin, Germany, 2010; Volume 130, pp. 235–260. [Google Scholar]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Capuccini, C.; Fini, M.; Mihailescu, I.N.; Ristoscu, C.; Sima, F.; Torricelli, P. Biofunctional alendronate-Hydroxyapatite thin films deposited by Matrix Assisted Pulsed Laser Evaporation. Biomaterials 2009, 30, 6168–6177. [Google Scholar]
- Rau, J.V.; Generosi, A.; Laureti, S.; Komlev, V.S.; Ferro, D.; Cesaro, S.N.; Paci, B.; Albertini, V.R.; Agostinelli, E.; Barinov, S.M. Physicochemical investigation of pulsed laser deposited carbonated hydroxyapatite films on titanium. ACS Appl. Mater. Interf. 2009, 1, 1813–1820. [Google Scholar] [CrossRef]
- Ding, L.; Zheng, Y.; Wan, Q.B.; Pei, X.B.; Chen, S.Y. Fluoridated hydroxyapatite/carbon nanotubes composite coating fabricated by radio frequency magnetron sputtering. Mater. Sci. Forum 2011, 675-677, 869–871. [Google Scholar] [CrossRef]
- Rau, J.V.; Smirnov, V.V.; Laureti, S.; Generosi, A.; Varvaro, G.; Fosca, M.; Ferro, D.; Cesaro, S.N.; Albertini, V.R.; Barinov, S.M. Properties of pulsed laser deposited fluorinated hydroxyapatite films on titanium. Mater. Res. Bull. 2012 45, 1304–1310.
- Yang, C.; Liu, F.; Ren, S.; Yang, G. Microstructure and magnetic properties of a two-phase alloy of α-Fe and metastable Fe3B. J. Magn. Magn. Mater. 2009, 321, 91–94. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional coatings or films for hard-tissue applications. Materials 2010, 3, 3994–4050. [Google Scholar] [CrossRef]
- Bai, X.; More, K.; Rouleau, C.M.; Rabiei, A. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 2010, 6, 2264–2273. [Google Scholar] [CrossRef]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 22–39. [Google Scholar] [CrossRef]
- Feng, Q.L.; Kim, T.N.; Wu, J.; Park, E.S.; Kim, J.O.; Lim, D.Y.; Cui, F.Z. Antibacterial effects of Ag-HAp thin films on alumina substrates. Thin Solid Films 1998, 335, 214–219. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Xie, Y.; Ji, H.; Ding, C.; Li, H.; Dai, K. Silver release from silver-containing hydroxyapatite coatings. Surf. Coatings Technol. 2010, 205, 1892–1896. [Google Scholar] [CrossRef]
- Shimazaki, T.; Miyamoto, H.; Ando, Y.; Noda, I.; Yonekura, Y.; Kawano, S.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. In vivo antibacterial and silver-releasing properties of novel thermal sprayed silver-containing hydroxyapatite coating. J. Biomed. Mater. Res. Appl. Biomater. 2010, 92, 386–389. [Google Scholar]
- De Jonge, L.T.; Leeuwenburgh, S.C.; Wolke, J.G.; Jansen, J.A. Organic-inorganic surface modifications for titanium implant surfaces. Pharm. Res. 2008, 25, 2357–2369. [Google Scholar] [CrossRef]
- Lynch, S.E.; Buser, D.; Hernandez, R.A.; Weber, H.P.; Stich, H.; Fox, C.H.; Williams, R.C. Effects of the platelet-derived growth factor/insulin-like growth factor-I combination on bone regeneration around titanium dental implants. Results of a pilot study in beagle dogs. J. Periodontol. 1991, 62, 710–716. [Google Scholar] [CrossRef]
- Sumner, D.R.; Turner, T.M.; Purchio, A.F.; Gombotz, W.R.; Urban, R.M.; Galante, J.O. Enhancement of bone ingrowth by transforming growth factor-β. J. Bone Jt. Surg. Ser. 1995, 77, 1135–1147. [Google Scholar]
- Puleo, D.A. Biochemical surface modification of Co-Cr-Mo. Biomaterials 1996, 17, 217–222. [Google Scholar] [CrossRef]
- Endo, K. Chemical modification of metallic implant surfaces with biofunctional proteins (Part 1). Molecular structure and biological activity of a modified NiTi alloy surface. Dent. Mater. J. 1995, 14, 185–198. [Google Scholar] [CrossRef]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. Can.Assoc. Radiol. J. 1998, 49, 324–335. [Google Scholar]
- Schliephake, H.; Scharnweber, D. Chemical and biological functionalization of titanium for dental implants. J. Mater. Chem. 2008, 18, 2404–2414. [Google Scholar] [CrossRef]
- Nijhuis, A.W.G.; Leeuwenburgh, S.C.G.; Jansen, J.A. Wet-Chemical deposition of functional coatings for bone implantology. Macromol. Biosci. 2010, 10, 1316–1329. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cell Mater. 2006, 12, 1–15. [Google Scholar]
- Elmengaard, B.; Bechtold, J.E.; Soballe, K. In vivo effects of RGD-coated titanium implants inserted in two bone-gap models. J. Biomed. Mater. Res. 2005, 75, 249–255. [Google Scholar]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Sewing, A.; Aref, A.; Roessler, S. Functionalization of dental implant surfaces using adhesion molecules. J. Biomed. Mater. Res. Appl. Biomater. 2005, 73, 88–96. [Google Scholar]
- Roessler, S.; Born, R.; Scharnweber, D.; Worch, H.; Sewing, A.; Dard, M. Biomimetic coatings functionalized with adhesion peptides for dental implants. J. Mater. Sci. Mater. Med. 2001, 12, 871–877. [Google Scholar] [CrossRef]
- Massia, S.P.; Hubbell, J.A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 1991, 114, 1089–1100. [Google Scholar] [CrossRef]
- LeBaron, R.G.; Athanasiou, K.A. Extracellular matrix cell adhesion peptides: Functional applications in orthopedic materials. Tissue Eng. 2000, 6, 85–103. [Google Scholar] [CrossRef]
- Sreejalekshmi, K.G.; Nair, P.D. Biomimeticity in tissue engineering scaffolds through synthetic peptide modifications Altering chemistry for enhanced biological response. J. Biomed. Mater. Res. Part A 2010, 96, 477–491. [Google Scholar]
- Solheim, E. Growth factors in bone. Int. Orthop. 1998, 22, 410–416. [Google Scholar] [CrossRef]
- Hall, J.; Sorensen, R.G.; Wozney, J.M.; Wikesjo, U.M. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J. Clin. Periodontol. 2007, 34, 444–451. [Google Scholar] [CrossRef]
- Siebers, M.C.; Walboomers, X.F.; Leewenburgh, S.C.; Wolke, J.C.; Boerman, O.C.; Jansen, J.A. Transforming growth factor-beta1 release from a porous electrostatic spray deposition-derived calcium phosphate coating. Tissue Eng. 2006, 12, 2449–2456. [Google Scholar] [CrossRef]
- De Jonge, L.T.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Hamers, A.A.J.; Wolke, J.G.C.; Jansen, J.A. In vitro responses to electrosprayed alkaline phosphatase/calcium phosphate composite coatings. Acta Biomater. 2009, 5, 2773–2782. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Cahalan, P.; Cahalan, L.; Fini, M.; Giardino, R. Surface engineering of titanium by collagen immobilization. Surface characterization and in vitro and in vivo studies. Biomaterials 2003, 24, 4639–4654. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Robetaler, S.; Sewing, A.; Huttmann, C. Biological performance of biomimetic calcium phosphate coating of titanium implants in the dog mandible. J. Biomed. Mater. Res. 2003, 64, 225–234. [Google Scholar]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell Mater. 2006, 11, 43–56. [Google Scholar]
- De Jonge, L.T.; Leeuwenburgh, S.C.; van den Beucken, J.J.; te Riet, J.; Daamen, W.F.; Wolke, J.G.; Scharnweber, D.; Jansen, J.A. The osteogenic effect of electrosprayed nanoscale collagen/calcium phosphate coatings on titanium. Biomaterials 2010, 31, 2461–2469. [Google Scholar]
- Fischer, U.; Hempel, U.; Becker, D.; Bierbaum, S.; Scharnweber, D.; Worch, H.; Wenzel, K.W. Transforming growth factor beta1 immobilized adsorptively on Ti6Al4V and collagen type I coated Ti6Al4V maintains its biological activity. Biomaterials 2003, 24, 2631–2641. [Google Scholar] [CrossRef]
- Cole, B.J.; Bostrom, M.P.; Pritchard, T.L.; Sumner, D.R.; Tomin, E.; Lane, J.M.; Weiland, A.J. Use of bone morphogenetic protein 2 on ectopic porous coated implants in the rat. Clin. Orthop. Relat. Res. 1997, 15, 219–228. [Google Scholar]
- Herr, G.; Hartwig, C.H.; Boll, C.; Kusswetter, W. Ectopic bone formation by composites of BMP and metal implants in rats. Acta Orthop. Scand. 1996, 67, 606–610. [Google Scholar] [CrossRef]
- Liu, Y.; Hunziker, E.B.; Layrolle, P.; de Bruijn, J.D.; de Groot, K. Bone morphogenetic protein 2 incorporated into biomimetic coatings retains its biological activity. Tissue Eng. 2004, 10, 101–108. [Google Scholar] [CrossRef]
- Liu, Y.; Huse, R.O.; de Groot, K.; Buser, D.; Hunziker, E.B. Delivery mode and efficacy of BMP-2 in association with implants. J. Dent. Search 2007, 86, 84–89. [Google Scholar]
- Liu, Y.; Hunziker, E.B.; Layrolle, P.; de Bruijn, J.D.; de Groot, K. Bone morphogenetic protein 2 incorporated into biomimetic coatings retains its biological activity. Tissue Eng. 2004, 10, 101–108. [Google Scholar] [CrossRef]
- Uludag, H.; Gao, T.; Porter, T.J.; Friess, W.; Wozney, J.M. Delivery systems for BMPs: Factors contributing to protein retention at an application site. J. Bone Jt. Surg. Am. 2001, 83, S128–S135. [Google Scholar]
- Uludag, H.; D’Augusta, D.; Palmer, R.; Timony, G.; Wozney, J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J. Biomed. Mater. Res. 1999, 46, 193–202. [Google Scholar] [CrossRef]
- Ewald, A.; Hösel, D.; Patel, S.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 2011, 7, 4064–4070. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Appl. Biomater. 2009, 91, 470–480. [Google Scholar]
- Jahoda, D.; Nyc, O.; Pokorny, D.; Landor, I.; Sosna, A. Antibiotic treatment for prevention of infectious complications in joint replacement. Acta Chir. Orthop. Traumatol. Cech. 2006, 73, 108–114. [Google Scholar]
- Alt, V.; Bitschnau, A.; Osterling, J.; Sewing, A.; Meyer, C.; Kraus, R.; Meissner, S.A.; Wenisch, S.; Domann, E.; Schnettler, R. The effects of combined gentamicin-hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 2006, 27, 4627–4634. [Google Scholar]
- Campbell, A.A.; Song, L.; Li, X.S.; Nelson, B.J.; Bottoni, C.; Brooks, D.E.; DeJong, E.S. Development, characterization, and anti-microbial efficacy of hydroxyapatite-chlorhexidine coatings produced by surface-induced mineralization. J. Biomed. Mater. Res. 2000, 53, 400–407. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Carpi, A.; Fini, M.; Giavaresi, G.; Giardino, R. Adsorption of cationic antibacterial on collagen-coated titanium implant devices. Biomed. Pharmacother. 2004, 58, 418–422. [Google Scholar]
- Kim, W.-H.; Lee, S.-B.; Oh, K.-T.; Moon, S.-K.; Kim, K.-M.; Kim, K.-N. The release behavior of CHX from polymer-coated titanium surfaces. Surf. Interf. Anal. 2008, 40, 202–204. [Google Scholar] [CrossRef]
- Harris, L.G.; Mead, L.; Muller-Oberlander, E.; Richards, R.G. Bacteria and cell cytocompatibility studies on coated medical grade titanium surfaces. J. Biomed. Mater. Res. 2006, 78, 50–58. [Google Scholar]
- Kozlovsky, A.; Artzi, Z.; Moses, O.; Kamin-Belsky, N.; Greenstein, R.B. Interaction of chlorhexidine with smooth and rough types of titanium surfaces. J. Periodontol. 2006, 77, 1194–1200. [Google Scholar] [CrossRef]
- Barbour, M.E.; O’Sullivan, D.J.; Jagger, D.C. Chlorhexidine adsorption to anatase and rutile titanium dioxide. Coll. Surf. Physicochem. Eng. Asp. 2007, 307, 116–120. [Google Scholar] [CrossRef]
- Darouiche, R.O.; Green, G.; Mansouri, M.D. Antimicrobial activity of antiseptic-coated orthopaedic devices. Int. J. Antimicrob. Agents 1998, 10, 83–86. [Google Scholar] [CrossRef]
- Jonathan, P.; Nazhat, S.N.; Blaker, J.J.; Boccaccini, A.R. In Vitro Attachment of Staphylococcus Epidermidis to Surgical Sutures with and without Ag-Containing Bioactive Glass Coating; Sage: London,UK, 2004; Volume 19, p. 11. [Google Scholar]
- Roy, M.; Bandyopadhyay, A.; Bose, S. In vitro antimicrobial and biological properties of laser assisted tricalcium phosphate coating on titanium for load bearing implant. Mater. Sci. Eng. 2009, 29, 1965–1968. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Gordon, S.; Rogers, M.J. Molecular mechanisms of action of bisphosphonates: Current status. Clin. Cancer Res. 2006, 12, 6222–6230. [Google Scholar] [CrossRef]
- Van beek, E.; Lowik, C.; van der Pluijm, G.; Papapoulos, S. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: A clue to the mechanism of action of nitrogen-containing bisphosphonates. J. Bone Miner. Res. 1999, 14, 722–729. [Google Scholar] [CrossRef]
- Wysowski, D.K. Reports of esophageal cancer with oral bisphosphonate use. Mass. Med. Soc. 2009, 360, 89–90. [Google Scholar]
- Marx, R.E. Oral and intravenous bisphosphonate-induced osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 2397–2410. [Google Scholar] [CrossRef]
- Denissen, H.; van Beek, E.; Lowik, C.; Papapoulos, S.; van Den Hooff, A. Ceramic hydroxyapatite implants for the release of bisphosphonate. Bone Miner. 1994, 25, 123–134. [Google Scholar] [CrossRef]
- Seshima, H.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Inoue, T.; Oda, Y. Control of bisphosphonate release using hydroxyapatite granules. J. Biomed. Mater. Res. Appl. Biomater. 2006, 78, 215–221. [Google Scholar]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Giardino, R.; Bigi, A. Alendronate-hydroxyapatite nanocomposites and their interaction with osteoclasts and osteoblast-like cells. Biomaterials 2008, 29, 790–796. [Google Scholar] [CrossRef]
- Peter, B.; Pioletti, D.P.; Laib, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.Y.; Bouler, J.M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Huang, Y.; Qu, Y.; Yang, B.; Li, W.; Zhang, B.; Zhang, X. In vivo biological responses of plasma sprayed hydroxyapatite coatings with an electric polarized treatment in alkaline solution. Mater. Sci. Eng. 2009, 29, 2411–2416. [Google Scholar] [CrossRef]
- Wu, G.M.; Hsiao, W.D.; Kung, S.F. Investigation of hydroxyapatite coated polyether ether ketone composites by gas plasma sprays. Surf. Coatings Technol. 2009, 203, 2755–2758. [Google Scholar] [CrossRef]
- Kozerski, S.; Pawlowski, L.; Jaworski, R.; Roudet, F.; Petit, F. Two zones microstructure of suspension plasma sprayed hydroxyapatite coatings. Surf. Coatings Technol. 2010, 204, 1380–1387. [Google Scholar] [CrossRef]
- D’Haese, R.; Pawlowski, L.; Bigan, M.; Jaworski, R.; Martel, M. Phase evolution of hydroxapatite coatings suspension plasma sprayed using variable parameters in simulated body fluid. Surf. Coatings Technol. 2010, 204, 1236–1246. [Google Scholar] [CrossRef]
- Cao, N.; Dong, J.; Wang, Q.; Ma, Q.; Xue, C.; Li, M. An experimental bone defect healing with hydroxyapatite coating plasma sprayed on carbon/carbon composite implants. Surf. Coatings Technol. 2010, 205, 1150–1156. [Google Scholar] [CrossRef]
- Noda, I.; Miyaji, F.; Ando, Y.; Miyamoto, H.; Shimazaki, T.; Yonekura, Y.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. Development of novel thermal sprayed antibacterial coating and evaluation of release properties of silver ions. J. Biomed. Mater. Res. Appl. Biomater. 2009, 89, 456–465. [Google Scholar]
- Hong, Z.; Mello, A.; Yoshida, T.; Luan, L.; Stern, P.H.; Rossi, A.; Ellis, D.E.; Ketterson, J.B. Osteoblast proliferation on hydroxyapatite coated substrates prepared by right angle magnetron sputtering. J. Biomed. Mater. Res. 2010, 93, 878–885. [Google Scholar]
- Jelinek, M.; Weiserova, M.; Kocourek, T.; Zezulova, M.; Strnad, J. Biomedical properties of laser prepared silver-doped hydroxyapatite. Laser Phys. 2011, 21, 1265–1269. [Google Scholar]
- Jelinek, M.; Kocourek, T.; Jurek, K.; Remsa, J.; Mikšovský, J.; Weiserova, M.; Strnad, J.; Luxbacher, T. Antibacterial properties of Ag-doped hydroxyapatite layers prepared by PLD method. Appl. Phys. Mater. Sci. Proc. 2010, 101, 615–620. [Google Scholar] [CrossRef]
- Koch, C.F.; Johnson, S.; Kumar, D.; Jelinek, M.; Chrisey, D.B.; Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mihailescu, I.N. Pulsed laser deposition of hydroxyapatite thin films. Mater. Sci. Eng. 2007, 27, 484–494. [Google Scholar] [CrossRef]
- Vasanthan, A.; Kim, H.; Drukteinis, S.; Lacefield, W. Implant surface modification using laser guided coatings: In vitro comparison of mechanical properties. J. Prosthodont. 2008, 17, 357–364. [Google Scholar] [CrossRef]
- Man, H.C.; Chiu, K.Y.; Cheng, F.T.; Wong, K.H. Adhesion study of pulsed laser deposited hydroxyapatite coating on laser surface nitrided titanium. Thin Solid Films 2009, 517, 5496–5501. [Google Scholar]
- Yang, S.; Xing, W.; Man, H.C. Pulsed laser deposition of hydroxyapatite film on laser gas nitriding NiTi substrate. Appl. Surf. Sci. 2009, 255, 9889–9892. [Google Scholar] [CrossRef]
- Socol, G.; Macovei, A.M.; Miroiu, F.; Stefan, N.; Duta, L.; Dorcioman, G.; Mihailescu, I.N.; Petrescu, S.M.; Stan, G.E.; Marcov, D.A.; et al. Hydroxyapatite thin films synthesized by pulsed laser deposition and magnetron sputtering on PMMA substrates for medical applications. Mater. Sci. Eng. Solid-State Mater. Adv. Technol. 2010, 169, 159–168. [Google Scholar]
- Rajesh, P.; Muraleedharan, C.V.; Komath, M.; Varma, H. Pulsed laser deposition of hydroxyapatite on titanium substrate with titania interlayer. J. Mater. Sci. Mater. Med. 2011, 22, 497–505. [Google Scholar] [CrossRef]
- Zeng, H.; Lacefield, W.R.; Mirov, S. Structural and morphological study of pulsed laser deposited calcium phosphate bioceramic coatings: Influence of deposition conditions, laser parameters, and target properties. J. Biomed. Mater. Res. 2000, 50, 248–258. [Google Scholar] [CrossRef]
- Garcia-Sanz, F.J.; Mayor, M.B.; Arias, J.L.; Pou, J.; Lean, B.; Perez-Amor, M. Hydroxyapatite coatings: A comparative study between plasma-spray and pulsed laser deposition techniques. J. Mater. Sci. Mater. Med. 1997, 8, 861–865. [Google Scholar] [CrossRef]
- Choi, J.M.; Kim, H.E.; Lee, I.S. Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials 2000, 21, 469–473. [Google Scholar] [CrossRef]
- Luo, Z.S.; Cui, F.Z.; Feng, Q.L.; Li, H.D.; Zhu, X.D.; Spector, M. In vitro and in vivo evaluation of degradability of hydroxyapatite coatings synthesized by ion beam-assisted deposition. Surf. Coatings Technol. 2000, 131, 192–195. [Google Scholar] [CrossRef]
- Kim, T.N.; Feng, Q.L.; Luo, Z.S.; Cui, F.Z.; Kim, J.O. Highly adhesive hydroxyapatite coatings on alumina substrates prepared by ion-beam assisted deposition. Surf. Coatings Technol. 1998, 99, 20–23. [Google Scholar] [CrossRef]
- Coelho, P.G.; Lemons, J.E. Physico/chemical characterization and in vivo evaluation of nanothickness bioceramic depositions on alumina-blasted/acid-etched Ti-6Al-4V implant surfaces. J. Biomed. Mater. Res. 2009, 90, 351–361. [Google Scholar]
- Rabiei, A.; Thomas, B.; Jin, C.; Narayan, R.; Cuomo, J.; Yang, Y.; Ong, J.L. A study on functionally graded HA coatings processed using ion beam assisted deposition with in situ heat treatment. Surf. Coatings Technol. 2006, 200, 6111–6116. [Google Scholar] [CrossRef]
- Coelho, P.G.; Cardaropoli, G.; Suzuki, M.; Lemons, J.E. Histomorphometric evaluation of a nanothickness bioceramic deposition on endosseous implants: A study in dogs. Clin. Implant Dent. Relat. Res. 2009, 11, 292–302. [Google Scholar] [CrossRef]
- Kim, H.; Choi, S.H.; Chung, S.M.; Li, L.H.; Lee, I.S. Enhanced bone forming ability of SLA-treated Ti coated with a calcium phosphate thin film formed by e-beam evaporation. Biomed. Mater. 2009, 5, 044106. [Google Scholar]
- Lee, I.S.; Whang, C.N.; Kim, H.E.; Park, J.C.; Song, J.H.; Kim, S.R. Various Ca/P ratios of thin calcium phosphate films. Mater. Sci. Eng. 2002, 22, 15–20. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kwon, T.Y.; Kim, K.H. Surface modification of titanium and titanium alloys by ion implantation. J. Biomed. Mater. Res. Appl. Biomater. 2011, 93, 581–591. [Google Scholar]
- Yang, J.X.; Jiao, Y.P.; Cui, F.Z.; Lee, I.S.; Yin, Q.S.; Zhang, Y. Modification of degradation behavior of magnesium alloy by IBAD coating of calcium phosphate. Surf. Coatings Technol. 2008, 202, 5733–5736. [Google Scholar] [CrossRef]
- Krout, A.; Wen, H.B.; Hippensteel, E.; Li, P. A hybrid coating of biomimetic apatite and osteocalcin. J. Biomed. Mater. Res. 2005, 73, 377–387. [Google Scholar]
- Chen, C.; Lee, I.-S.; Zhang, S.-M.; Yang, H.C. Biomimetic apatite formation on calcium phosphate-coated titanium in Dulbecco’s phosphate-buffered saline solution containing CaCl2 with and without fibronectin. Acta Biomater. 2010, 6, 2274–2281. [Google Scholar] [CrossRef]
- Ryu, H.S.; Hong, S.-H. Hybrid coatings of poly(L-lysine) and apatite on micro-arc oxidized titania. Mater. Lett. 2009, 63, 2107–2110. [Google Scholar] [CrossRef]
- Liu, Y.; de Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [CrossRef]
- Ishibe, T.; Goto, T.; Kodama, T.; Miyazaki, T.; Kobayashi, S.; Takahashi, T. Bone formation on apatite-coated titanium with incorporated BMP-2/heparin in vivo. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 867–875. [Google Scholar] [CrossRef]
- Leivo, J.; Meretoja, V.; Vippola, M.; Levanen, E.; Vallittu, P.; Mantyla, T.A. Sol-gel derived aluminosilicate coatings on alumina as substrate for osteoblasts. Acta Biomater. 2006, 2, 659–668. [Google Scholar] [CrossRef]
- Cheng, K.; Weng, W.; Wang, H.; Zhang, S. In vitro behavior of osteoblast-like cells on fluoridated hydroxyapatite coatings. Biomaterials 2005, 26, 6288–6295. [Google Scholar] [CrossRef]
- Balamurugan, A.; Rebelo, A.H.S.; Lemos, A.F.; Rocha, J.H.G.; Ventura, J.M.G.; Ferreira, J.M.F. Suitability evaluation of sol-gel derived Si-substituted hydroxyapatite for dental and maxillofacial applications through in vitro osteoblasts response. Dent. Mater. 2008, 24, 1374–1380. [Google Scholar] [CrossRef]
- Liu, J.; Miao, X. Sol-gel derived bioglass as a coating material for porous alumina scaffolds. Ceram. Int. 2004, 30, 1781–1785. [Google Scholar] [CrossRef]
- Fathi, M.H.; Doost Mohammadi, A. Preparation and characterization of sol-gel bioactive glass coating for improvement of biocompatibility of human body implant. Mater. Sci. Eng. 2008, 474, 128–133. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Crouse, P.L.; Li, L.; Garrod, D. Combined laser/sol-gel synthesis of calcium silicate coating on Ti6Al4V substrates for improved cell integration. Appl. Surf. Sci. 2007, 253, 7998–8002. [Google Scholar] [CrossRef]
- Pang, X.; Casagrande, T.; Zhitomirsky, I. Electrophoretic deposition of hydroxyapatite-CaSiO3-chitosan composite coatings. J. Coll. Interf. Sci. 2009, 330, 323–329. [Google Scholar] [CrossRef]
- Sun, F.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-chitosan-heparin coatings. J. Mater. Proc. Technol. 2009, 209, 1597–1606. [Google Scholar] [CrossRef]
- Grandfield, K.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-silica-chitosan coatings. Mater. Charact. 2008, 59, 61–67. [Google Scholar] [CrossRef]
- Schouten, C.; Meijer, G.J.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; de Jonge, L.T.; Wolke, J.G.C.; Spauwen, P.H.M.; Jansen, J.A. In vivo bone response and mechanical evaluation of electrosprayed CaP nanoparticle coatings using the iliac crest of goats as an implantation model. Acta Biomater. 2010, 6, 2227–2236. [Google Scholar]
- Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Siebers, M.C.; Schoonman, J.; Jansen, J.A. In vitro and in vivo reactivity of porous, electrosprayed calcium phosphate coatings. Biomaterials 2006, 27, 3368–3378. [Google Scholar] [CrossRef]
- Iafisco, M.; Bosco, R.; Leeuwenburgh, S.C.G.; van den Beucken, J.J.J.P.; Jansen, J.A.; Prat, M.; Roveri, N. Electrostatic spray deposition of biomimetic nanocrystalline apatite coatings onto titanium. Adv. Eng. Mater. 2012, 14, B13–B20. [Google Scholar]
- De Jonge, L.T.; Leeuwenburgh, S.C.G.; van den Beucken, J.J.J.P.; Wolke, J.G.C.; Jansen, J.A. Electrosprayed enzyme coatings as bioinspired alternatives to bioceramic coatings for orthopedic and oral implants. Adv. Funct. Mater. 2009, 19, 755–762. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; van Oirschot, B.; Bosco, R.; den Beucken, J.J.J.P.; Aldosari, A.A.F.; Anil, S.; Jansen, J.A. Biological response to titanium implants coated with nanocrystals calcium phosphate or type 1 collagen in a dog model. Clin. Oral Implant. Res. 2012. [Google Scholar] [CrossRef]
- Gross, K.A.; Saber-Samandari, S. Revealing mechanical properties of a suspension plasma sprayed coating with nanoindentation. Surf. Coatings Technol. 2009, 203, 2995–2999. [Google Scholar] [CrossRef]
- Huang, Y.; Song, L.; Liu, X.; Xiao, Y.; Wu, Y.; Chen, J.; Wu, F.; Gu, Z. Hydroxyapatite coatings deposited by liquid precursor plasma spraying: Controlled dense and porous microstructures and osteoblastic cell responses. Biofabrication 2010, 2, 045003. [Google Scholar] [CrossRef]
- Sobieszczyk, S.; Zielinski, A. Coatings in arthroplasty. Adv. Mater. Sci. 2008, 8, 35–54. [Google Scholar]
- Khor, K.A.; Li, H.; Cheang, P. Significance of melt-fraction in HVOF sprayed hydroxyapatite particles, splats and coatings. Biomaterials 2004, 25, 1177–1186. [Google Scholar] [CrossRef]
- Morks, M.F.; Fahim, N.F.; Kobayashi, A. Structure, mechanical performance and electrochemical characterization of plasma sprayed SiO2/Ti-reinforced hydroxyapatite biomedical coatings. Appl. Surf. Sci. 2008, 255, 3426–3433. [Google Scholar]
- Hasan, S.; Stokes, J. Design of experiment analysis of the Sulzer Metco DJ high velocity oxy-fuel coating of hydroxyapatite for orthopedic applications. J. Therm. Spray Technol. 2010, 20, 186–194. [Google Scholar] [CrossRef]
- Morks, M.F. Fabrication and characterization of plasma-sprayed HA/SiO2 coatings for biomedical application. J. Mech. Behav. Biomed. Mater. 2008, 1, 105–111. [Google Scholar] [CrossRef]
- Morks, M.F.; Kobayashi, A.; Fahim, N.F. Abrasive wear behavior of sprayed hydroxyapitite coatings by gas tunnel type plasma spraying. Wear 2007, 262, 204–209. [Google Scholar] [CrossRef]
- Heimann, R.B. Thermal spraying of biomaterials. Surf. Coatings Technol. 2006, 201, 2012–2019. [Google Scholar] [CrossRef]
- Lima, R.S.; Dimitrievska, S.; Bureau, M.N.; Marple, B.R.; Petit, A.; Mwale, F.; Antoniou, J. HVOF-sprayed Nano TiO2-HA coatings exhibiting enhanced biocompatibility. J. Therm. Spray Technol. 2010, 19, 336–343. [Google Scholar] [CrossRef]
- Yamashita, K.; Arashi, T.; Kitagaki, K.; Yamada, S.; Umegaki, T.; Ogawa, K. Preparation of apatite thin films through rf-sputtering from calcium phosphate glasses. J. Am. Ceram. Soc. 1994, 77, 2401–2407. [Google Scholar] [CrossRef]
- Van Der Wal, E.; Oldenburg, S.J.; Heij, T.; Denier van Der Gon, A.W.; Brongersma, H.H.; Wolke, J.G.C.; Jansen, J.A.; Vredenberg, A.M. Adsorption and desorption of Ca and PO4 species from SBFs on RF-sputtered calcium phosphate thin films. Appl. Surf. Sci. 2006, 252, 3843–3854. [Google Scholar]
- Jansen, J.A.; Wolke, J.G.C.; Swann, S.; van Der Waerden, J.P.C.M.; de Groof, K. Application of magnetron sputtering for producing ceramic coatings on implant materials. Clin. Oral Implants Res. 1993, 4, 28–34. [Google Scholar]
- Van Dijk, K.; Schaeken, H.G.; Wolke, J.G.C.; Jansen, J.A. Influence of annealing temperature on RF magnetron sputtered calcium phosphate coatings. Biomaterials 1996, 17, 405–410. [Google Scholar] [CrossRef]
- Van Dijk, K.; Schaeken, H.G.; Wolke, J.C.G.; Maree, C.H.M.; Habraken, F.H.P.M.; Verhoeven, J.; Jansen, J.A. Influence of discharge power level on the properties of hydroxyapatite films deposited on Ti6A14V with RF magnetron sputtering. J. Biomed. Mater. Res. 1995, 29, 269–276. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.Z.; Lee, I.S.; Wang, X. Plasma surface modification of magnesium alloy for biomedical application. Surf. Coatings Technol. 2010, 205, S182–S187. [Google Scholar] [CrossRef]
- Cotell, C.M.; Chrisey, D.B.; Grabowski, K.S.; Sprague, J.A.; Gossett, C.R. Pulsed laser deposition of hydroxylapatite thin films on Ti-6Al-4V. J. Appl. Biomater. 1992, 3, 87–93. [Google Scholar] [CrossRef]
- Leon, B. Pulsed Laser Deposition of Thin Calcium Phosphate Coatings; Springer: New York, NY, USA, 2009; p. 101. [Google Scholar]
- Jedynski, M.; Hoffman, J.; Mroz, W.; Szymanski, Z. Plasma plume induced during ArF laser ablation of hydroxyapatite. Appl. Surf. Sci. 2008, 255, 2230–2236. [Google Scholar] [CrossRef]
- Jelinek, M.; Weiserova, M.; Kocourek, T.; Zezulova, M.; Strnad, J. Biomedical properties of laser prepared silver-doped hydroxyapatite. Laser Phys. 2011, 21, 1265–1269. [Google Scholar]
- Mroz, V. Functional properties of nanostructured materials. NATO Sci. Ser. 2006, 223, 183–196. [Google Scholar] [CrossRef]
- Johnson, S.; Haluska, M.; Narayan, R.J.; Snyder, R.L. In situ annealing of hydroxyapatite thin films. Mater. Sci. Eng. 2006, 26, 1312–1316. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coatings Technol. 2011, 205, 2785–2792. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface nano-functionalization of biomaterials. Mater. Sci. Eng. Rep. 2011, 70, 275–302. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Kwok, C.T.; Wong, P.K.; Cheng, F.T.; Man, H.C. Characterization and corrosion behavior of hydroxyapatite coatings on Ti6Al4V fabricated by electrophoretic deposition. Appl. Surf. Sci. 2009, 255, 6736–6744. [Google Scholar]
- Stoch, A.; Brożek, A.; Kmita, G.; Stoch, J.; Jastrzębski, W.; Rakowska, A. Electrophoretic coating of hydroxyapatite on titanium implants. J. Mol. Struct. 2001, 596, 191–200. [Google Scholar] [CrossRef]
- Siefert, W. Corona spray pyrolysis: A new coating technique with an extremely enhanced deposition efficiency. Thin Solid Films 1984, 120, 267–274. [Google Scholar] [CrossRef]
- Wilhelm, O.; Mädler, L.; Pratsinis, S.E. Electrospray evaporation and deposition. J. Aerosol. Sci. 2003, 34, 815–836. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface Engineering for Bone Implants: A Trend from Passive to Active Surfaces. Coatings 2012, 2, 95-119. https://doi.org/10.3390/coatings2030095
Bosco R, Van Den Beucken J, Leeuwenburgh S, Jansen J. Surface Engineering for Bone Implants: A Trend from Passive to Active Surfaces. Coatings. 2012; 2(3):95-119. https://doi.org/10.3390/coatings2030095
Chicago/Turabian StyleBosco, Ruggero, Jeroen Van Den Beucken, Sander Leeuwenburgh, and John Jansen. 2012. "Surface Engineering for Bone Implants: A Trend from Passive to Active Surfaces" Coatings 2, no. 3: 95-119. https://doi.org/10.3390/coatings2030095
APA StyleBosco, R., Van Den Beucken, J., Leeuwenburgh, S., & Jansen, J. (2012). Surface Engineering for Bone Implants: A Trend from Passive to Active Surfaces. Coatings, 2(3), 95-119. https://doi.org/10.3390/coatings2030095




