Ink-Jet Printing of Gluconobacter oxydans: Micropatterned Coatings As High Surface-to-Volume Ratio Bio-Reactive Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gluconobacter oxydans Ink Preparation and Characterization
2.1.1. Cultivation of G. oxydans
2.1.2. Ink Formulation and Rheology
2.2. Ink-Jet Printing of G. oxydans Inks
2.2.1. Description of the Ink-Jet Plotter
2.2.2. Profilometry Measurements and Estimation of Microstructure Dimensions
2.3. Measurement of Microstructure Reactivity
3. Results and Discussion
3.1. Viscosity of G. oxydans Latex Emulsions
3.2. Study of Ink-Jet Printed G. oxydans Micropatterned Coatings
3.2.1. Profilometry of Deposited Microstructures


| Parameter | Numerical value | Unit | |
|---|---|---|---|
| Emulsion dilution factor | 4 | 2 | – |
| Number of droplets per dot | 2 | 3 | – |
| Base diameter (a) | 241 ± 9 | 233 ± 4 | µm |
| Top diameter (b) | 174 ± 7 | 176 ± 5 | – |
| Well diameter (c) | 114 ± 10 | – | – |
| Volume (V) | (54 ± 5) × 103 | (100 ± 4) × 103 | µm3 |
| Lateral surface area (S) 1 | (46 ± 4) × 103 | (43 ± 2) × 103 | µm2 |
| Height (h1, h2) 2 | 0.89 ± 0.04, 2.2 ± 0.1 | –, 3.0 ± 0.2 | µm |
| Cell concentration | 0.38 0.45 20.6 × 103 | 0.62 1.45 61.8 × 103 | cells·µm−3 cells·µm−2 cells·(dot) −1 |
| L-sorbose reaction rate 3 | not determined | 435 | g·L−1·h−1 |
| – | not determined | 1.02 | g·m−2·h−1 |
3.2.2. Reactivity
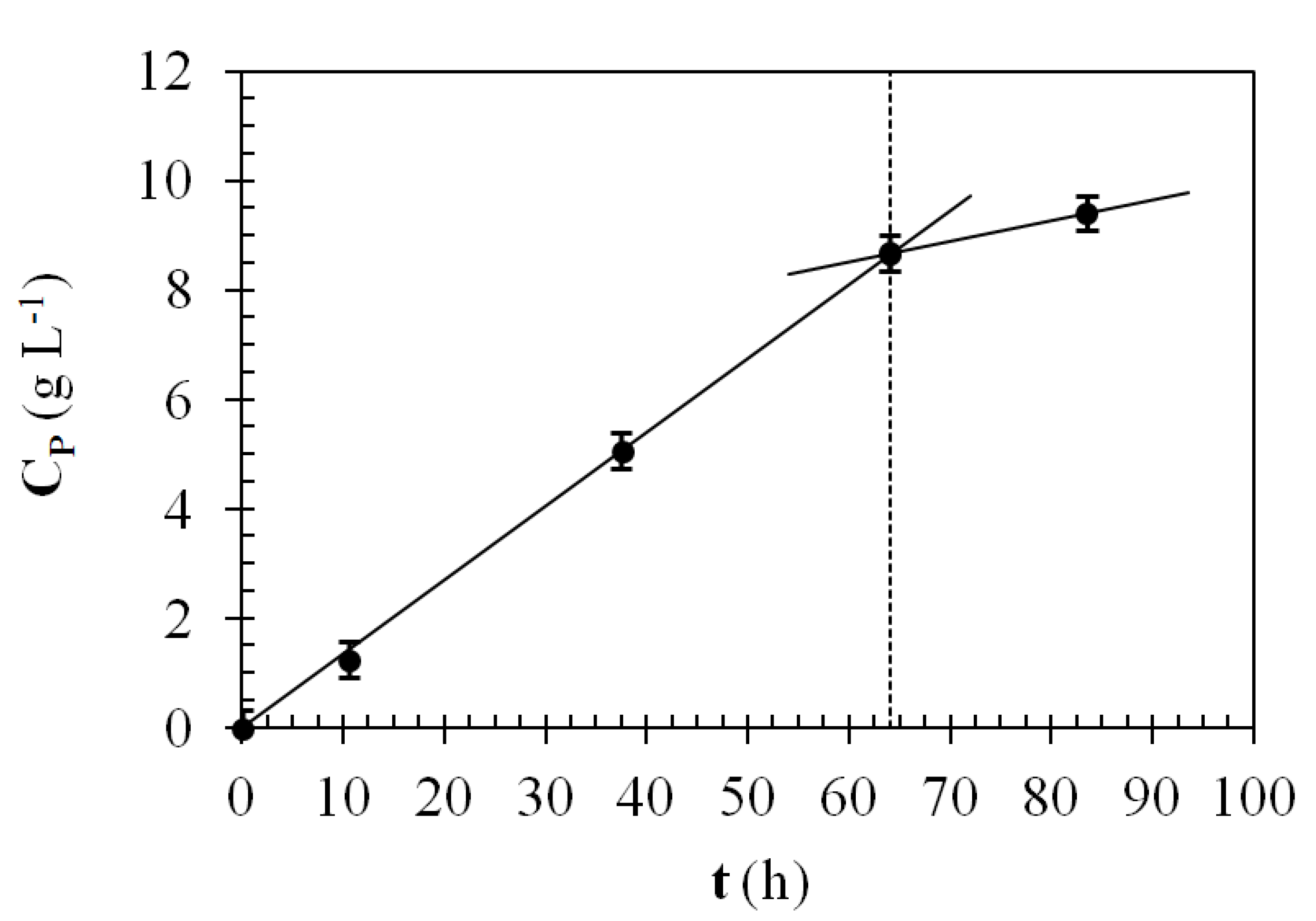
3.2.3. Coating Volumetric and Superficial Cell Concentration
3.2.4. Generation of Increased Surface Area Biocoatings Using Ink Jet Microstructure Pillar Arrays
| Coating type | Aspect ratio | S (m2) | N |
|---|---|---|---|
| Monolayer of cells | – | 1 | 3.1 × 1011 |
| Coating (this work) | 0.013 | 0.57 | 1.1 × 1012 |
| Coating | 1 | 2.7 | 8.4 × 1013 |
| Coating | 3 | 7.1 | 2.5 × 1014 |
| Coating | 5 | 11 | 4.2 × 1014 |
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Alper, J. Biology and the inkjets. Science 2004, 305, 1895. [Google Scholar] [CrossRef]
- Gratson, G.M.; Xu, M.; Lewis, J.A. Microperiodic structures: Direct writing of three-dimensional webs. Nature 2004, 428. [Google Scholar] [CrossRef]
- Li, Q.; Lewis, J.A. Nanoparticle inks for directed assembly of three-dimensional structures. Adv. Mater. 2003, 15, 1634–1641. [Google Scholar]
- Phamduy, T.B.; Corr, D.T.; Chrisey, D.B. Bioprinting. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; Flickinger, M.C., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2010; Volume 2, pp. 732–741. [Google Scholar]
- Saunders, R.; Derby, B. Bioprinting, Inkjet Deposition. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; Flickinger, M.C., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2010; Volume 2, pp. 732–741. [Google Scholar]
- Flickinger, M.C.; Freeman, E.A.; Anderson, C.R.; Lyngberg, O.K.; Laudon, M.C.; Scriven, L.E. Formulation of reactive microbial latex inks for ink-jet deposition of living bacteria or yeasts. In Proceedings of the Power of Ink-Jet Materials III, Berlin, Germany, 1–2 December 2005.
- Flickinger, M.C.; Schottel, J.L.; Bond, R.D.; Aksan, A.; Scriven, L.E. Painting and printing living bacteria: Engineering nanoporous biocatalytic coatings to preserve microbial viability and intensify reactivity. Biotechol. Prog. 2007, 23, 2–17. [Google Scholar] [CrossRef]
- Flickinger, M.C.; Lyngberg, O.K.; Freeman, E.A.; Anderson, C.R.; Laudon, M.C. Formulation of reactive nanostructured adhesive microbial ink-jet inks for miniature biosensors and biocatalysis. In Nanotechnology Applications in Coatings; Fernando, R.H., Sung, L.-P., Eds.; American Chemical Society: Washington, DC, USA, 2009; Volume 1008, pp. 156–187. [Google Scholar]
- Lyngberg, O.K.; Thiagarajan, V.; Stemke, D.J.; Schottel, J.L.; Scriven, L.E.; Flickinger, M.C. A patch coating method for preparing biocatalytic films of Escherichia coli. Biotechnol. Bioeng. 1999, 62, 44–55. [Google Scholar] [CrossRef]
- Lyngberg, O.K.; Stemke, D.J.; Schottel, J.L.; Flickinger, M.C. A simple single use luciferase based mercury biosensor using latex-film immobilized Escherichia coli HB101. J. Ind. Microbiol. Biotechnol. 1999, 23, 668–676. [Google Scholar] [CrossRef]
- Lyngberg, O.K. Development of Thin Biocatalytic Composite Coatings Consisting of Latex and Metabolically Active Bacterial Cells. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2000. [Google Scholar]
- Lyngberg, O.K.; Ng, C.P.; Thiagarajan, V.; Scriven, L.E.; Flickinger, M.C. Engineering the microstructure and permeability of thin multilayer latex biocatalytic coatings containing E. coli. Biotechnol. Prog. 2001, 17, 1169–1179. [Google Scholar] [CrossRef]
- Lyngberg, O.K.; Solheid, C.; Charaniya, S.; Ma, Y.; Thiagarajan, V.; Scriven, L.E.; Flickinger, M.C. Permeability and reactivity of Thermatoga maritima in latex bimodal blend coatings at 80°C: A model high temperature biocatalytic coating. Extremophiles 2005, 9, 197–207. [Google Scholar] [CrossRef]
- Swope, K.L.; Flickinger, M.C. Activation and regeneration of whole cell biocatalysts: Initial and periodic induction behavior in starved Escherichia coli after immobilization in thin synthetic films. Biotechnol. Bioeng. 1996, 51, 360–370. [Google Scholar] [CrossRef]
- Swope, K.L.; Flickinger, M.C. The use of confocal scanning laser microscopy and other tools to characterize Escherichia coli in high-cell-density synthetic biofilms. Biotechnol. Bioeng. 1996, 52, 340–356. [Google Scholar] [CrossRef]
- Gosse, J.L.; Engel, B.J.; Rey, F.E.; Harwood, C.S.; Scriven, L.E.; Flickinger, M.C. Hydrogen production by photoreactive nanoporous latex coatings of nongrowing Rhodopseudomonas palustris CGA009. Biotechnol. Prog. 2007, 23, 124–130. [Google Scholar] [CrossRef]
- Gosse, J.L.; Engel, B.J.; Hui, J.C.; Harwood, C.S.; Flickinger, M.C. Progress toward a biomimetic leaf: 4000 h of hydrogen production by coating-stabilized nongrowing photosynthetic Rhodopseudomonas palustris. Biotechnol. Prog. 2010, 26, 907–918. [Google Scholar]
- Gosse, J.L; Flickinger, M.C. Uniform lab-scale biocatalytic nanoporous latex coatings for reactive microorganisms. Methods Mol. Biol. 2011, 743, 213–222. [Google Scholar] [CrossRef]
- Gosse, J.L.; Chinn, M.S.; Grunden, A.M.; Bernal, O.I.; Jenkins, J.S.; Yeager, C.; Kosourov, S.; Seibert, M.; Flickinger, M.C. A versatile method for preparation of hydrated microbial-latex biocatalytic coatings for gas absorption and gas evolution. J. Ind. Microbiol. Biotechnol. 2012, 39, 1269–1278. [Google Scholar] [CrossRef]
- Jenkins, J.; Velev, O.; Flickinger, M.C. Deposition of composite coatings from particle-particle and particle-yeast blends by convective-sedimentation assembly. J. Colloid Interface Sci. 2012, 380, 192–200. [Google Scholar] [CrossRef]
- Huang, Z.; Thiagarajan, V.S; Lyngberg, O.K; Scriven, L.E.; Flickinger, M.C. Microstructure evolution in polymer latex coatings for whole-cell biocatalyst application. J. Colloid Interface Sci. 1999, 215, 226–243. [Google Scholar] [CrossRef]
- Thiagarajan, V.S.; Huang, Z.; Scriven, L.E.; Schottel, J.L.; Flickinger, M.C. Microstructure of a biocatalytic latex coating containing Escherichia coli cells. J. Colloid Interface Sci. 1999, 215, 244–257. [Google Scholar] [CrossRef]
- Mota, M.; Yelshin, A.; Fidaleo, M.; Flickinger, M.C. Modelling diffusivity in porous polymeric membranes with an intermediate layer containing microbial cells. Biochem. Eng. J. 2007, 37, 285–293. [Google Scholar] [CrossRef]
- Bhatti, A.; Mott, M.; Evans, J.; Edirisinghe, M. PZT pillars for 1–3 composites prepared by ink-jet printing. J. Mater. Sci. Lett. 2001, 20, 1245–1248. [Google Scholar] [CrossRef]
- Zhao, X.; Evans, J.; Edirisinghe, M.; Song, J. Direct ink-jet printing of vertical walls. J. Am. Ceram. Soc. 2002, 85, 2113–2115. [Google Scholar] [CrossRef]
- Ko, S.H.; Chung, J.; Hotz, N.; Nam, K.H.; Grigoropoulos, C.P. Metal nanoparticle direct inkjet printing for low-temperature 3D micro metal structure fabrication. J. Micromech. Microeng. 2010, 20. [Google Scholar] [CrossRef]
- Kullmann, C.; Schirmer, N.C.; Lee, M.; Ko, S.H.; Hotz, N.; Grigoropoulos, C.P.; Poulikakos, D. 3D micro-structures by piezoelectric inkjet printing of gold nanofluids. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Maleksaeedi, S.; Wang, J.K.; El-Hajje, A.; Harb, L.; Guneta, V.; He, Z.; Wiria, F.E.; Choong, C.; Ruys, A.J. Toward 3D printed bioactive titanium scaffolds with bimodal pore size distribution for bone ingrowth. Procedia CIRP 2013, 5, 158–163. [Google Scholar] [CrossRef]
- Fidaleo, M.; Charaniya, S.; Solheid, C.; Diel, U.; Laudon, M.; Ge, H; Scriven, L.E.; Flickinger, M.C. A model system for increasing the intensity of whole-cell biocatalysis: Investigation of the rate of oxidation of D-sorbitol to L-sorbose by thin bi-layer latex coatings of non-growing Gluconobacter oxydans. Biotechnol. Bioeng. 2006, 95, 446–458. [Google Scholar] [CrossRef]
- Fidaleo, M.; Flickinger, M.C. Engineering and modeling of thin, adhesive, microbial biocatalytic coatings for high intensity oxidations in multi-phase microchannel bioreactors. Chem. Eng. Sci. 2011, 66, 3251–3257. [Google Scholar] [CrossRef]
- Reshetilov, A.N.; Donova, M.V.; Dovbnya, D.V.; Il’yasov, P.V.; Boronin, A.M.; Leasers, T.; Green, R. Membrane-bound dehydrogenases of Gluconobacter oxydans: Sensors for measuring sugars, alcohols and polyoles. B. Exp. Biol. Med. 1998, 126, 702–704. [Google Scholar] [CrossRef]
- Deppenmeier, U.; Ehrenreich, A. Physiology of acetic acid bacteria in light of the genome sequence of Gluconobacter oxydans. J. Mol. Microbiol. Biotechnol. 2009, 16, 69–80. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Mulchandani, A.; Groom, C.A. The development of an amperometric microbial biosensor using Acetobacter pasteurianus for lactic acid. J. Biotechnol. 1989, 10, 241–252. [Google Scholar] [CrossRef]
- Takayama, K.; Kurosaki, T.; Ikeda, T.J. Mediated electrocatalysis at a biocatalyst electrode based on a bacterium, Gluconobacter industrius. J. Electroanal. Chem. 1993, 356, 295–301. [Google Scholar] [CrossRef]
- Karube, I.; Kiyoko, Y. BOD Sensor and BOD Measuring Method. World Patent No. 95,06,242, 2 March 1995. [Google Scholar]
- Reshetilov, A.N.; Iliasov, P.V.; Donova, M.V.; Dovbnya, D.V.; Boronin, A.M.; Leathers, T.D.; Greene, R.V. Evaluation of a Gluconobacter oxydans whole cell biosensor for amperometric detection of xylose. Biosens. Bioelectron. 1997, 12, 241–247. [Google Scholar] [CrossRef]
- Reshetilov, A.N.; Lobanov, A.V.; Morozova, N.O.; Gordon, S.H.; Greene, R.V.; Leathers, T.D. Detection of ethanol in a two-component glucose/ethanol mixture using a nonselective microbial sensor and a glucose enzyme electrode. Biosens. Bioelectron. 1998, 13, 787–793. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Borisov, I.A.; Gordon, S.H.; Greene, R.V.; Leathers, T.D.; Reshetilov, A.N. Analysis of ethanol–glucose mixtures by two microbial sensors: Application of chemometrics and artificial neural networks for data processing. Biosens. Bioelectron. 2001, 16, 1001–1007. [Google Scholar] [CrossRef]
- Švitel, J.; Čurilla, O.; Tkáč, J. Microbial cell-based biosensor for sensing glucose, sucrose or lactose. Biotechnol. Appl. Biochem. 1998, 27, 153–158. [Google Scholar]
- Švitel, J.; Tkáč, J.; Voštiar, I.; Navrátil, M.; Štefuca, V.; Bučko, M.; Gemeiner, P. Gluconobacter in biosensors: Applications of whole cells and enzymes isolated from gluconobacter and acetobacter to biosensor construction. Biotechnol. Lett. 2006, 28, 2003–2010. [Google Scholar] [CrossRef]
- Tkáč, J.; Štefuca, V.; Gemeiner, P. Focus on Biotechnology. In Applications of Cell Immobilisation Biotechnology; Nedovič, V., Willaert, R., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 8, pp. 549–566. [Google Scholar]
- Fromm, J.E. Numerical calculation of the fluid dynamics of drop-on-demand jets. IBM J. Res.Dev. 1984, 28, 322–333. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter 2008, 4, 703–713. [Google Scholar] [CrossRef]
- Friederich, A.; Binder, J.R.; Bauer, W. Rheological control of the coffee stain effect for inkjet printing of ceramics. J. Am. Ceram. Soc. 2013, 96, 2093–2099. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R. Contact line deposits in an evaporating drop. Phys. Rev. E 2000, 62, 756–765. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Du, G.; Zhou, J.; Chen, J. Efficient production of L-sorbose from D-sorbitol by whole cell immobilization of Gluconobacter oxydans WSH-003. Biochem. Eng. J. 2013, 77, 171–176. [Google Scholar] [CrossRef]
- Schottel, J.L.; Orwin, P.M.; Anderson, C.R.; Flickinger, M.C. Spatial expression of a mercury-inducible green fluorescent protein within a nanoporous latex-based biosensor coating. J. Ind. Microbiol. Biotechnol. 2008, 35, 283–290. [Google Scholar] [CrossRef]
- Piskorska, M.; Soule, T.; Gosse, J.L.; Milliken, C.; Flickinger, M.C.; Smith, G.W.; Yeager, C.M. Preservation of H2 production activity in nanoporous latex coatings of Rhodopseudomonas palustris CGA009 during dry storage at ambient temperatures. Microb. Biotechnol. 2013, 6, 515–525. [Google Scholar] [CrossRef]
- Chini, S.F.; Amirfazli, A. Understanding pattern collapse in photolithography process due to capillary forces. Langmuir 2010, 26, 13707–13714. [Google Scholar] [CrossRef]
- Jenkins, J.; Flickinger, M.C.; Velev, O. Continuous convective-sedimentation assembly of colloidal microsphere coatings for biotechnology applications. Coatings 2013, 3, 26–48. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Tkáč, J.; Vŏstiar, I.; Gorton, L.; Gemeiner, P.; Šturdik, E. Improved selectivity of microbial biosensor using membrane coating. Application to the analysis of ethanol during fermentation. Biosens. Bioelectron. 2003, 18, 1125–1134. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fidaleo, M.; Bortone, N.; Schulte, M.; Flickinger, M.C. Ink-Jet Printing of Gluconobacter oxydans: Micropatterned Coatings As High Surface-to-Volume Ratio Bio-Reactive Coatings. Coatings 2014, 4, 1-17. https://doi.org/10.3390/coatings4010001
Fidaleo M, Bortone N, Schulte M, Flickinger MC. Ink-Jet Printing of Gluconobacter oxydans: Micropatterned Coatings As High Surface-to-Volume Ratio Bio-Reactive Coatings. Coatings. 2014; 4(1):1-17. https://doi.org/10.3390/coatings4010001
Chicago/Turabian StyleFidaleo, Marcello, Nadia Bortone, Mark Schulte, and Michael C. Flickinger. 2014. "Ink-Jet Printing of Gluconobacter oxydans: Micropatterned Coatings As High Surface-to-Volume Ratio Bio-Reactive Coatings" Coatings 4, no. 1: 1-17. https://doi.org/10.3390/coatings4010001
APA StyleFidaleo, M., Bortone, N., Schulte, M., & Flickinger, M. C. (2014). Ink-Jet Printing of Gluconobacter oxydans: Micropatterned Coatings As High Surface-to-Volume Ratio Bio-Reactive Coatings. Coatings, 4(1), 1-17. https://doi.org/10.3390/coatings4010001




