Development of Antibacterial Composite Films Based on Isotactic Polypropylene and Coated ZnO Particles for Active Food Packaging
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Sample Preparation
2.2. Composite Preparation
| Sample | iPP (wt %) | ZnOc (wt %) |
|---|---|---|
| iPP | 100 | − |
| iPP/2%ZnOc | 98 | 2 |
| iPP/5%ZnOc | 95 | 5 |
2.3. Characterization
3. Results and Discussion
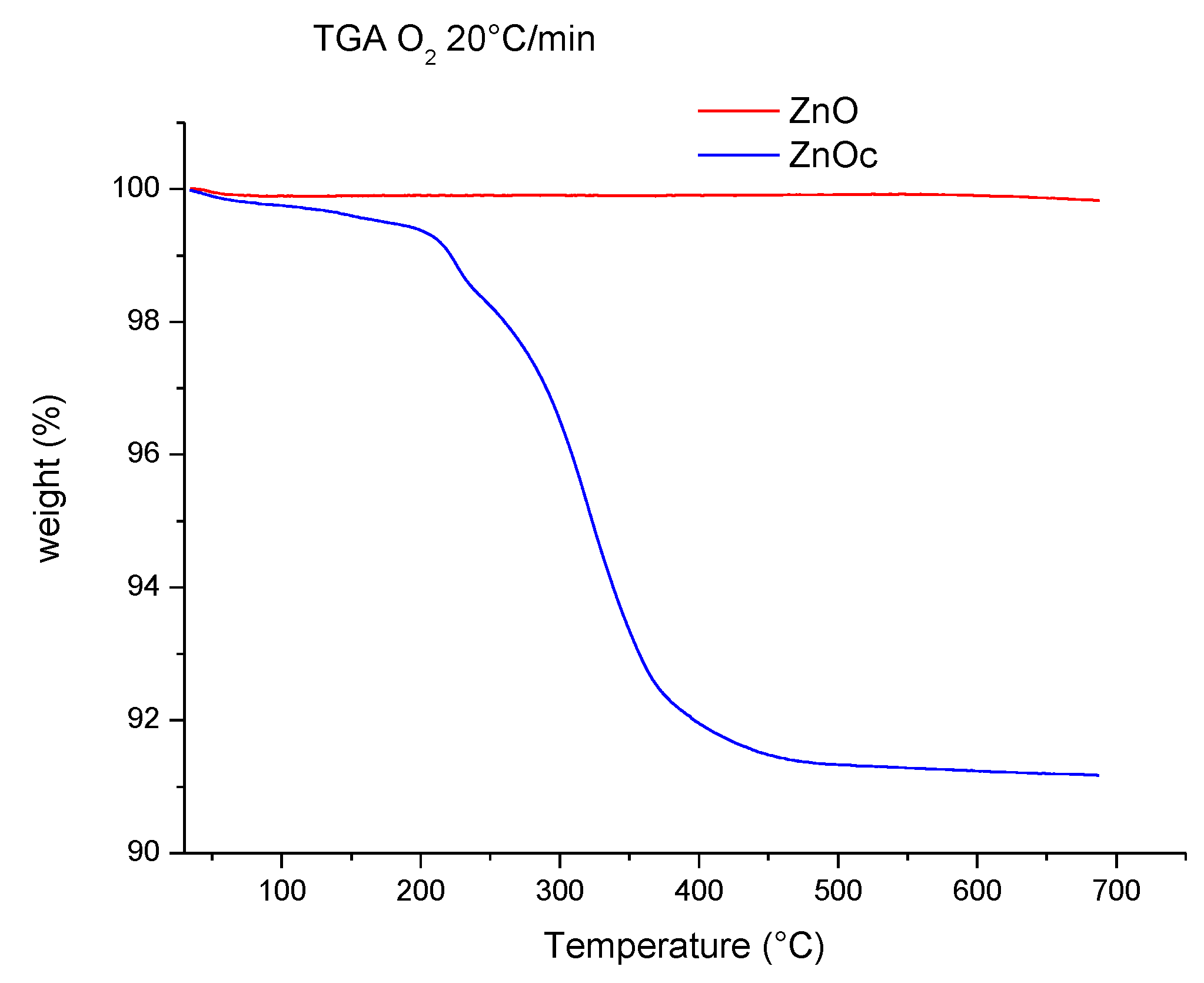
3.1. Analysis of ZnO and ZnOc Particles

- at 2916 and 2848 cm−1: these vibration bands can be assigned to the “stretching” of the symmetric and asymmetric aliphatic group CH2;
- at 1460 cm−1: this band is assigned to the vibration of “bending” of aliphatic groups CH2 and CH3 of stearic acid;
- at 1540 and 1384 cm−1: these bands are assigned to the asymmetric and symmetric vibrations of the carboxylate group of the stearic acid;
- at around 454 cm−1: These bands give information about the shape of the particles. It is interesting to go deeper into the bands at points 3 and 4.

- if Δ(experimental) Δ(sodium salt) is bidentate chelating coordination;
- if Δ(experimental) ≤ Δ(sodium salt) is bidentate coordination to bridge;
- if Δ(experimental) > Δ(sodium salt) coordination is monodentate.

3.2. Analysis of the iPP/ZnOc Composites
3.2.1. Structure and Morphology



3.2.2. Thermostability

| Sample | Tmax (°C) |
|---|---|
| iPP | 335 |
| iPP/2%ZnOc | 375 |
| iPP/5%ZnOc | 381 |
3.2.3. Mechanical and Impact Properties

| Sample | E (MPa) | σy (MPa) | εy (%) | σb (MPa) | εb (%) |
|---|---|---|---|---|---|
| iPP | 1350 ± 100 | 19 ± 3 | 7 ± 2 | 30 ± 3 | 890 ± 65 |
| iPP/2%ZnOc | 1537 ± 44 | 25 ± 2 | 7 ± 1 | 28 ± 4 | 645 ± 54 |
| iPP/5%ZnOc | 1515 ± 79 | 26 ± 1 | 7 ± 1 | 28 ± 3 | 605 ± 76 |
| Sample | F (N) | E (J) | T (kJ/m2) |
|---|---|---|---|
| iPP | 73 ± 7 | 0.032 ± 0.008 | 1.91 ± 0.35 |
| iPP/2%ZnOc | 84 ± 3 | 0.033 ± 0.005 | 2.07 ± 0.22 |
| iPP/5%ZnOc | 89 ± 5 | 0.042 ± 0.004 | 2.41 ± 0.14 |
3.2.4. Antibacterial Properties

| Sample | %R (t = 1 h) | %R (t = 24 h) | %R (t = 48 h) | %R (t = 5 days) | %R (t = 10 days) |
|---|---|---|---|---|---|
| iPP | 0 | 0 | 0 | 0 | 0 |
| iPP/2%ZnOc | 0 | 55.61 ± 0.01 | 94.00 ± 0.01 | 99.99 ± 0.01 | 99.99 ± 0.01 |
| iPP/5%ZnOc | 0 | 91.12 ± 0.01 | 99.99 ± 0.01 | 99.99 ± 0.01 | 99.99 ± 0.01 |
- in the iPP/ZnOc system the ZnOc particles maintain their antibacterial properties against E. coli, with respect to the uncoated particles;
- in the system iPP/PPgMA/ZnO, the ZnO particles that are linked to the maleic anhydride groups of PPgMA [24], do not display similar antibacterial activity at least up to 48 h. Probably, the PP chains of the PPgMA, due to the link between MA and ZnO, cover the ZnO particles and hinder the antibacterial activity.
| Sample | %R (t = 48 h) | %R (t = 5 days) |
|---|---|---|
| iPP | 0 | 0 |
| iPP/2%ZnO | 90 | 99.99 |
| iPP/2%ZnOc | 94 | 99.99 |
| iPP/PP(9k)gMA(4.8) */2%ZnO | 65 | NA |
| iPP/PP(65k)gMA(1.4) **/2%ZnO | 60 | NA |
| iPP/PP(95k)gMA(0.5) ***/2%ZnO | 31 ± 5 | NA |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Languimuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Habsah, H.; Dasmawati, M. Review on Zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O.; Sawai, J.; Sasamoto, T. Change in antibacterial characteristics with doping amount of ZnO in MgO-ZnO solid solution. Int. J. Inorg. Mater. 2000, 2, 451–454. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Silvestre, C.; Cimmino, S. Ecosustainable Polymer Nanomaterials for Food Packaging: Innovative Solutions, Characterization Needs, Safety and Environmental Issues; Silvestre, C., Cimmino, S., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Lagaron, M.; Ocio, M.J.; Lopez-Rubio, A.J. Antimicrobial Polymers; Yam, K.L., Lee, D.S., Eds.; John Wiley & Son: Hoboken, NJ, USA, 2012. [Google Scholar]
- Matei, A.; Cernica, I.; Cadar, O.; Roman, C.; Schiopu, V. Synthesis and characterization of ZnO—Polymer nanocomposites. Int. J. Mater. Form. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Wei, W.C.J. Processing and property improvement of polymeric composites with added ZnO nanoparticles through microinjection molding. J. Appl. Polym. Sci. 2006, 102, 6009–6016. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Solimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on reducing pasteurization temperature of orange juice. J. Food Process. Preserv. 2012, 36, 104–112. [Google Scholar] [CrossRef]
- Droval, G.; Aranberri, I.; Bilbao, A.; German, L.; Verelst, M.; Dexpert-Ghys, J. Antimicrobial activity of nanocomposites: Poly(amide) 6 and low density poly(ethylene) filled with zinc oxide. E-Polymers 2008, 128, 1–13. [Google Scholar] [CrossRef]
- Lepot, N.; van Bael, M.K.; van den Rul, H.; D’Haen, J.; Peeters, R.; Franco, D.; Mullens, J. Influence of incorporation of ZnO nanoparticles and biaxial orientation on mechanical and oxygen barrier properties of polypropylene films for food packaging. J. Appl. Polym. Sci. 2015, 120, 1616–1623. [Google Scholar] [CrossRef]
- Chandramouleeswaran, S.; Mhaske, S.T.; Kathe, A.A.; Varadarajan, P.V.; Prasad, V.; Vigneshwaran, N. Functional behaviour of polypropylene/ZnO-soluble starch nanocomposites. Nanotechnolohy 2007, 18. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Y.; Liu, H.; Belfiore, A. Effects of organic nucleating agents and zinc oxide nanoparticles on isotactic polypropylene crystallization. Polymer 2004, 45, 2081–2091. [Google Scholar] [CrossRef]
- Silvestre, C.; Cimmino, S.; Pezzuto, M.; Marra, A.; Ambrogi, V.; Dexpert-Ghys, J.; Verelst, M.; Augier, S.; Romano, I.; Duraccio, D. Preparation and characterization of isotactic polypropylene/zinc oxide microcomposites with antibacterial activity. Polym. J. 2013, 45, 938–945. [Google Scholar] [CrossRef]
- Duraccio, D.; Silvestre, C.; Pezzuto, M.; Cimmino, S.; Marra, A. Polypropylene and polyethylene-based nanocomposites for food packaging applications. In Ecosustainable Polymer Nanomaterials for Food Packaging; Silvestre, C., Cimmino, S., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013; pp. 143–167. [Google Scholar]
- Cimmino, S.; Duraccio, D.; Marra, A.; Pezzuto, M.; Romano, I.; Silvestre, C. Effect of compatibilisers on mechanical, barrier and antimicrobial properties of iPP/ZnO nano/microcomposites for food packaging application. J. Appl. Packag. Res. 2015, 7, 108–127. [Google Scholar]
- Erem, A.D.; Ozcan, G.; Skrifvars, M. Antibacterial activity of PA6/ZnO nanocomposite fibers. Text. Res. J. 2011, 81, 1638–1646. [Google Scholar] [CrossRef]
- Murariu, M.; Paint, Y.; Murariu, O.; Raquez, J.M.; Bonnaud, L.; Dubois, P. Current progress in the production of PLA–ZnO nanocomposites: Beneficial effects of chain extender addition on key properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Krunks, M.; Mellikov, E. Zinc oxide thin films by the spray pirolysis method. Thin Solid Films 1995, 270, 33–36. [Google Scholar] [CrossRef]
- Alavi, S.; Caussat, B.; Couderc, J.P.; Dexpert-Ghys, J.; Joffin, N.; Neumeyer, D.; Verelst, M. Spray pyrolysis synthesis of submicronic particles. Possibilities and limits. Adv. Sci. Technol. 2003, 30, 417–424. [Google Scholar]
- Cimmino, S.; Silvestre, C.; Duraccio, D.; Pezzuto, M. Effect of hydrocarbon resin on the morphology and mechanical properties of isotactic polypropylene/clay composites. J. Appl. Polym. Sci. 2011, 119, 1135–1143. [Google Scholar] [CrossRef]
- Kaci, M.; Benhamida, A.; Cimmino, S.; Silvestre, C.; Carfagna, C. Waste and virgin LDPE/PET blends compatibilized with an Ethylene-Butyl Acrylate-Glycidyl Methacrylate (EBAGMA) Terpolymer, 1. Macromol. Mater. Eng. 2005, 290, 987–995. [Google Scholar] [CrossRef]
- Utracki, L.A. Compatibilization of polymer blends. Can. J. Chem. Eng. 2002, 80, 1008–1016. [Google Scholar] [CrossRef]
- Akbar, B.; Bagheri, R. Influence of compatibilizer and processing conditions on morphology, mechanical properties, and deformation mechanism of PP/Clay nanocomposite. J. Nanomater. 2012, 8. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.; Goddard, J.M. Active packaging coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef]
- ASTM E 2149-10: Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents under Dynamic Contact Conditions; ASTM: West Conshohocken, PA, USA, 2001.
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, 829–858. [Google Scholar] [CrossRef]
- Zeleňák, V.; Vargová, Z.; Gyӧryová, K. Correletion of infrared spectra of zinc(II) carboxtlates with their structures. Spectrochim. Acta Part A 2007, 66, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Capelle, H.A.; Britcher, L.G.; Morris, G.E. Sodium stearate absorbtion onto titania pigment. J. Colloid Interface Sci. 2003, 268, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Andrés Vergés, M.; Mifsud, A.; Serna, C.J. Formation of rod-like zinc oxide microcrystals in homogeneous solution. J. Chem. Soc. Farday Trans. 1990, 86, 959–963. [Google Scholar] [CrossRef]
- Silvestre, C.; Cimmino, S.; di Pace, E. Morphology of polyolefins. In Handbook of Polyolefins, 2nd ed.; Vasile, C., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 175–206. [Google Scholar]
- Silvestre, C.; Cimmino, S.; Triolo, R. Structure, morphology and crystallization of a random ethylene-propylene copolymer. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 493–500. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestre, C.; Duraccio, D.; Marra, A.; Strongone, V.; Cimmino, S. Development of Antibacterial Composite Films Based on Isotactic Polypropylene and Coated ZnO Particles for Active Food Packaging. Coatings 2016, 6, 4. https://doi.org/10.3390/coatings6010004
Silvestre C, Duraccio D, Marra A, Strongone V, Cimmino S. Development of Antibacterial Composite Films Based on Isotactic Polypropylene and Coated ZnO Particles for Active Food Packaging. Coatings. 2016; 6(1):4. https://doi.org/10.3390/coatings6010004
Chicago/Turabian StyleSilvestre, Clara, Donatella Duraccio, Antonella Marra, Valentina Strongone, and Sossio Cimmino. 2016. "Development of Antibacterial Composite Films Based on Isotactic Polypropylene and Coated ZnO Particles for Active Food Packaging" Coatings 6, no. 1: 4. https://doi.org/10.3390/coatings6010004
APA StyleSilvestre, C., Duraccio, D., Marra, A., Strongone, V., & Cimmino, S. (2016). Development of Antibacterial Composite Films Based on Isotactic Polypropylene and Coated ZnO Particles for Active Food Packaging. Coatings, 6(1), 4. https://doi.org/10.3390/coatings6010004







