Novel Thiol-Ene Hybrid Coating for Metal Protection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of 1,4-di (vinylimidazolium) Butane Bisbromide
2.3. Fabrication of Hybrid Material by Thiol-Ene Reaction
2.4. Preparation of Hybrid Coating on Metal
2.5. Preparation of Sol-Gel Coating
2.6. Characterization
3. Results and Discussion
3.1. Structural Evolution of the PIL Hybrid Film
3.2. Thermal Stability of the PIL Hybrid Film
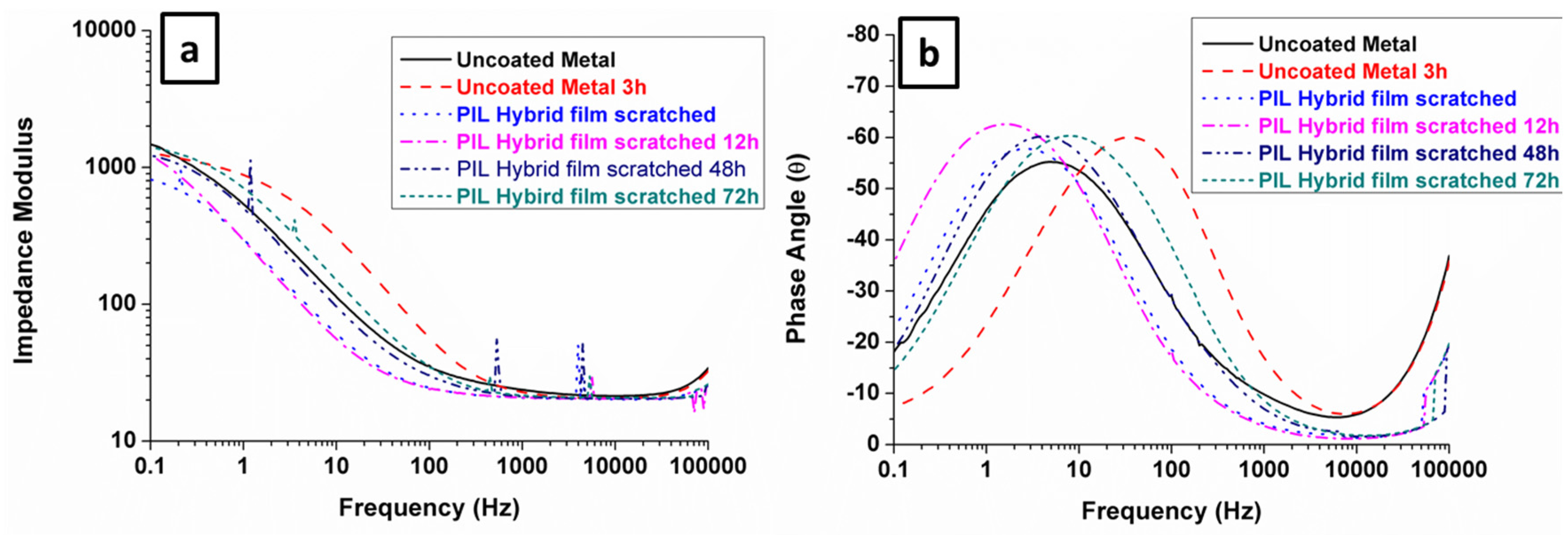
3.3. Electrochemical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khramov, A.N.; Balbyshev, V.N.; Voevodin, N.N.; Donley, M.S. Nanostructured sol-gel derived conversion coatings based on epoxy- and amino-silanes. Prog. Org. Coat. 2003, 47, 207–213. [Google Scholar] [CrossRef]
- Rahimi, H.; Mozaffarinia, R.; Hojjati Najafabadi, A. Corrosion and wear resistance characterization of environmentally friendly sol-gel hybrid nanocomposite coating on AA5083. J. Mater. Sci. Technol. 2013, 29, 603–608. [Google Scholar] [CrossRef]
- Rahimi, S.K.; Potrekar, R.; Dutta, N.K.; Choudhury, N.R. Anticorrosive interfacial coatings for metallic substrates. Surf. Innov. 2013, 1, 112–137. [Google Scholar] [CrossRef]
- Vijayalakshmi, U.; Rajeswari, S. Synthesis and characterization of sol-gel derived glass-ceramic and its corrosion protection on 316L SS. J. Sol-Gel Sci. Technol. 2007, 43, 251–258. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G.S. Sol-gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099–5111. [Google Scholar] [CrossRef]
- He, X.; Shi, X. Self-repairing coating for corrosion protection of aluminum alloys. Prog. Org. Coat. 2009, 65, 37–43. [Google Scholar] [CrossRef]
- Metroke, T.L.; Parkhill, R.L.; Knobbe, E.T. Passivation of metal alloys using sol-gel-derived materials—A review. Prog. Org. Coat. 2001, 41, 233–238. [Google Scholar] [CrossRef]
- Sanchez, C.; de A. A. Soler-Illia, G.J.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed hybrid organic-inorganic nanocomposites from functional nanobuilding blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. Synthesis and characterization of methacrylate phospho-silicate hybrid for thin film applications. Polymer 2007, 48, 7078–7086. [Google Scholar] [CrossRef]
- Vreugdenhil, A.J.; Balbyshev, V.N.; Donley, M.S. Nanostructured silicon sol-gel surface treatments for Al 2024-T3 protection. J. Coat. Technol. 2001, 73, 35–43. [Google Scholar] [CrossRef]
- Donley, M.S.; Mantz, R.A.; Khramov, A.N.; Balbyshev, V.N.; Kasten, L.S.; Gaspar, D.J. The self-assembled nanophase particle (SNAP) process: A nanoscience approach to coatings. Prog. Org. Coat. 2003, 47, 401–415. [Google Scholar] [CrossRef]
- Sinapi, F.; Forget, L.; Delhalle, J.; Mekhalif, Z. Self-assembly of (3-mercaptopropyl)trimethoxysilane on polycrystalline zinc substrates towards corrosion protection. Appl. Surf. Sci. 2003, 464–471. [Google Scholar] [CrossRef]
- Sinapi, F.; Delhalle, J.; Mekhalif, Z. XPS and electrochemical evaluation of two-dimensional organic films obtained by chemical modification of self-assembled monolayers of (3-mercaptopropyl)trimethoxysilane on copper surfaces. Mater. Sci. Eng. 2002, 22, 345–353. [Google Scholar] [CrossRef]
- Bagiyan, G.A.; Koroleva, I.K.; Soroka, N.V.; Ufimtsev, A.V. Oxidation of thiol compounds by molecular oxygen in aqueous solutions. Russ. Chem. Bull. 2003, 52, 1135–1141. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid) latexes prepared by dispersion polymerization of ionic liquid monomers. Macromolecules 2011, 44, 744–750. [Google Scholar] [CrossRef]
- Taghavikish, M.; Subianto, S.; Dutta, N.K.; Choudhury, N.R. Facile fabrication of polymerizable ionic liquid based-gel beads via thiol-ene chemistry. ACS Appl. Mater. Interfaces 2015, 7, 17298–17306. [Google Scholar] [CrossRef] [PubMed]
- Tremont, R.; de Jesús-Cardona, H.; García-Orozco, J.; Castro, R.J.; Cabrera, C.R. 3-mercaptopropyltrimethoxysilane as a Cu corrosion inhibitor in KCL solution. J. Appl. Electrochem. 2000, 30, 737–743. [Google Scholar] [CrossRef]
- Damia, C.; Sarda, S.; Deydier, E.; Sharrock, P. Study of two hydroxyapatite/poly(alkoxysilane) implant coatings. Surf. Coat. Technol. 2006, 201, 3008–3015. [Google Scholar] [CrossRef]
- Piwoński, I.; Grobelny, J.; Cichomski, M.; Celichowski, G.; Rogowski, J. Investigation of 3-mercaptopropyltrimethoxysilane self-assembled monolayers on Au(111) surface. Appl. Surf. Sci. 2005, 242, 147–153. [Google Scholar] [CrossRef]
- Bauer, F.; Ernst, H.; Decker, U.; Findeisen, M.; Gläsel, H.-J.; Langguth, H.; Hartmann, E.; Mehnert, R.; Peuker, C. Preparation of scratch and abrasion resistant polymeric nanocomposites by monomer grafting onto nanoparticles, 1 FTIR and multi-nuclear NMR spectroscopy to the characterization of methacryl grafting. Macromol. Chem. Phys. 2000, 201, 2654–2659. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, F.Z.; Jin, F.; Zhang, R.; Luo, B.; Zhang, T. The evaluation of coating performance by analyzing the intersection of bode plots. Int. J. Electrochem. Sci. 2014, 9, 5116–5125. [Google Scholar]
- Messaadia, L.; ID El mouden, O.; Anejjar, A.; Messali, M.; Salghi, R.; Benali, O.; Cherkaoui, O.; Lallam, A. Adsorption and corrosion inhibition of new synthesized pyridazinium-based ionic liquid on carbon steel in 0.5 M·H2SO4. J. Mater. Environ. Sci. 2015, 6, 598–606. [Google Scholar]
- Galio, A.F.; Lamaka, S.V.; Zheludkevich, M.L.; Dick, L.F.P.; Müller, I.L.; Ferreira, M.G.S. Inhibitor-doped sol-gel coatings for corrosion protection of magnesium alloy AZ31. Surf. Coat. Technol. 2010, 204, 1479–1486. [Google Scholar] [CrossRef]










| Metal Sample | Ecorr (V) | Icorr (A) | EI (%) |
|---|---|---|---|
| Blank metal | −0.674 | 7.9 × 10−6 | – |
| SNAP coated | −0.312 | 7.4 × 10−8 | – |
| PIL/SNAP Hybrid film coated | −0.395 | 1.3 × 10−7 | – |
| Blank metal 24 h | −0.937 | 4.9 × 10−6 | – |
| SNAP coated 24 h | −0.613 | 4.1 × 10−6 | 16.3 |
| PIL/SNAP Hybrid film coated 24 h | −0.479 | 1.5 × 10−7 | 96.9 |
| SAMPLE | Rsolution (Ω) | Rcoating (Ω) | Ccoating (F) | Rinterface (Ω) | Cinterface (F) |
|---|---|---|---|---|---|
| SNAP only | 21 | 158 | 4.16 × 10−5 | 1.17 × 106 | 2.08 × 10−5 |
| SNAP only 24 h | 12 | 18 | 8.44 × 10−6 | 2613 | 4.08 × 10−4 |
| PIL/SNAP hybrid film | 23 | 15,257 | 7.04 × 10−6 | 1.54 × 105 | 1.44 × 10−5 |
| PIL/SNAP hybrid film 24 h | 21 | 15,009 | 4.45 × 10−6 | 3.19 × 105 | 1.84 × 10−5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghavikish, M.; Subianto, S.; Dutta, N.K.; Roy Choudhury, N. Novel Thiol-Ene Hybrid Coating for Metal Protection. Coatings 2016, 6, 17. https://doi.org/10.3390/coatings6020017
Taghavikish M, Subianto S, Dutta NK, Roy Choudhury N. Novel Thiol-Ene Hybrid Coating for Metal Protection. Coatings. 2016; 6(2):17. https://doi.org/10.3390/coatings6020017
Chicago/Turabian StyleTaghavikish, Mona, Surya Subianto, Naba Kumar Dutta, and Namita Roy Choudhury. 2016. "Novel Thiol-Ene Hybrid Coating for Metal Protection" Coatings 6, no. 2: 17. https://doi.org/10.3390/coatings6020017
APA StyleTaghavikish, M., Subianto, S., Dutta, N. K., & Roy Choudhury, N. (2016). Novel Thiol-Ene Hybrid Coating for Metal Protection. Coatings, 6(2), 17. https://doi.org/10.3390/coatings6020017





