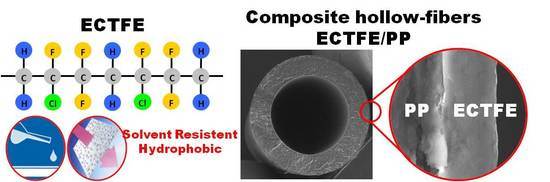
Development of Novel ECTFE Coated PP Composite Hollow-Fiber Membranes
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of the Polymeric Solution
2.3. Dip-Coating
2.4. Characterization
2.4.1. Morphology and Thickness
2.4.2. Hydrophobicity
2.4.3. Pore Size
2.4.4. Water Permeability
3. Results and Discussion
3.1. ECTFE Solubility
3.2. PP Hollow-Fibers
3.3. ECTFE/PP Composite Hollow Fiber
- 114°–111° for PP membrane dipped in a solution containing 1 wt % ECTFE;
- 109°–100° for PP membrane dipped in a solution containing 3 wt % ECTFE;
- 95°–94° for PP membrane dipped in a solution containing 5 wt % ECTFE.
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Basu, S.; Maes, M.; Cano-Odena, A.; Alaerts, L.; De Vos, D.E.; Vankelecom, I.F.J. Solvent resistant nanofiltration (SRNF) membranes based on metal-organic frameworks. J. Membr. Sci. 2009, 344, 190–198. [Google Scholar] [CrossRef]
- Baker, R.W. Overview of Membrane Science and Technology. In Membrane Technology and Applications, 3rd ed.; Baker, R.W., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 1–14. [Google Scholar]
- Uemura, T.; Henmi, M. Thin-Film Composite Membranes for Reverse Osmosis. In Advanced Membrane Technology and Applications; Li, N.N., Fane, A.G., Ho, W.S.W., Matsuura, T., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 1–19. [Google Scholar]
- Koutsou, C.P.; Karabelas, A.J.; Kostoglou, M. Membrane desalination under constant water recovery—The effect of module design parameters on system performance. Sep. Purif. Technol. 2015, 147, 90–113. [Google Scholar] [CrossRef]
- Figoli, A.; Santoro, S.; Galiano, F.; Basile, A. Pervaporation membranes: Preparation and characterization. In Vapour Permeation and Membrane Distillation Principles and Applications; Basile, A., Figoli, A., Khayet, M., Eds.; Elsevier Woodhead Publishing: Kidlington, UK, 2015; pp. 19–63. [Google Scholar]
- Othman, R.; Mohammad, A.W.; Ismail, M.; Salimon, J. Application of polymeric solvent resistant nanofiltration membranes for biodiesel production. J. Membr. Sci. 2010, 348, 287–297. [Google Scholar] [CrossRef]
- Smitha, B.; Suhanya, D.; Sridhar, S.; Ramakrishna, M. Separation of organic–organic mixtures by pervaporation—A review. J. Membr. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Drobek, M.; Figoli, A.; Santoro, S.; Navascués, N.; Motuzas, J.; Simone, S.; Algieri, C.; Gaeta, N.; Querze, L.; Trotta, A.; et al. PVDF-MFI mixed matrix membranes as VOCs adsorbers. Microporous Mesoporous Mater. 2015, 207, 126–133. [Google Scholar] [CrossRef]
- Ramaiah, K.P.; Satyasri, D.; Sridhar, S.; Krishnaiah, A. Removal of hazardous chlorinated VOCs from aqueous solutions using novel ZSM-5 loaded PDMS/PVDF composite membrane consisting of three hydrophobic layers. J. Hazard. Mater. 2013, 261, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Halar® ECTFE Ethylene-Chlorotrifluoroethylene Design and Processing Guide. Solvay Technical Sheets. Available online: http://www.solvaysolexis.com (accessed on 10 July 2016).
- Carretier, S.; Chen, L.-A.; Venault, A.; Yang, Z.-R.; Aimar, P.; Chang, Y. Design of PVDF/PEGMA-b-PS-b-PEGMA membranes by VIPS for improved biofouling mitigation. J. Membr. Sci. 2016, 510, 355–369. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Zhang, X.; Zheng, X.; Wu, Z. Enhanced antifouling behaviours of polyvinylidene fluoride membrane modified through blending with nano-TiO2/polyethylene glycol mixture. Appl. Surf. Sci. 2015, 345, 418–427. [Google Scholar] [CrossRef]
- Reid, K.; Dixon, M.; Pelekani, C.; Jarvis, K.; Willis, M.; Yu, Y. Biofouling control by hydrophilic surface modification of polypropylene feed spacers by plasma polymerisation. Desalination 2014, 335, 108–118. [Google Scholar] [CrossRef]
- Hausman, R.; Gullinkala, T.; Escobar, I.C. Development of copper-charged polypropylene feed spacers for biofouling control. J. Membr. Sci. 2010, 358, 114–121. [Google Scholar] [CrossRef]
- Roh, I.J.; Ramaswamy, S.; Krantz, W.B.; Greenberg, A.R. Poly(ethylene chlorotrifluoroethylene) membrane formation via thermally induced phase separation (TIPS). J. Membr. Sci. 2010, 362, 211–220. [Google Scholar] [CrossRef]
- Müller, H.-J. A new solvent resistant membrane based on ECTFE. Desalination 2006, 199, 191–192. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Greenberg, A.R.; Krantz, W.B. Fabrication of poly (ECTFE) membranes via thermally induced phase separation. J. Membr. Sci. 2002, 210, 175–180. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Santoro, S.; Galiano, F.; Alfadul, S.M.; Al-Harbi, O.A.; Drioli, E. Preparation and characterization of ECTFE solvent resistant membranes and their application in pervaporation of toluene/water mixtures. Sep. Purif. Technol. 2012, 90, 147–161. [Google Scholar] [CrossRef]
- Drioli, E.; Santoro, S.; Simone, S.; Barbieri, G.; Brunetti, A.; Macedonio, F.; Figoli, A. ECTFE membrane preparation for recovery of humidified gas streams using membrane condenser. React. Funct. Polym. 2014, 79, 1–7. [Google Scholar] [CrossRef]
- Ursino, C.; Simone, S.; Donato, L.; Santoro, S.; De Santo, M.P.; Drioli, E.; Di Nicolò, E.; Figoli, A. ECTFE membranes produced by non-toxic diluents for organic solvent filtration separation. RSC Adv. 2016, 6, 81001–81012. [Google Scholar] [CrossRef]
- Pan, J.; Xiao, C.; Huang, Q.; Wang, C.; Liu, H.; Hu, J. ECTFE porous membranes with conveniently controlled microstructures for vacuum membrane distillation. J. Mater. Chem. A 2015, 3, 45249–45257. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Q.; Tang, Y.; Lin, Y.; Wang, X. Preparation of ECTFE membranes with bicontinuous structure via TIPS method by a binary diluent. Desalin. Water Treat. 2016, 57, 17646–17657. [Google Scholar] [CrossRef]
- Wang, K.; Chung, T.; Gryta, M. Hydrophobic PVDF hollow fiber membranes with narrow pore size distribution and ultra-skin for the fresh water production through membrane distillation. Chem. Eng. Sci. 2008, 63, 2587–2594. [Google Scholar] [CrossRef]
- Atkinson, P.M.; Lloyd, D.R. Anisotropic flat sheet membrane formation via TIPS: thermal effects. J. Membr. Sci. 2000, 171, 1–18. [Google Scholar] [CrossRef]
- Lu, K.J.; Zuo, J.; Chung, T.S. Tri-bore PVDF hollow fibers with a super-hydrophobic coating for membrane distillation. J. Membr. Sci. 2016, 514, 165–175. [Google Scholar] [CrossRef]
- Hou, D.; Wang, J.; Sun, X.; Ji, Z.; Luan, Z. Preparation and properties of PVDF composite hollow fiber membranes for desalination through direct contact membrane distillation. J. Membr. Sci. 2012, 405–406, 185–200. [Google Scholar] [CrossRef]
- Rafat, M.; De, D.; Khulbe, K.C.; Nguyen, T.; Matsuura, T. Surface characterization of hollow fiber membranes used in artificial kidney. J. Appl. Polym. Sci. 2006, 101, 4386–4400. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surface to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadrzadeh, M.; Sudhölter, E.J.R.; de Smet, L.C.P.M. Surface modification methods of organic solvent nanofiltration membranes. Chem. Eng. J. 2010, 289, 562–582. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; Mahmoud, M.H.H.; Sanad, M.M.S.; Criscuoli, A.; Figoli, A.; Drioli, E. Membrane contactor as a novel technique for separation of iron ions from ilmenite leachant. Int. J. Miner. Process 2010, 96, 62–69. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, X.; Zhao, L. Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis. Membranes 2014, 4, 81–95. [Google Scholar] [CrossRef] [PubMed]









| ECTFE (wt%) | t (s) | CA (°) | dp (μm) | dLp (μm) |
|---|---|---|---|---|
| 0 | 0 | 126 ± 3 | 0.401 ± 0.006 | 0.493 ± 0.017 |
| 1 | 1 | 114 ± 2 | 0.175 ± 0.011 | 0.441 ± 0.027 |
| 1 | 3 | 113 ± 3 | 0.150 ± 0.009 | 0.464 ± 0.012 |
| 1 | 5 | 111 ± 3 | 0.133 ± 0.008 | 0.486 ± 0.024 |
| 3 | 1 | 104 ± 4 | 0.138 ± 0.010 | 0.461 ± 0.027 |
| 3 | 3 | 107 ± 5 | 0.088 ± 0.007 | 0.447 ± 0.012 |
| 3 | 5 | 100 ± 1 | 0.055 ± 0.003 | 0.511 ± 0.033 |
| 5 | 1 | 95 ± 2 | 0.095 ± 0.008 | 0.501 ± 0.019 |
| 5 | 3 | 94 ± 3 | 0.053 ± 0.003 | 0.488 ± 0.022 |
| 5 | 5 | 93 ± 2 | 0.047 ± 0.004 | 0.448 ± 0.021 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, S.; Drioli, E.; Figoli, A. Development of Novel ECTFE Coated PP Composite Hollow-Fiber Membranes. Coatings 2016, 6, 40. https://doi.org/10.3390/coatings6030040
Santoro S, Drioli E, Figoli A. Development of Novel ECTFE Coated PP Composite Hollow-Fiber Membranes. Coatings. 2016; 6(3):40. https://doi.org/10.3390/coatings6030040
Chicago/Turabian StyleSantoro, Sergio, Enrico Drioli, and Alberto Figoli. 2016. "Development of Novel ECTFE Coated PP Composite Hollow-Fiber Membranes" Coatings 6, no. 3: 40. https://doi.org/10.3390/coatings6030040