Gelatin-Based Films and Coatings for Food Packaging Applications
Abstract
:1. Introduction
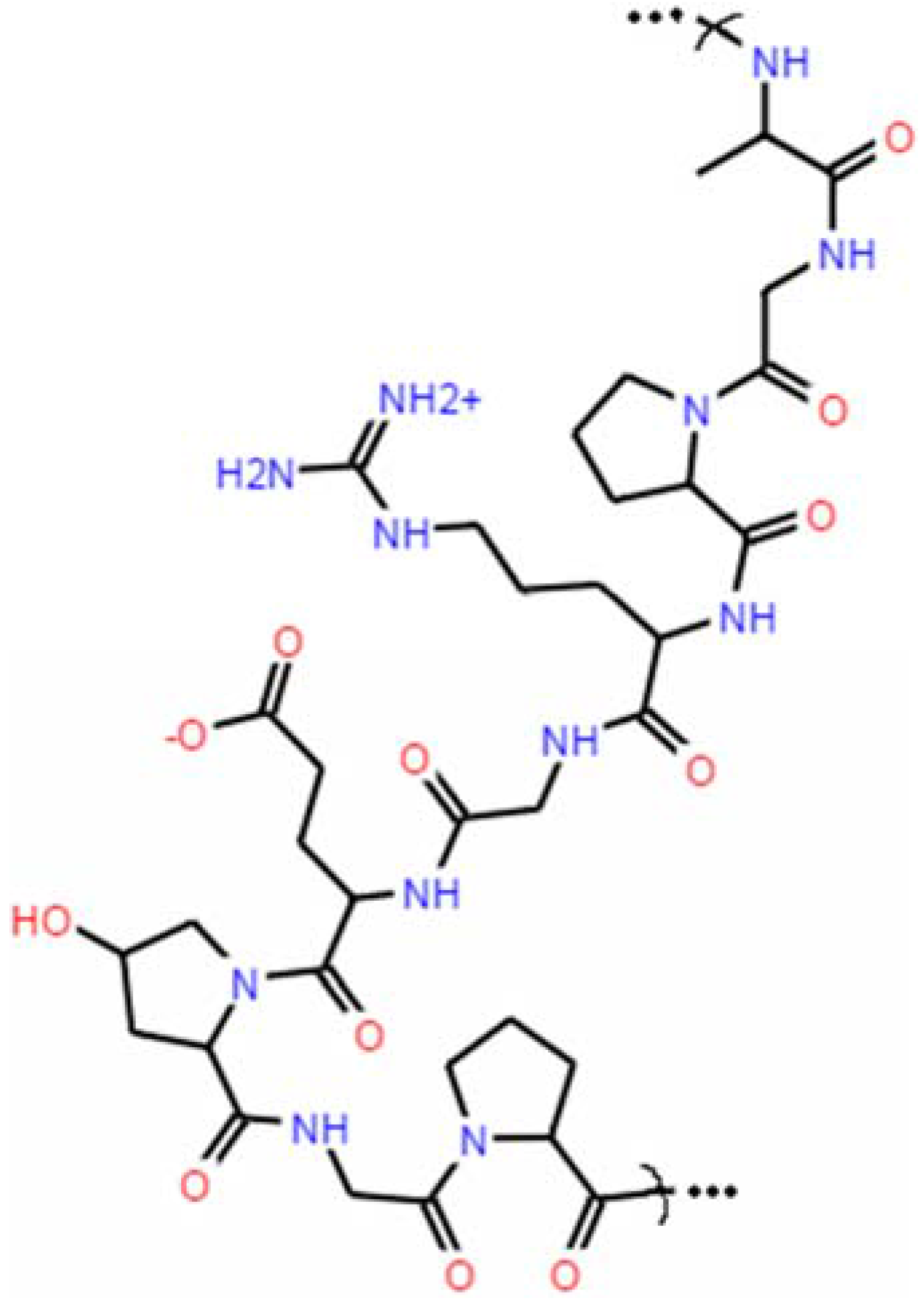
Gelatin and Film-Forming Properties
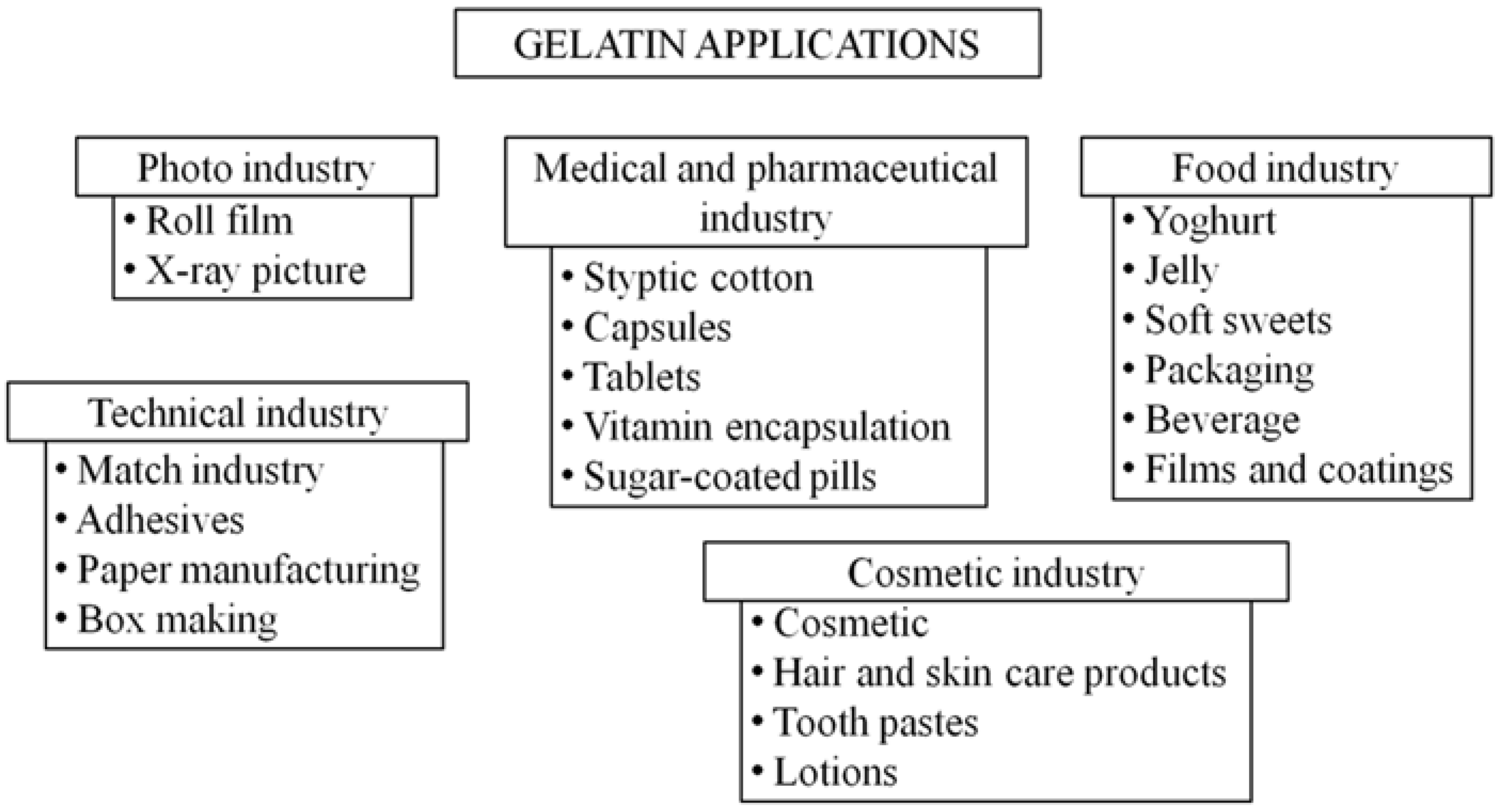
2. Gelatin-Based Films and Coatings for Food Packaging
2.1. Antimicrobial Agents
2.2. Antioxidant Agents
2.3. Other Agents
3. Edible Film and Coating Applications
3.1. Meat Products
3.2. Fishery Products
3.3. Other Food Products
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Natural polymer based cling films for food packaging. Int. J. Pharm. Pharm. Sci. 2015, 7, 10–18. [Google Scholar]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated red crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Plackett, D. Biopolymers—New Materials for Sustainable Films and Coatings; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Biscarat, J.; Charmette, C.; Sanchez, J.; Pochat-Bohatier, C. Development of a new family of food packaging bioplastics from cross-linked gelatin based films. Can. J. Chem. Eng. 2015, 93, 176–182. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; López, J.; Garrigós, M.D.C.; Valente, A.J.M.; Jiménez, A. Functional properties of sodium and calcium caseinate antimicrobial active films containing carvacrol. J. Food Eng. 2014, 121, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarzadeh, B.; Almasi, H. Biodegradable polymers. In Biodegradation—Life of Science; Chamy, R., Rosenkranz, F., Eds.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Pérez-Gago, M.B.; Rhim, J.W. Edible coating and film materials: Lipid bilayers and lipid emulsions. In Innovations in Food Packaging, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 325–350. [Google Scholar]
- Shankar, S.; Jaiswal, L.; Rhim, J.W. Gelatin-based nanocomposite films: Potential use in antimicrobial active packaging. In Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 339–348. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, A.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish gelatin: Characteristics, functional properties, applications and future potentials. Food Eng. Rev. 2014, 7, 33–44. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gareis, H.; Schrieber, R. Gelatine Handbook: Theory and Industrial Practice; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Del Carmen Garrigós, M.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Zarama, M.A.; Jiménez-Aparicio, A.R.; Solorza-Feria, J. Obtainment and partial characterization of biodegradable gelatin films with tannic acid, bentonite and glycerol. J. Sci. Food Agric. 2016, 96, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.; Yang, W.; Xue, C.; Wang, Y.; Dong, J.; Xue, Y. Modification of gelatine with galla chinensis extract, a natural crosslinker. Int. J. Food Prop. 2016, 19, 731–744. [Google Scholar] [CrossRef]
- Taylor, M.M.; Lee, J.; Bumanlag, L.P.; Latona, R.J.; Brown, E.M. Biopolymers produced from gelatin and whey protein concentrate using polyphenols. J. Am. Leather Chem. Assoc. 2014, 109, 82–88. [Google Scholar]
- Benbettaïeb, N.; Chambin, O.; Assifaoui, A.; Al-Assaf, S.; Karbowiak, T.; Debeaufort, F. Release of coumarin incorporated into chitosan-gelatin irradiated films. Food Hydrocoll. 2016, 56, 266–276. [Google Scholar] [CrossRef]
- Alemán, A.; González, F.; Arancibia, M.Y.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Comparative study between film and coating packaging based on shrimp concentrate obtained from marine industrial waste for fish sausage preservation. Food Control 2016, 70, 325–332. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Karbowiak, T.; Debeaufort, F. Release behavior of quercetin from chitosan-fish gelatin edible films influenced by electron beam irradiation. Food Control 2016, 66, 315–319. [Google Scholar] [CrossRef]
- Gupta, B.; Tummalapalli, M.; Deopura, B.L.; Alam, M.S. Preparation and characterization of in-situ crosslinked pectin-gelatin hydrogels. Carbohydr. Polym. 2014, 106, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of nile perch (lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocoll. 2012, 29, 144–151. [Google Scholar] [CrossRef]
- Jeya Shakila, R.; Jeevithan, E.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Comparison of the properties of multi-composite fish gelatin films with that of mammalian gelatin films. Food Chem. 2012, 135, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Ramos, M.; García-Serna, E.; Carmen Garrigós, M.D.; Jiménez, A. Polymers extracted from biomass. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Iahnke, A.O.S.; Costa, T.M.H.; Rios, A.O.; Flôres, S.H. Residues of minimally processed carrot and gelatin capsules: Potential materials for packaging films. Ind. Crops Prod. 2015, 76, 1071–1078. [Google Scholar] [CrossRef]
- Valdes, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jimenez, A.; Garrigos, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Sung, S.Y.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crops Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Alparslan, Y.; Yapıcı, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT Food Sci. Technol. 2016, 72, 457–466. [Google Scholar] [CrossRef]
- Yanwong, S.; Threepopnatkul, P. Effect of Peppermint and citronella essential oils on properties of fish skin gelatin edible films. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012064. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Ge, S.; Wang, S.; Qin, Z.; Chen, L.; Zheng, Q.; Liu, Q.; Zhang, Q. The preparation, characterization, antimicrobial stability and in vitro release evaluation of fish gelatin films incorporated with cinnamon essential oil nanoliposomes. Food Hydrocoll. 2015, 43, 427–435. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Ibarguren, C.; Céliz, G.; Díaz, A.S.; Bertuzzi, M.A.; Daz, M.; Audisio, M.C. Gelatine based films added with bacteriocins and a flavonoid ester active against food-borne pathogens. Innov. Food Sci. Emerg. Technol. 2015, 28, 66–72. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Rhim, J.W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing agnps and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.W. Preparation, characterization, and antimicrobial activity of gelatin/zno nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, J.H.; Yang, H.J.; Song, K.B. Production and characterisation of skate skin gelatin films incorporated with thyme essential oil and their application in chicken tenderloin packaging. Int. J. Food Sci. Technol. 2016, 51, 1465–1472. [Google Scholar] [CrossRef]
- Shakila, R.J.; Jeevithan, E.; Arumugam, V.; Jeyasekaran, G. Suitability of antimicrobial grouper bone gelatin films as edible coatings for vacuum-packaged fish steaks. J. Aquat. Food Prod. Technol. 2016, 25, 724–734. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Kadam, S.U.; Pankaj, S.K.; Tiwari, B.K.; Cullen, P.J.; O’Donnell, C.P. Development of biopolymer-based gelatin and casein films incorporating brown seaweed ascophyllum nodosum extract. Food Packag. Shelf Life 2015, 6, 68–74. [Google Scholar] [CrossRef]
- Iahnke, A.O.E.S.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Antioxidant films based on gelatin capsules and minimally processed beet root (Beta vulgaris L. Var. Conditiva) residues. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Biendl, M. Physicochemical and antioxidant properties of biopolymer/candelilla wax emulsion films containing hop extract—A comparative study. Food Hydrocoll. 2016, 60, 384–392. [Google Scholar] [CrossRef]
- Liu, F.; Antoniou, J.; Li, Y.; Yi, J.; Yokoyama, W.; Ma, J.; Zhong, F. Preparation of gelatin films incorporated with tea polyphenol nanoparticles for enhancing controlled-release antioxidant properties. J. Agric. Food Chem. 2015, 63, 3987–3995. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, A.; Ye, R.; Li, X.; Han, Y.; Liu, C. The production of gelatin-calcium carbonate composite films with different antioxidants. Int. J. Food Prop. 2015, 18, 2442–2456. [Google Scholar] [CrossRef]
- Peña-Rodriguez, C.; Martucci, J.F.; Neira, L.M.; Arbelaiz, A.; Eceiza, A.; Ruseckaite, R.A. Functional properties and in vitro antioxidant and antibacterial effectiveness of pigskin gelatin films incorporated with hydrolysable chestnut tannin. Food Sci. Technol. Int. 2015, 21, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.; Molinaro, S.; Tyuftin, A.; Bolton, D.; Fanning, S.; Kerry, J.P. Incorporation of commercially-derived antimicrobials into gelatin-based films and assessment of their antimicrobial activity and impact on physical film properties. Food Control 2016, 64, 202–211. [Google Scholar] [CrossRef]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Garrigós, M.C. Chapter 26 Carvacrol-based films: Usage and potential in antimicrobial packaging. In Antimicrobial Food Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 329–338. [Google Scholar]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Antioxidant potential of spices and their active constituents. Crit. Rev. Food Sci. Nutr. 2012, 54, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Ngamakeue, N.; Chitprasert, P. Encapsulation of holy basil essential oil in gelatin: Effects of palmitic acid in carboxymethyl cellulose emulsion coating on antioxidant and antimicrobial activities. Food Bioprocess Technol. 2016, 9, 1735–1745. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as agricultural polymers for packaging production. Cereal Chem. J. 1998, 75, 1–9. [Google Scholar] [CrossRef]
- Limpisophon, K.; Tanaka, M.; Osako, K. Characterisation of gelatin-fatty acid emulsion films based on blue shark (prionace glauca) skin gelatin. Food Chem. 2010, 122, 1095–1101. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Emulsion film based on fish skin gelatin and palm oil: Physical, structural and thermal properties. Food Hydrocoll. 2015, 48, 248–259. [Google Scholar] [CrossRef]
- Bertan, L.C.; Tanada-Palmu, P.S.; Siani, A.C.; Grosso, C.R.F. Effect of fatty acids and “brazilian elemi” on composite films based on gelatin. Food Hydrocoll. 2005, 19, 73–82. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Wang, Q.; Liu, F.; Wei, Z.-H. Characterization of gelatin-based edible films incorporated with olive oil. Food Res. Int. 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, W.; Wang, K.; Liu, Y.; Liu, A.; Zhang, S.; Zhao, Y. Impact of melting point of palm oil on mechanical and water barrier properties of gelatin-palm oil emulsion film. Food Hydrocoll. 2016, 60, 243–251. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Emulsion stability and properties of fish gelatin-based films as affected by palm oil and surfactants. J. Sci. Food Agric. 2016, 96, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, morphological and thermal behaviour characterisations of fish gelatin film incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Wang, L.; Auty, M.A.E.; Rau, A.; Kerry, J.F.; Kerry, J.P. Effect of ph and addition of corn oil on the properties of gelatin-based biopolymer films. J. Food Eng. 2009, 90, 11–19. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-anan, M. An approach for the enhancement of the mechanical properties and film coating efficiency of shellac by the formation of composite films based on shellac and gelatin. J. Food Eng. 2012, 108, 94–102. [Google Scholar] [CrossRef]
- Al-Hassan, A.A.; Norziah, M.H. Starch-gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Tao, Z.; Weng, W.-Y.; Cao, M.-J.; Liu, G.-M.; Su, W.-J.; Osako, K.; Tanaka, M. Effect of blend ratio and pH on the physical properties of edible composite films prepared from silver carp surimi and skin gelatin. J. Food Sci. Technol. 2015, 52, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, X.; Lu, Y.; Dong, S.; Zhao, Y.; Zeng, M. Effects of chitosan molecular weight and degree of deacetylation on the properties of gelatine-based films. Food Hydrocoll. 2012, 26, 311–317. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin–chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Physical and functional characterization of active fish gelatin films incorporated with lignin. Food Hydrocoll. 2013, 30, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Role of lignosulphonate in properties of fish gelatin films. Food Hydrocoll. 2012, 27, 60–71. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Osako, K. Development and characterisation of blend films based on fish protein isolate and fish skin gelatin. Food Hydrocoll. 2014, 39, 58–67. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khanjanzadeh, H.; Salari, A. Effect of using walnut/almond shells on the physical, mechanical properties and formaldehyde emission of particleboard. Compos. Part B 2013, 45, 858–863. [Google Scholar] [CrossRef]
- Valdés, A.; Fenollar, O.; Beltrán, A.; Balart, R.; Fortunati, E.; Kenny, J.M.; Garrigós, M.C. Characterization and enzymatic degradation study of poly(ε-caprolactone)-based biocomposites from almond agricultural by-products. Polym. Degrad. Stab. 2016, 132, 181–190. [Google Scholar] [CrossRef]
- Valdés García, A.; Ramos Santonja, M.; Sanahuja, A.B.; Del Carmen Garrigós Selva, M. Characterization and degradation characteristics of poly(ε-caprolactone)-based composites reinforced with almond skin residues. Polym. Degrad. Stab. 2014, 108, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Beltrán, A.; Garrigós, M.C. Potential Use of Nut Agricultural by-Products in Polymer Materials: A Review. In Agricultural Wastes: Characteristics, Types and Management; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 87–106. [Google Scholar]
- Vázquez, H.; Canché-Escamilla, G.; Cruz-Ramos, C.A. Coconut husk lignin. I extraction and characterisation. J. Appl. Polym. Sci. 1992, 45, 633–644. [Google Scholar]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties and characteristics of nanocomposite films from tilapia skin gelatin incorporated with ethanolic extract from coconut husk. J. Food Sci. Technol. 2015, 52, 7669–7682. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W.; Kim, Y.-T. Biopolymer-based composite packaging materials with nanoparticles. In Innovations in Food Packaging; Elsevier Academic Press: London, UK, 2014. [Google Scholar]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Preparation and characterization of chitosan nanoparticles-loaded fish gelatin-based edible films. J. Food Proc. Eng. 2015, 39, 521–530. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Panzavolta, S.; Gioffrè, M.; Bracci, B.; Rubini, K.; Bigi, A. Montmorillonite reinforced type a gelatin nanocomposites. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Coronado Jorge, M.F.; Alexandre, E.M.C.; Caicedo Flaker, C.H.; Bittante, A.M.Q.B.; Sobral, P.J.D.A. Biodegradable films based on gelatin and montmorillonite produced by spreading. Int. J. Polym. Sci. 2015, 2015, 806791. [Google Scholar] [CrossRef]
- Ge, L.; Li, X.; Zhang, R.; Yang, T.; Ye, X.; Li, D.; Mu, C. Development and characterization of dialdehyde xanthan gum crosslinked gelatin based edible films incorporated with amino-functionalized montmorillonite. Food Hydrocoll. 2015, 51, 129–135. [Google Scholar] [CrossRef]
- Li, X.; Liu, A.; Ye, R.; Wang, Y.; Wang, W. Fabrication of gelatin-laponite composite films: Effect of the concentration of laponite on physical properties and the freshness of meat during storage. Food Hydrocoll. 2015, 44, 390–398. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Gelatin based films capable of modifying its color against environmental ph changes. Food Hydrocoll. 2016, 61, 523–530. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.D.L.S.; Gomide, L.A.D.M.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kerry, J.F.; Kerry, J.P. Application and assessment of extruded edible casings manufactured from pectin and gelatin/sodium alginate blends for use with breakfast pork sausage. Meat Sci. 2007, 75, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Herring, J.L.; Jonnalongadda, S.C.; Narayanan, V.C.; Coleman, S.M. Oxidative stability of gelatin coated pork at refrigerated storage. Meat Sci. 2010, 85, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Tyburcy, A.; Kozyra, D. Effects of composite surface coating and pre-drying on the properties of kabanosy dry sausage. Meat Sci. 2010, 86, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Yang, H.-J.; Lee, K.-Y.; Song, K.B. Physical properties and application of a red pepper seed meal protein composite film containing oregano oil. Food Hydrocoll. 2016, 55, 136–143. [Google Scholar] [CrossRef]
- De Oliveira, M.M.M.; Brugnera, D.F.; Piccoli, R.H. Essential oils of thyme and rosemary in the control of listeria monocytogenes in raw beef. Braz. J. Microbiol. 2013, 44, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Min, B.J.; Han, I.Y.; Dawson, P.L. Antimicrobial gelatin films reduce listeria monocytogenes on turkey bologna. Poult. Sci. 2010, 89, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; López de Lacey, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and ziziphora clinopodioides essential oil on survival of listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Alparslan, Y.; Baygar, T.; Hasanhocaoglu, H.; Metin, C. Effects of gelatin-based edible films enriched with laurel essential oil on the quality of rainbow trout (oncorhynchus mykiss) fillets during refrigerated storage. Food Technol. Biotechnol. 2014, 52, 325–333. [Google Scholar]
- Ojagh, S.M.; Núñez-Flores, R.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Lessening of high-pressure-induced changes in atlantic salmon muscle by the combined use of a fish gelatin-lignin film. Food Chem. 2011, 125, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Song, H.Y.; Shin, Y.J.; Song, K.B. Preparation of a barley bran protein-gelatin composite film containing grapefruit seed extract and its application in salmon packaging. J. Food Eng. 2012, 113, 541–547. [Google Scholar] [CrossRef]
- Song, N.-B.; Lee, J.-H.; Al Mijan, M.; Song, K.B. Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT Food Sci. Technol. 2014, 57, 453–460. [Google Scholar] [CrossRef]
- Davis, C.G.; Lin, B.-H. Factors Affecting US Pork Consumption; United States Department of Agriculture Economic Research Service (USDA/ERS): Washington, DC, USA, 2005.
- Mor-Mur, M.; Yuste, J. Emerging bacterial pathogens in meat and poultry: An overview. Food Bioprocess Technol. 2010, 3, 24–35. [Google Scholar] [CrossRef]
- Chung, C.-K.; Zuo, L. Method for Refreshing Preserved Pork by Using Edible Composite Antibacterial Film. Patent CN 102487988B, 29 April 2013. [Google Scholar]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Welt, B.A.; Ralat, M.; Marshall, M.R. Effect of high pressure processing and cooking treatment on the quality of atlantic salmon. Food Chem. 2009, 116, 828–835. [Google Scholar] [CrossRef]
- Rotariu, O.; Thomas, D.J.I.; Goodburn, K.E.; Hutchison, M.L.; Strachan, N.J.C. Smoked salmon industry practices and their association with listeria monocytogenes. Food Control 2014, 35, 284–292. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Ranadheera, C.S.; Wijerathne, T.D.; Udayangani, R.M.C.; Himali, S.M.C.; Pickova, J. Applications of seafood by-products in the food industry and human nutrition. In Seafood Processing by-Products: Trends and Applications; Kim, S.-K., Ed.; Springer: New York, NY, USA, 2014; pp. 463–528. [Google Scholar]
- Song, L. Edible Red Bean Film. Patent CN 103589173A, 19 February 2014. [Google Scholar]
- Song, L. Edible Packaging Film for Garlic. Patent CN 103589168A, 19 February 2014. [Google Scholar]
- Wang, X.; Kong, D.; Ma, Z.; Zhao, R. Effect of carrot puree edible films on quality preservation of fresh-cut carrots. Irish J. Agric. Food Res. 2015, 54, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Feng, X.; Han, P.; Duan, X. Effect of propolis/nano-silica composite coating on activities of ripening and senescence related enzymes in cherry tomato fruits. J. Chin. Inst. Food Sci. Technol. 2016, 16, 159–165. [Google Scholar]
- Licodiedoff, S.; Koslowski, L.A.D.; Scartazzini, L.; Monteiro, A.R.; Ninow, J.L.; Borges, C.D. Conservation of physalis by edible coating of gelatin and calcium chloride. Int. Food Res. J. 2016, 23, 1629–1634. [Google Scholar]
- Youssef, A.R.M.; Ali, E.A.M.; Emam, H.E. Influence of postharvest applications of some edible coating on storage life and quality attributes of navel orange fruit during cold storage. Int. J. Chem. Technol. Res. 2015, 8, 2189–2200. [Google Scholar]
- Andrade, R.; Skurtys, O.; Osorio, F. Drop impact of gelatin coating formulated with cellulose nanofibers on banana and eggplant epicarps. LWT Food Sci. Technol. 2015, 61, 422–429. [Google Scholar] [CrossRef]
- Poverenov, E.; Rutenberg, R.; Danino, S.; Horev, B.; Rodov, V. Gelatin-chitosan composite films and edible coatings to enhance the quality of food products: Layer-by-layer vs. Blended formulations. Food Bioprocess Technol. 2014, 7, 3319–3327. [Google Scholar] [CrossRef]
- Poverenov, E.; Zaitsev, Y.; Arnon, H.; Granit, R.; Alkalai-Tuvia, S.; Perzelan, Y.; Weinberg, T.; Fallik, E. Effects of a composite chitosan-gelatin edible coating on postharvest quality and storability of red bell peppers. Postharvest Biol. Technol. 2014, 96, 106–109. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Casari, A.C.A.; Mariano, M.; Yamashita, F.; Mei, L.H.I.; Soldi, V.; Martelli, S.M. Effect of a gelatin-based edible coating containing cellulose nanocrystals (CNC) on the quality and nutrient retention of fresh strawberries during storage. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64. [Google Scholar] [CrossRef]
- Feng, D.; Zhengguang, W.; Yimei, Z.; Xiang, Z.; Meng, G.X.; Xu, Y.; Bi, Y. Effect of chitosan composite coating on chinese blueberry fruit (Vaccinium uliginosum L.). Acta Hortic. 2014, 1053, 207–214. [Google Scholar] [CrossRef]
- Bizura Hasida, M.R.; Nur Aida, M.P.; Zaipun, M.Z.; Hairiyah, M. Quality evaluation of fresh-cut “josapine” pineapple coated with hydrocolloid based edible coating using gelatin. Acta Hortic. 2013, 1012, 1037–1042. [Google Scholar] [CrossRef]
- Neves, A.C.V., Jr.; Coneglian, R.C.C.; Soares, A.G.; Freitas, D.G.C.; Fonseca, M.J.O.; Barreira, F.R.; De Miranda, A.F.M. Physical and sensory characterization of edible coatings applied to minimally processed persimmon. Acta Hortic. 2012, 934, 537–542. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- De Moraes Crizel, T.; Haas Costa, T.M.; de Oliveira Rios, A.; Hickmann Flôres, S. Valorization of food-grade industrial waste in the obtaining active biodegradable films for packaging. Ind. Crops Prod. 2016, 87, 218–228. [Google Scholar] [CrossRef]

| Gelatin | Active Additive | Application | Main Benefits | Ref. |
|---|---|---|---|---|
| Fish gelatin | Origanum vulgare L. essential oil | Films | Enhancement in WVP, solubility, barrier capability to ultraviolet light | [37] |
| Enhancement in antimicrobial properties | ||||
| Fish gelatin | Nanoencapsulated Origanum vulgare L. essential oil | Films | Maintenance of initial thermal stability | [38] |
| Less resistant and more flexible films | ||||
| Decrease in WVP | ||||
| Exhibited antimicrobial activity | ||||
| Bovine gelatin | Bacteriocins and flavonoid ester prunin laurate | Films | Maintenance of functional properties | [39] |
| Enhancement in antimicrobial properties and synergistic effect | ||||
| Gelatin | Silver nanoparticles | Films | Enhancement in hydrophobicity, water | [40,41] |
| vapour and UV barrier | ||||
| Compact surface structure | ||||
| Strong antibacterial activity | ||||
| Gelatin | Zinc oxide nanoparticles | Films | Crystalline structure | [42] |
| Enhancement in thermal stability, moisture content, water contact angle, WVP and elongation at break | ||||
| Strong antibacterial activity | ||||
| Skate skin gelatin | Thyme essential oil | Chicken tenderloin (wrap) | Enhancement in antimicrobial properties | [43] |
| Extend shelf-life of chicken tenderloin | ||||
| Increase in elongation at break | ||||
| Grouper bone gelatin | Chitosan, clove and pepper essential oils | Fish steaks (coating) | Enhancement in antimicrobial properties | [44] |
| Extend the shelf-life of fish steaks | ||||
| Fish gelatin | Cinnamon essential oil nanoliposomes | Films | Decrease in tensile strength, water soluble, water content and WVP | [36] |
| Sustained release effect and improvement in antimicrobial stability | ||||
| Fish skin gelatin | Peppermint and citronella essential oils | Films | Enhancement in antimicrobial properties | [33] |
| Fish gelatin | Green tea, grape seed, ginger or gingko leaf | Films | Enhancement in antioxidant properties | [45] |
| Bovine gelatin | Brown seaweed Ascophyllum nodosum | Films | Increase in hydrophilicity | [46] |
| Enhancement in antioxidant properties | ||||
| Residues of gelatin capsules | Beet root residue powder | Films | Enhancement in antioxidant properties | [47] |
| Maintenance of initial thermal stability | ||||
| Bovine gelatin residue | Carrot residue fibre derived from minimally processed carrots | Films | High barrier, optical and thermal properties | [27] |
| Capacity for protecting sunflower oil from primary rancidity reactions | ||||
| Pork gelatin | Ethanolic hop extract | Films | Enhancement in antioxidant properties | [48] |
| Gelatin | Free/encapsulated tea polyphenols | Sunflower oil packaging | No significant differences in visual aspect | [49] |
| Enhancement in antioxidant properties | ||||
| Good oxidation inhibitory effect over 6 weeks of storage | ||||
| Gelatin | Tea polyphenols | Films | Enhancement in antioxidant properties | [50] |
| Pig skin gelatin | Hydrolysable chestnut tannin | Films | Enhancement in antimicrobial and antioxidant properties | [51] |
| Beef gelatin | Articoat DLP 02, Artemix Consa 152/NL, Auranta FV and sodium octanoate | Films | Enhancement in antimicrobial and antioxidant properties at different degrees | [52] |
| Enhancement in oxygen transmission rate | ||||
| Food grade gelatin | Orange leaf essential oil | Shrimps (coating) | Shelf-life extension | [32] |
| Enhancement in antimicrobial and antioxidant properties | ||||
| Bovine hide gelatin | Oregano and lavender essential oils | Films | Enhancement in antimicrobial and antioxidant properties | [31] |
| Food Applicability | Product | Matrix | Processing Method | Final Product | Ref. |
|---|---|---|---|---|---|
| Meat products | Beef steaks | Bovine gelatin type B mixed with chitosan | Dipping into matrix solution | Coating | [92] |
| Pork sausages | Gelatin, pectin and sodium alginate blends | Extrusion | Film | [93] | |
| Pork loin | Porcine gelatin | Dipping into gelatin matrix | Coating | [94] | |
| Kabanosy dry sausages | Pork gelatin, kappa-carrageenan and glycerol | Dipping into matrix solution | Coating | [95] | |
| Chicken tenderloin | Skate skin gelatin with thyme essential oil | Casting | Film | [96] | |
| Raw beef | Gelatin, Tween 80 and essential oils of Thymus vulgaris and Rosmarinus officinalis | Dipping into matrix solution | Coating | [97] | |
| Turkey bologna | Gelatin, glycerol and Nisaplin and Guardian CS1-50 antimicrobial additives | Casting | Film | [98] | |
| Bacon | Gelatin | Casting | Film | [99] | |
| Fishery products | Rainbow trout | Cold water fish skin, chitosan and glycerol | Casting and dipping into matrix solution | Film and coating | [100] |
| Cod fillets | Bovine hide gelatin, chitosan, sorbitol and glycerol with clove essential oil | Casting | Film | [99] | |
| Minced trout fillets | Cold water fish skin gelatin, chitosan, glycerol, red grape seed extract and Ziziphora linopodioides essential oil | Casting | Film | [101] | |
| Rainbow trout fillets | Food grade gelatin, glycerol, sorbitol, Tween 20 and laurel leaf essential oil | Casting | Film | [102] | |
| Tuna meat | Gelatin, red pepper seed meal protein and several plasticizers (glycerol, sorbitol, fructose and sucrose) | Casting | Film | [96] | |
| Fish sausages | Warm-water fish gelatin, chitosan, shrimp concentrate, Tween 80 and glycerol | Casting | Film | [19] | |
| Atlantic Salmon | Warm-water fish gelatin, lignin, sorbitol and glycerol | Casting | Film | [103] | |
| Salmon | Porcine skin gelatin, barley bran protein, sorbitol and grapefruit seed extract | Casting | Film | [104] | |
| Cold smoked Salmon | Pork gelatin, chicken feather protein, sorbitol and clove oil | Casting | Film | [105] | |
| Shrimps | Gelatin, glycerol, sorbitol, Tween 20 and orange leaf extract | Dipping into matrix solution | Coating | [32] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.C. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. https://doi.org/10.3390/coatings6040041
Ramos M, Valdés A, Beltrán A, Garrigós MC. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings. 2016; 6(4):41. https://doi.org/10.3390/coatings6040041
Chicago/Turabian StyleRamos, Marina, Arantzazu Valdés, Ana Beltrán, and María Carmen Garrigós. 2016. "Gelatin-Based Films and Coatings for Food Packaging Applications" Coatings 6, no. 4: 41. https://doi.org/10.3390/coatings6040041







