Evaluation of Accelerated Ageing Tests for Metallic and Non-Metallic Graffiti Paints Applied to Stone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials: Graffiti Spray Paint and Building Stone
- Blanco Cristal granite (igneous rock) from Cadalso de los Vidrios (Madrid, Spain), a white leucogranite, heterogranular-panallatriomorphic medium-grained biotite adamellitic granite. The essential components of this granite are quartz (26%), K-feldspar (29%), plagioclases (27.5%), biotite (9%), muscovite (2%) and chlorite (4.5%) as an accessory mineral [16,17]. Granite is particularly common in Galicia (where this lithotype constitutes almost 50% of the geological substrate), northern Portugal, Brittany and Ireland. Many of the buildings and monuments in these areas are built from this material.
- Rhyolitic ignimbrite (igneous rock), extrusive or volcanic rock equivalent to granite from Central America. Its composition is very varied, consisting of a matrix based on silicates and Na-feldspar, K-feldspar and Ca-feldspar, with some pyroclastic products in the form of quartz, pumice and volcanic glass and some accessory mineral grains such as chlorite, sericite, hematite and leucoxene. It was used very extensively in prehispanic Mexican monuments, e.g., the Tiristán monuments (Quinceo, Michoacan) and a cosmogonic stone, which is on display in the Section of Archeology of the Michoacan State Museum.
- The stone of Puerto de Santa María (sedimentary rock) from Cádiz (Spain) is a calcarenite bio-esparithic or fossiliferous limestone (biocalcarenite) with a highly variable silica/calcium carbonate ratio, of colour ranging between greyish and yellowish. The texture is grainy, with fine to medium grain size, and a higher proportion of bioclasts and quartz grains [18,19]. This type of stone is found e.g., in the Cathedral of Seville and Cadiz (Andalusia, Spain). In the 17th century, this type of stone was considered the strongest in the area and was used for constructing pillars and columns and not as ornamental stone.
2.2. Ageing Trials
2.2.1. Humidity Cycles
2.2.2. Freeze-Thawing Cycles
2.2.3. NaCl and Na2SO4 Salt Cycles
- Full immersion of the test pieces in a 14% salt solution for 2 h;
- Removal of the specimens from the solution and transfer to an oven at 40 °C for 16 h;
- Removal of the test pieces from the oven and maintenance at room temperature for 3 h in a room that does not exceed 80% relative humidity (RH).
2.3. Analytical Techniques Used to Evaluate the Effects of the Accelerated Ageing
2.3.1. Characterization of the Physical Properties
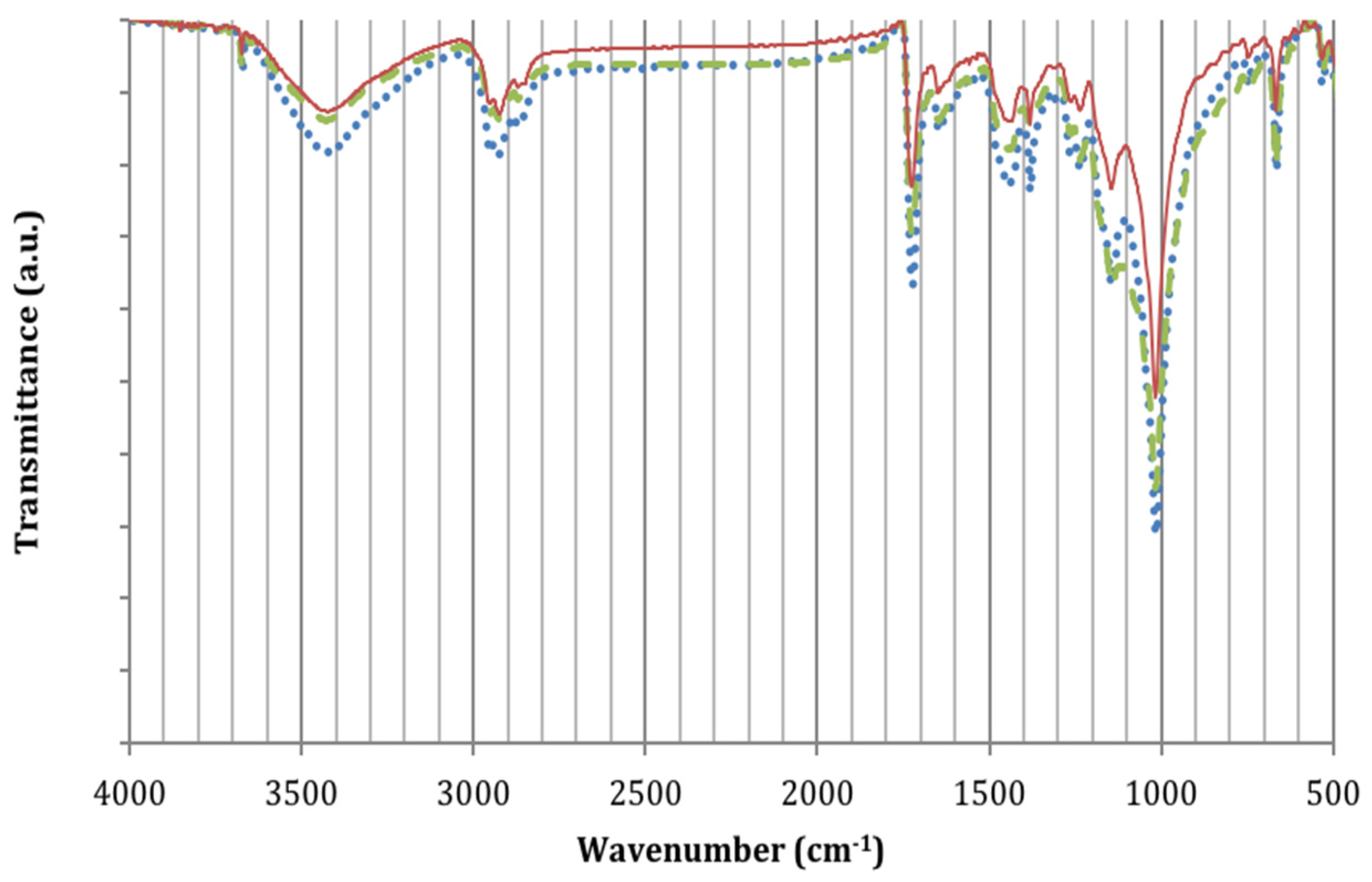
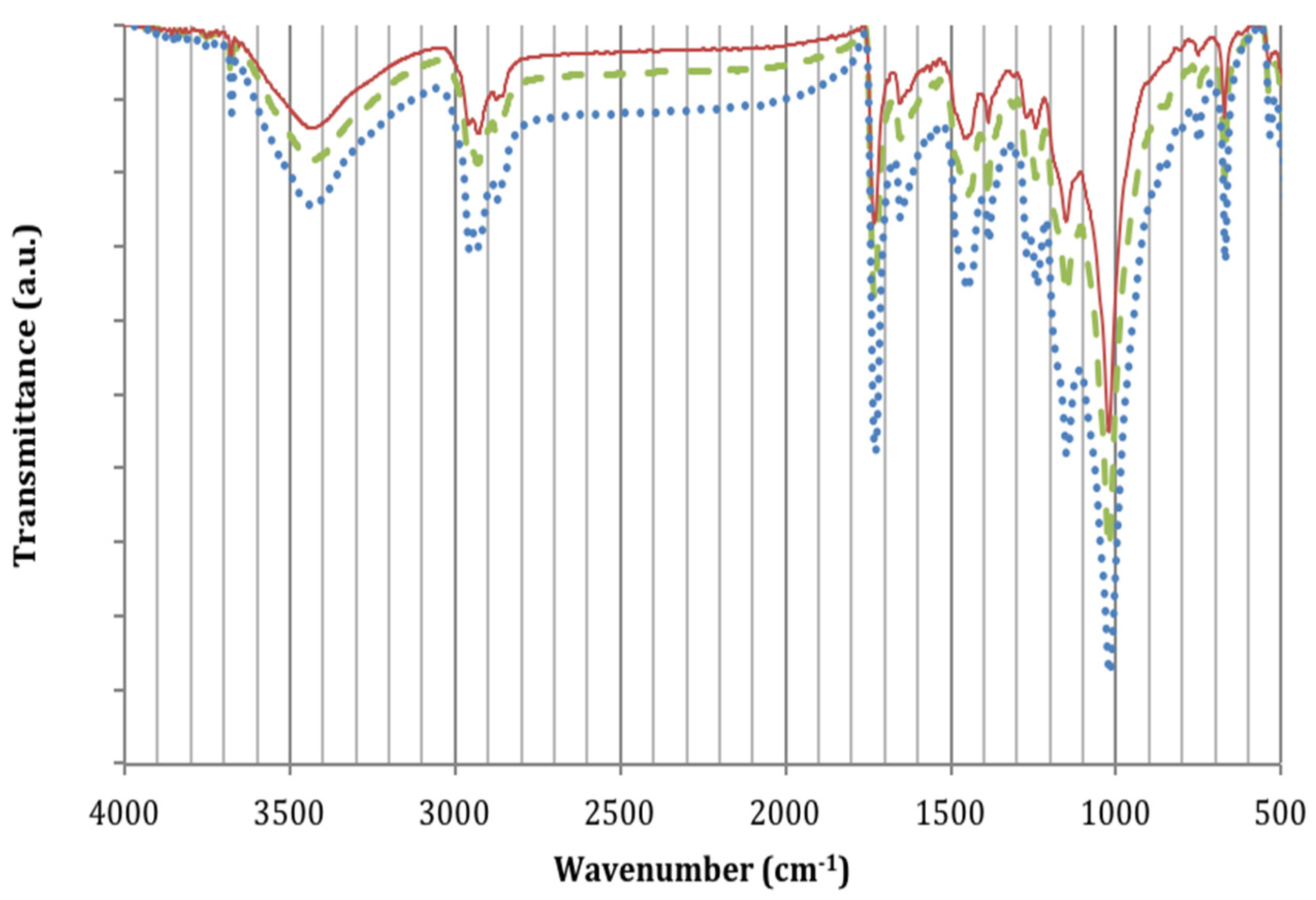
2.3.2. Infrared Spectroscopy
2.3.3. Statistical Analysis
3. Results and Discussion
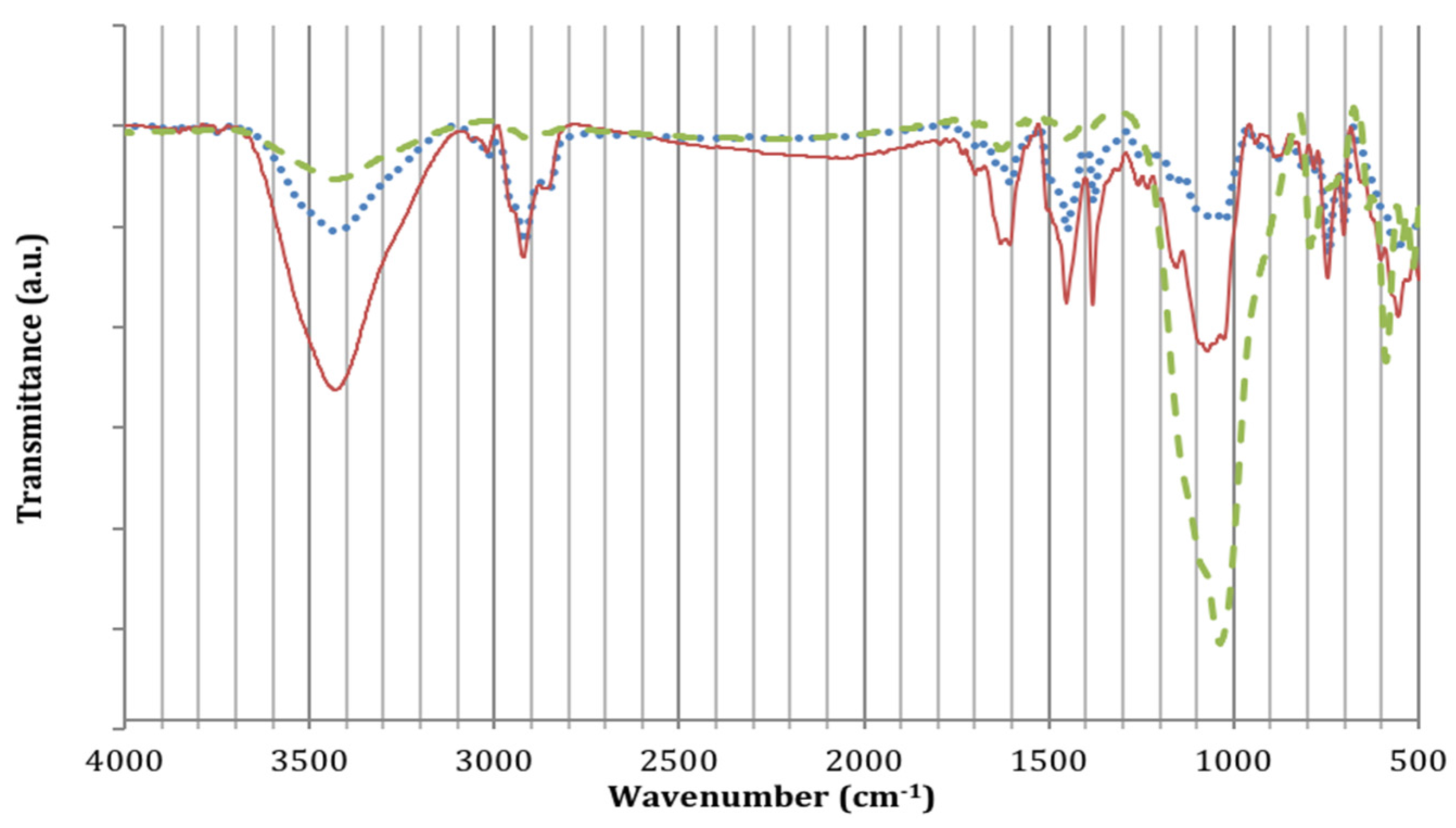
3.1. Characterization of Graffiti Paints
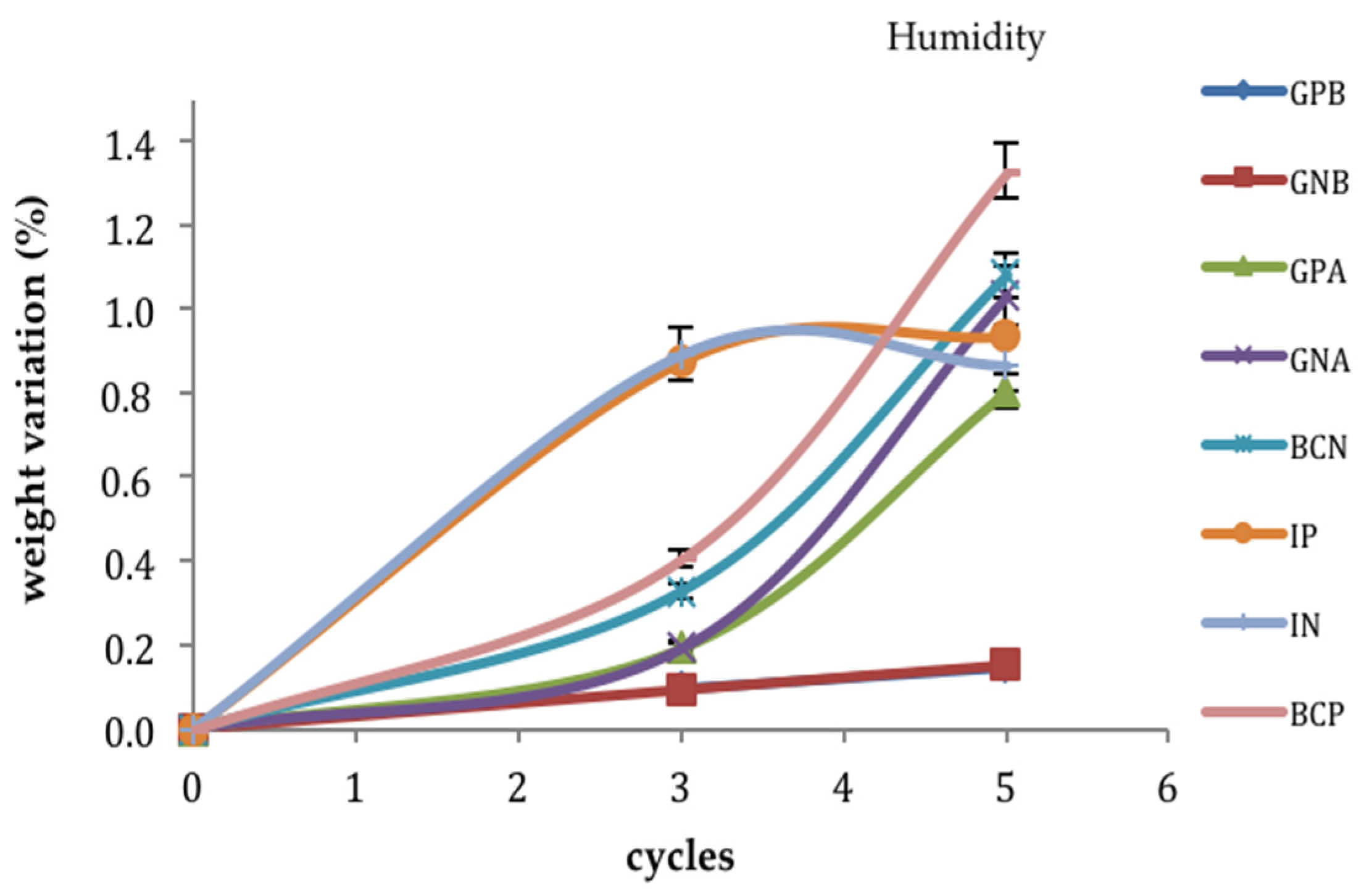
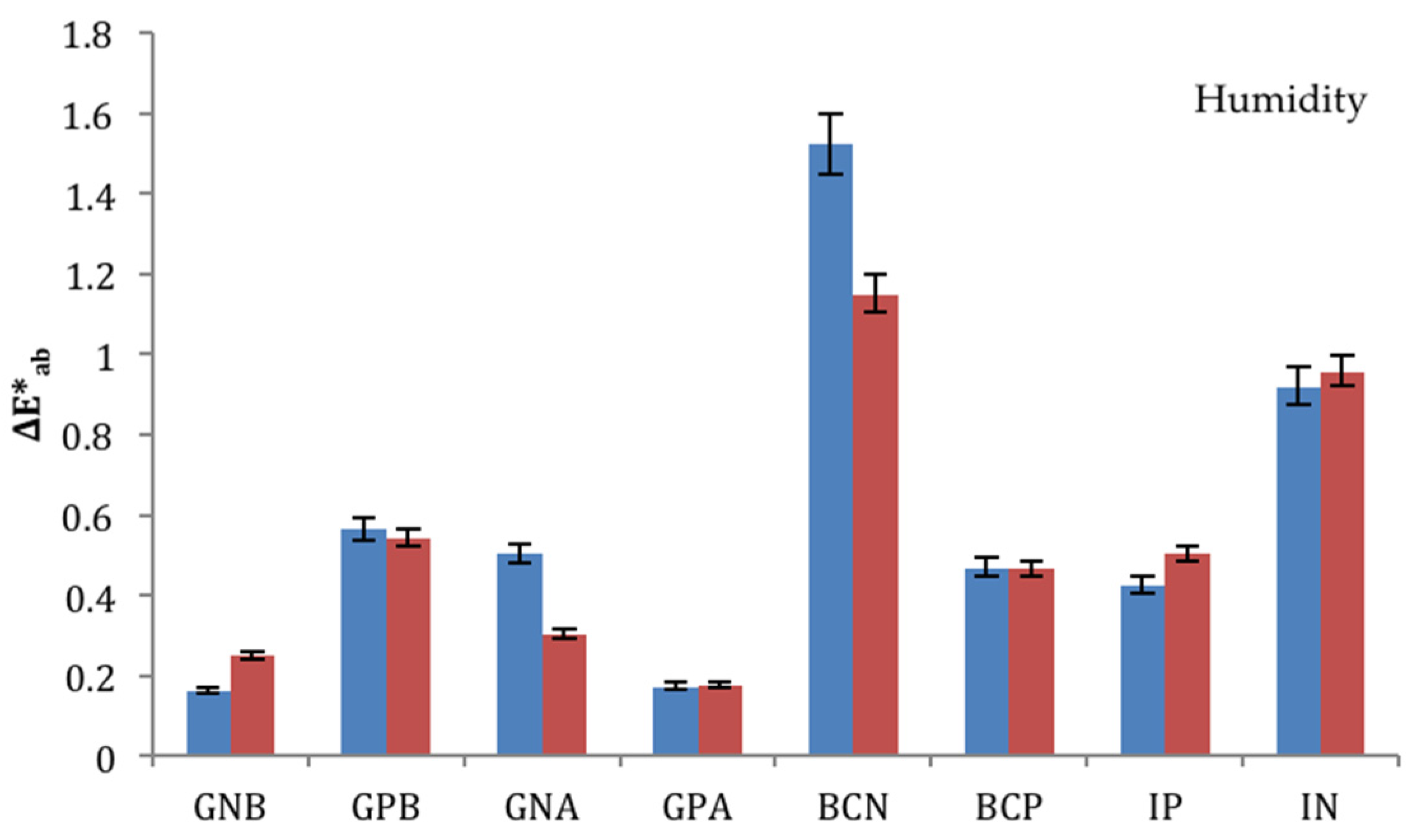
3.2. Ageing Trials: Humidity
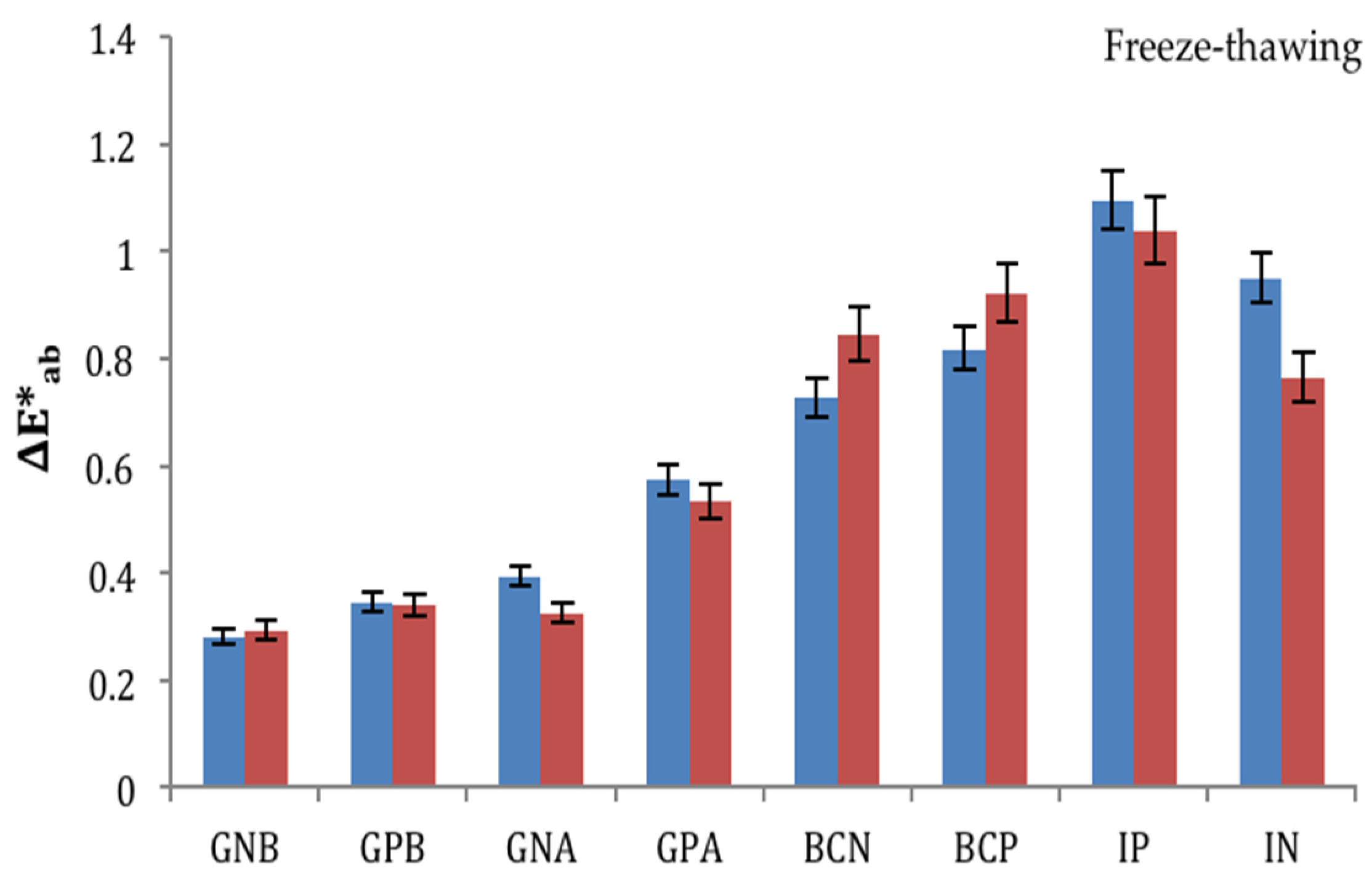
3.3. Ageing Trials: Freeze-Thawing
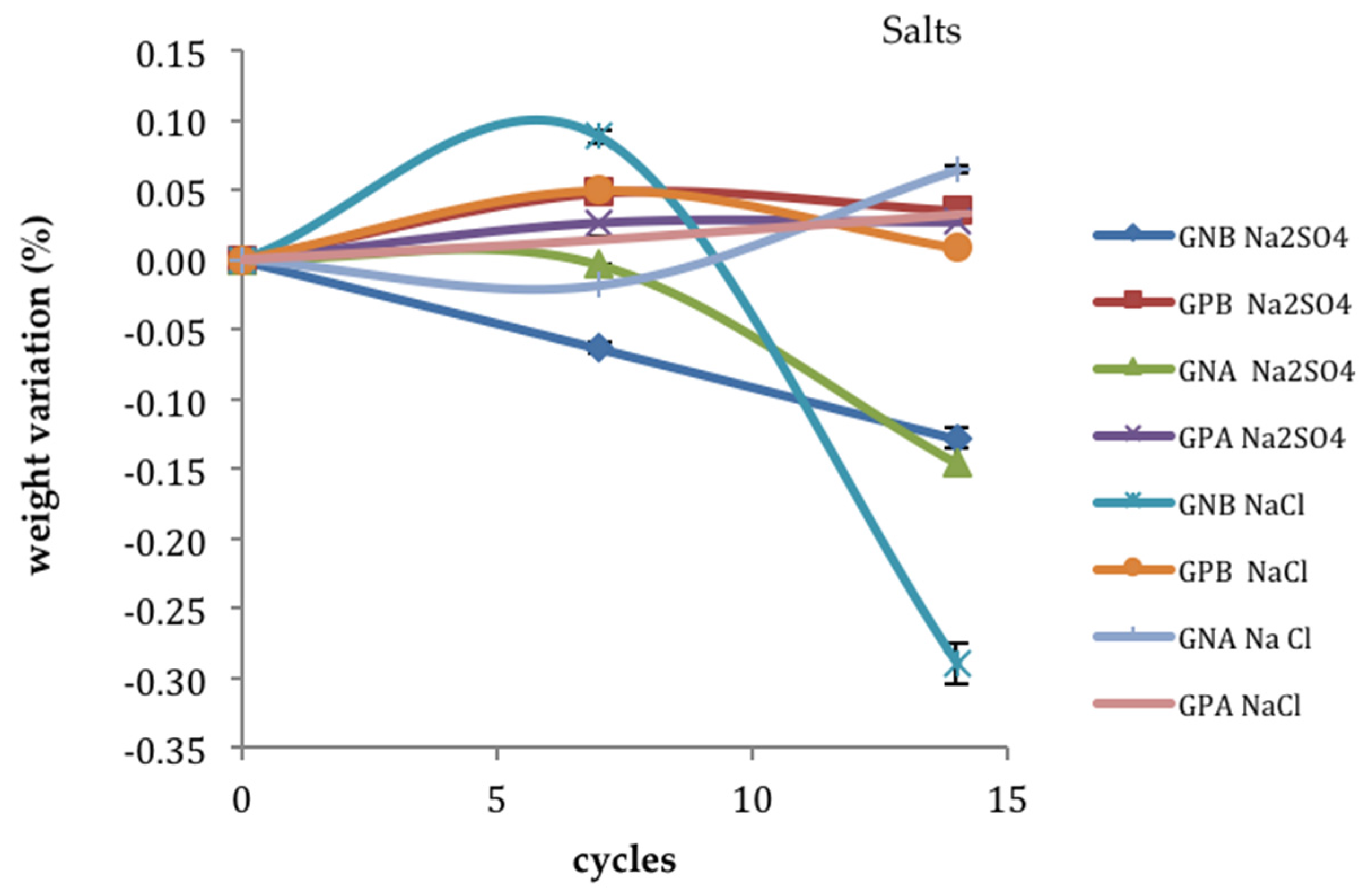
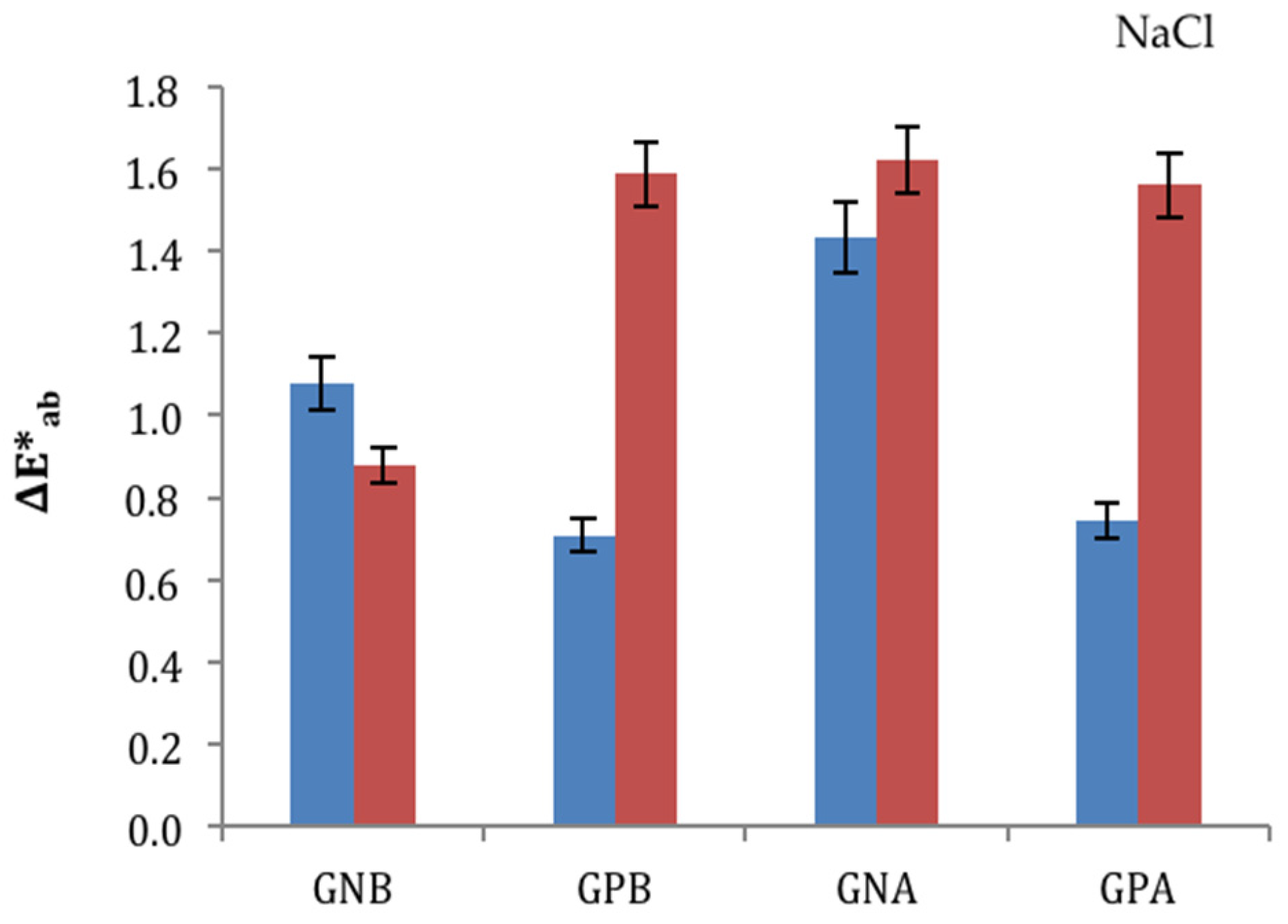
3.4. Ageing Trials: NaCl and Na2SO4 Salts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- A concelleira de Servizos condena os actos vandálicos e pide responsabilidade aos traballadores do servizo de limpeza (In Galician). Available online: http://santiagodecompostela.gal (accessed on 27 October 2017).
- Rabea, A.M.; Mirabedini, S.M.; Mohseni, M. Investigating the surface properties of polyurethane based anti-graffiti coating against UV exposure. J. Appl. Polym. Sci. 2012, 124, 3082–3091. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Blanco-Varela, M.T.; Martínez-Ramírez, S. Freeze-Thaw and UV Resistance in Building Stone Coated with Two Permanent Anti-graffiti Treatments. In Engineering Geology for Society and Territory; Lollino, G., Giordan, D., Eds.; Springer: Cham, Switzerland, 2015; Volume 8, pp. 531–534. [Google Scholar]
- Carmona-Quiroga, P.M.; Jacobs, R.M.J.; Viles, H.A. Weathering of two anti-graffiti protective coatings on concrete paving slabs. Coatings 2017, 7, 1. [Google Scholar] [CrossRef]
- Feller, R.L. Accelerated Aging: Photochemical and Thermal Aspects; Getty Conservation Institute: Los Angeles, CA, USA, 1994. [Google Scholar]
- Izzo, F.C.; Balliana, E.; Pinton, F.; Zendri, E. A preliminary study of the composition of commercial oil, acrylic and vinyl paints and their behaviour after accelerated ageing conditions. Conserv. Sci. Cult. Herit. 2014, 14, 353–369. [Google Scholar]
- Cortea, I.M.; Radvan, A.; Vasiliu, C.; Puscas, N. Preliminary results of accelerated ageing tests on acrylic art paints. Univ. Politeh. Buchar. Sci. Bull. Ser. 2014, 76, 215–222. [Google Scholar]
- Zhang, H.; Dun, Y.; Tang, Y.; Zuo, Y.; Zhao, X. Correlation between natural exposure and artificial ageing test for typical marine coating systems. J. Appl. Polym. Sci. 2016, 133, 43893. [Google Scholar] [CrossRef]
- Ecco, L.G.; Rossi, S.; Fedel, M.; Deflorian, F. Color variation of electrophoretic styrene-acrylic paints under field and accelerated ultraviolet exposure. Mater. Des. 2017, 116, 554–564. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Jacobs, R.M.J.; Martínez-Ramírez, S.; Viles, H.A. Durability of anti-graffiti coatings on stone: Natural vs. accelerated weathering. PLoS ONE 2017, 12, e0172347. [Google Scholar] [CrossRef] [PubMed]
- Doehne, E.; Price, C.A. Stone Conservation. In An Overview of Current Research, 2nd ed.; Getty Publications: Los Angeles, CA, USA, 2010. [Google Scholar]
- Sanmartín, P.; Cappitelli, F.; Mitchell, R. Current methods of graffiti removal: A review. Constr. Build. Mater. 2014, 71, 363–374. [Google Scholar] [CrossRef]
- Sanmartín, P.; Mitchell, R.; Cappitelli, F. Evaluation of Cleaning Methods for Graffiti Removal. In Urban Pollution and Changes to Materials and Building Surfaces; Brimblecombe, P., Ed.; Imperial College Press: London, UK, 2016; pp. 291–312. [Google Scholar]
- Sanmartín, P.; DeAraujo, A.; Vasanthakumar, A.; Mitchell, R. Feasibility study involving the search for natural strains of microorganisms capable of degrading graffiti from heritage materials. Int. Biodeterior. Biodegr. 2015, 103, 186–190. [Google Scholar] [CrossRef]
- Montana Colors®. Available online: http://www.montanacolors.com (accessed on 27 October 2017).
- Sanmartín, P.; Silva, B.; Prieto, B. Effect of surface finish on roughness, color and gloss of ornamental granites. J. Mater. Civ. Eng. 2011, 23, 1239–1248. [Google Scholar] [CrossRef]
- Sanmartín, P.; Chorro, E.; Vázquez-Nion, D.; Martínez-Verdú, F.M.; Prieto, B. Conversion of a digital camera into a non-contact colorimeter for use in stone cultural heritage: The application case to Spanish granites. Measurement 2014, 56, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Esbert, R.M.; Ordaz, J.; Alonso, F.J.; Ruiz De Argandoña, V.G.; Montoto, M.; Marcos, R.; Valdeón, L. Petrophysical caracterization and weatherability of the stones of the Seville Cathedral. Mater. Constr. 1988, 38, 5–23. [Google Scholar] [CrossRef]
- Sanmartín, P.; Rivas, T.; De los Santos, D.M.; Silva, B.; Mosquera, M.J. Effectiveness of a new water-repellent and consolidant nanomaterial applied to a biocalcareous stone. In Proceedings of the 11th International Congress on Deterioration and Conservation of Stone, Torun, Polonia, 15–20 September 2008; Volume II, pp. 1045–1054. [Google Scholar]
- Giacomucci, L.; Toja, F.; Sanmartín, P.; Toniolo, L.; Prieto, B.; Villa, F.; Cappitelli, F. Degradation of nitrocellulose-based paint by D. desulfuricans ATCC 13541. Biodegradation 2012, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- ASTM D7089 Determination of the Effectiveness of Anti-Graffiti Coating for Use on Concrete, Masonry and Natural Stone Surfaces by Pressure Washing; American Society for Testing and Materials: West Conshohocken, PA, USA, 2006.
- ASTM D 870–02 Standard Practice for Testing Water Resistance of Coatings Using Water Immersion, Annual Book of ASTM Standard; American Society for Testing and Materials: West Conshohocken, PA, USA, 2002.
- Bhargava, S.; Kubota, M.; Lewis, R.; Advani, S.; Prasad, A.; Deitzel, J. Ultraviolet, water, and thermal aging studies of a waterborne polyurethane elastomer-based high reflectivity coating. Prog. Org. Coat. 2015, 79, 75–82. [Google Scholar] [CrossRef]
- ASTM D 6944–03 Standard Test Method for Resistance for Cured Coatings to Thermal Cycling, Annual Book of ASTM Standard; American Society for Testing and Materials: West Conshohocken, PA, USA, 2003.
- Shahidzadeh-Bonn, N.; Desarnaud, J.; Bertrand, F.; Chateau, X.; Bonn, D. Damage in porous media due to salt crystallization. Phys. Rev. 2010, E81, 0066110. [Google Scholar] [CrossRef] [PubMed]
- Reunion Internationale des Laboratoires d’Essais et de Recherche sur Les Materiaux et Les Constructions (R.I.L.E.M.) Paris. Crystallization Test by Total Inmersion (Test V.1). Crystallization Test by Partial Inmersion (Test V.2). In Proceedings of the International Symposium on Deterioration and Conservation of Stone Monuments (UNESCO-RILEM), Paris, France, 5–9 June 1978.
- Sanmartín, P. Evaluación de dos Tratamientos Consolidantes Aplicados a Granitos y Calcarenitas, Work Submitted for Obtain the Research Sufficiency Diploma; University of Santiago de Compostela: Santiago, Spain, 2007; p. 103. (In Spanish) [Google Scholar]
- Prieto, B.; Sanmartín, P.; Silva, B.; Martinez-Verdú, F. Measuring the color of granite rocks. A proposed procedure. Color Res. Appl. 2010, 35, 368–375. [Google Scholar] [CrossRef]
- Removing Graffiti from Historic Properties. Available online: http://www.mass.gov/eea/docs/dcr/stewardship/rmp/bmps/graffiti-removal.pdf (accessed on 27 October 2017).
- Pozo-Antonio, J.S.; Rivas, T.; Jacobs, R.M.J.; Viles, H.A.; Carmona-Quiroga, P.M. Effectiveness of commercial anti-graffiti treatments in two granites of different texture and mineralogy. Prog. Org. Coat. Submitted.
- Wyszecki, G.; Stiles, W.S. Color Science. Concepts and Methods. Quantitative Data and Formulae; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Völz, H.G. Industrial Color Testing; Wiley VCH: Weinheim, Germany, 2001. [Google Scholar]
- Berns, R.S. Billmeyer and Saltzman ́s Principles of Color Technology, 3rd ed.; John Wiley and Sons: New York, NY, USA, 2000. [Google Scholar]
- Sodium Chloride (NaCl). Available online: https://www.crystran.co.uk/optical-materials/sodium-chloride-nacl (accessed on 27 October 2017).
- Periasamy, A.; Muruganand, S.; Palaniswamy, M. Vibrational studies of Na2SO4, K2SO4, NaHSO4 and KHSO4 crystals. Rasayan J. Chem. 2009, 2, 981–989. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, Tables and Charts; John Wiley and Sons, Ltd.: New York, NY, USA, 2004. [Google Scholar]
- Bayer, I.S. On the durability and wear resistance of transparent superhydrophobic coatings. Coatings 2017, 7, 12. [Google Scholar] [CrossRef]
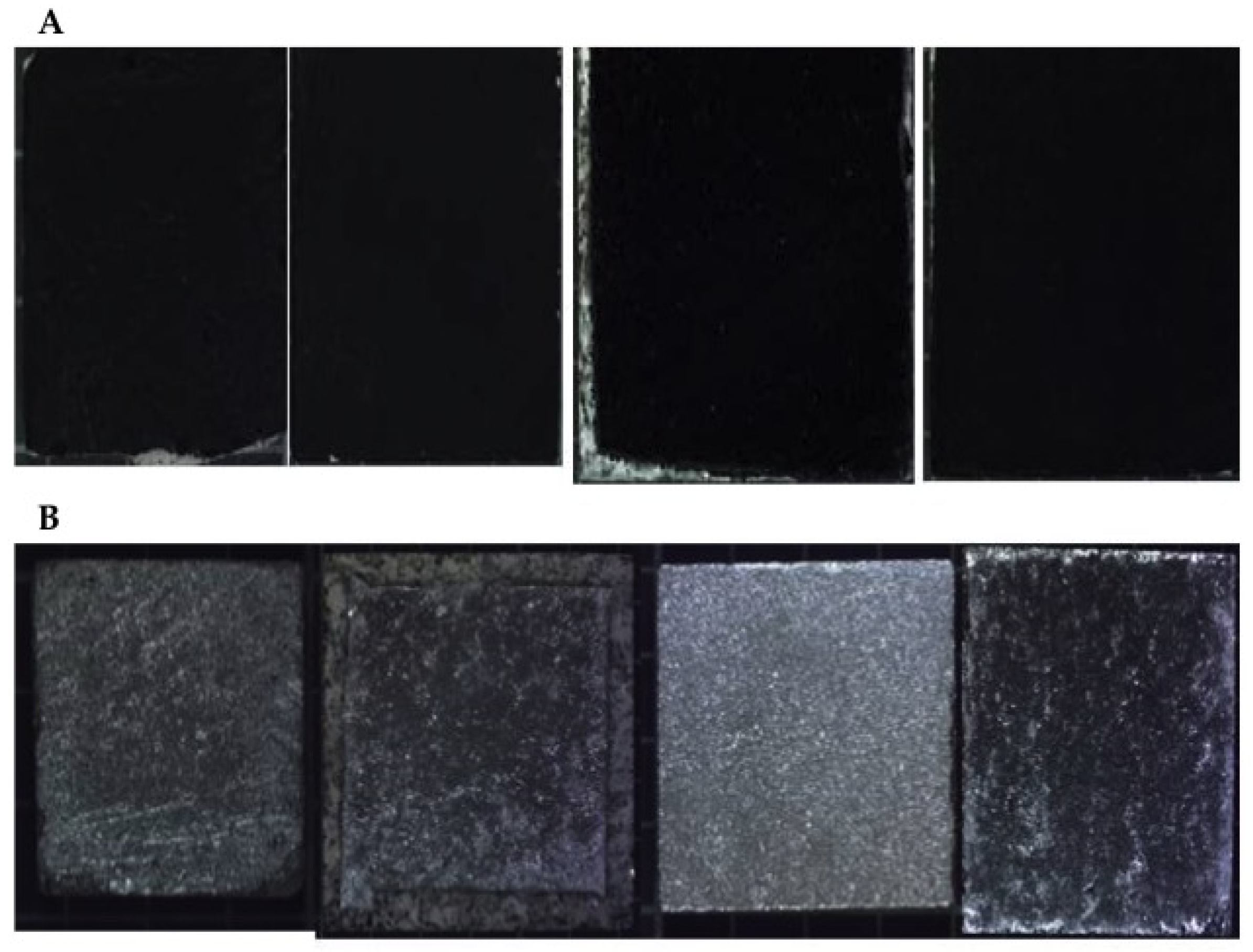




| Code | Description | Ageing Test * |
|---|---|---|
| GNA | Blanco Cristal granite with non-metallic (black) paint: one year old | Humidity (3; 5), freeze-thawing (10; 20), NaCl and Na2SO4 salts (7; 14) |
| GNB | Blanco Cristal granite with non-metallic (black) paint | Humidity (3; 5), freeze-thawing (10; 20), NaCl and Na2SO4 salts (7; 14) |
| GPA | Blanco Cristal granite with metallic (silver) paint: one year old | Humidity (3; 5), freeze-thawing (10; 20), NaCl and Na2SO4 salts (7; 14) |
| GPB | Blanco Cristal granite with metallic (silver) paint | Humidity (3; 5), freeze-thawing (10; 20), NaCl and Na2SO4 salts (7; 14) |
| IN | Rhyolitic ignimbrite with non-metallic (black) paint | Humidity (3; 5), freeze-thawing (10; 20) |
| IP | Rhyolitic ignimbrite with metallic (silver) paint | Humidity (3; 5), freeze-thawing (10; 20) |
| BCN | Biocalcarenite of Puerto de Santa María with non-metallic (black) paint | Humidity (3; 5), freeze-thawing (10; 20) |
| BCP | Biocalcarenite of Puerto de Santa María with metallic (silver) paint | Humidity (3; 5), freeze-thawing (10; 20) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartín, P.; Cappitelli, F. Evaluation of Accelerated Ageing Tests for Metallic and Non-Metallic Graffiti Paints Applied to Stone. Coatings 2017, 7, 180. https://doi.org/10.3390/coatings7110180
Sanmartín P, Cappitelli F. Evaluation of Accelerated Ageing Tests for Metallic and Non-Metallic Graffiti Paints Applied to Stone. Coatings. 2017; 7(11):180. https://doi.org/10.3390/coatings7110180
Chicago/Turabian StyleSanmartín, Patricia, and Francesca Cappitelli. 2017. "Evaluation of Accelerated Ageing Tests for Metallic and Non-Metallic Graffiti Paints Applied to Stone" Coatings 7, no. 11: 180. https://doi.org/10.3390/coatings7110180







