Study on the Formation of Reaction Phase to Si Addition in Boron Steel Hot-Dipped in Al–7Ni Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
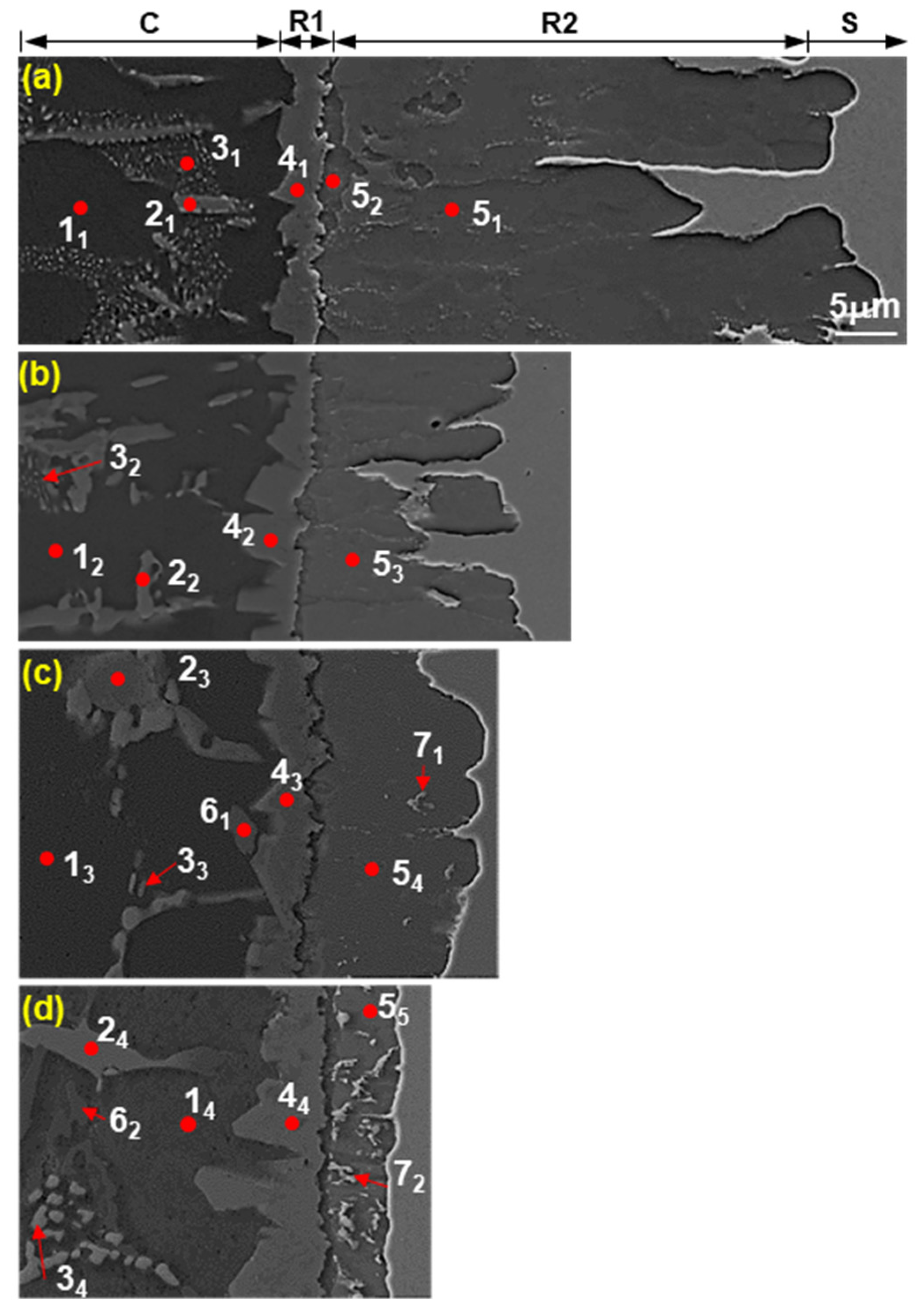
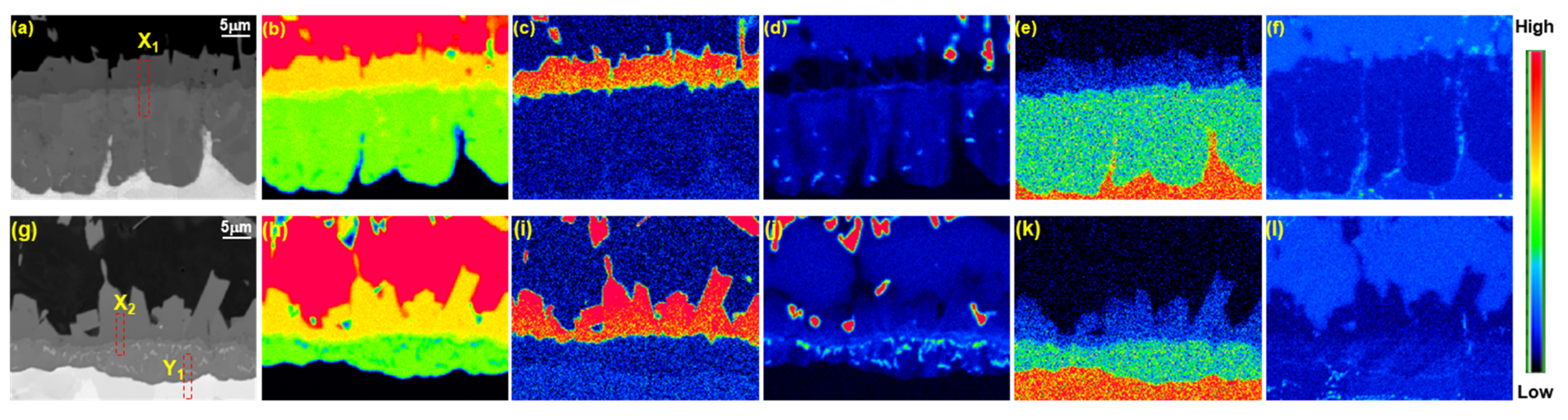
3.1. Classification of Microstructure of Coating Layer and Reaction Layer in Al–7 wt % Ni–xSi Hot Dipping
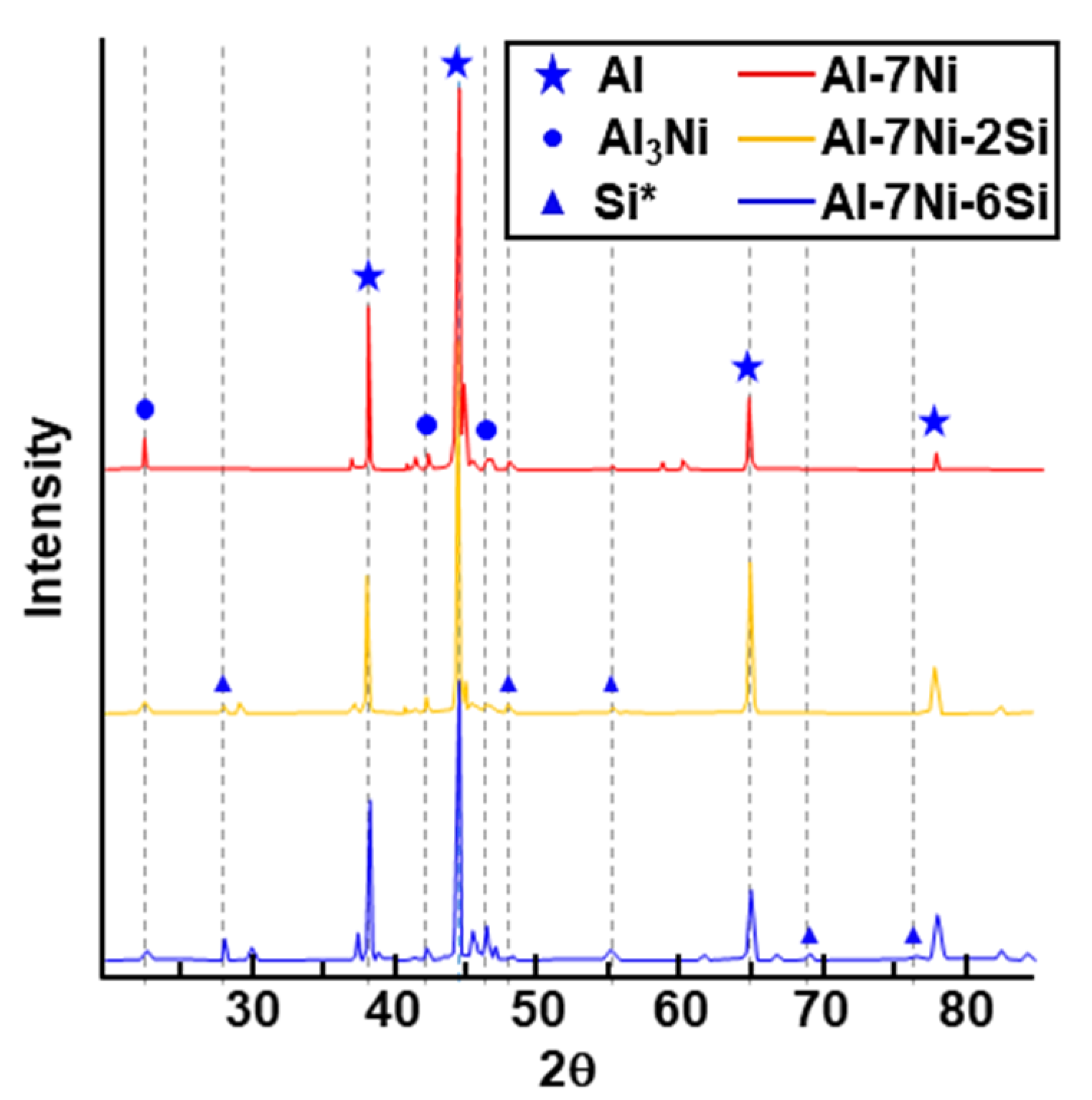
3.2. Identification of Phases on Pure Coating Layers Depending on Various Levels of Si Addition in Coating Compositon
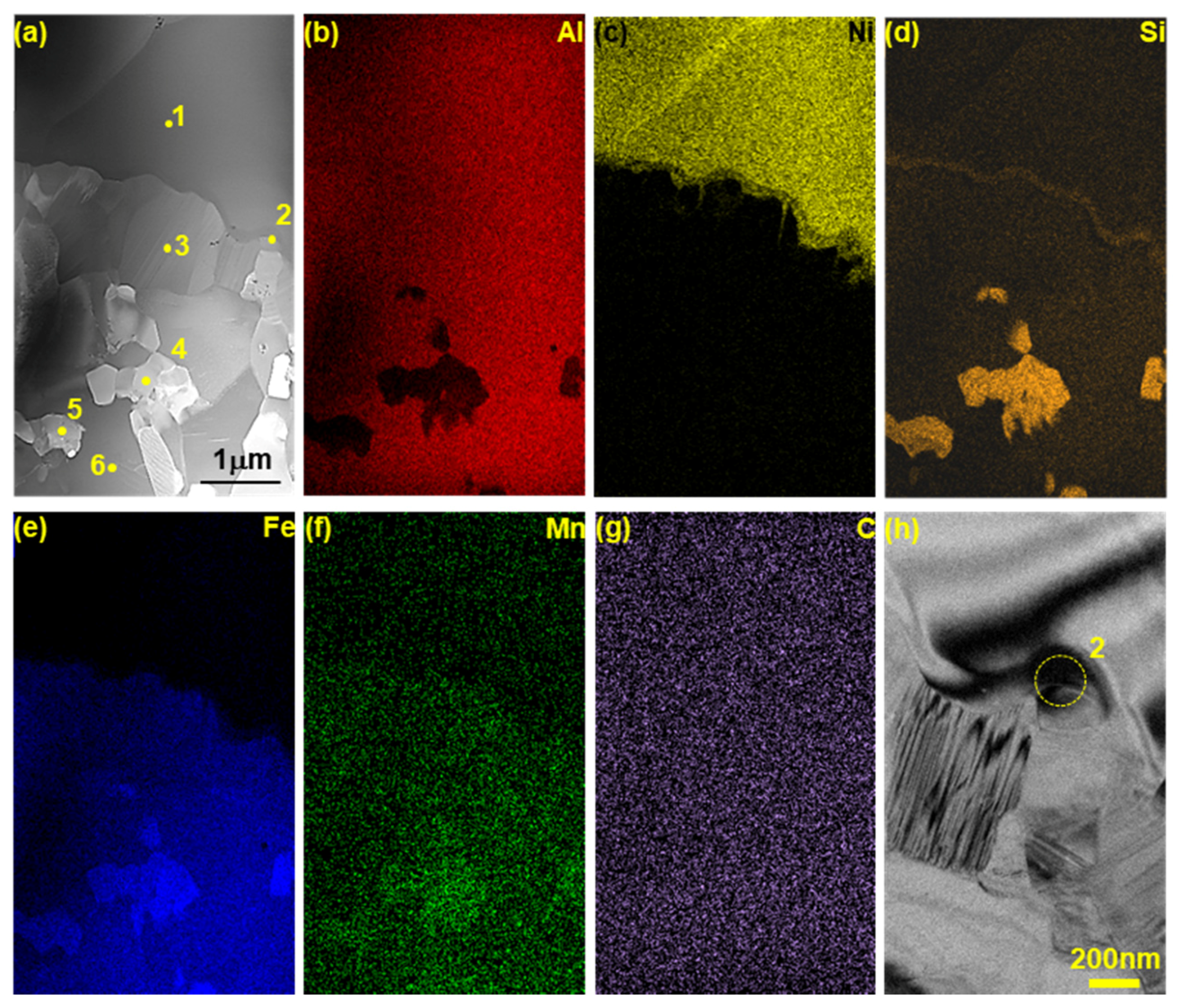
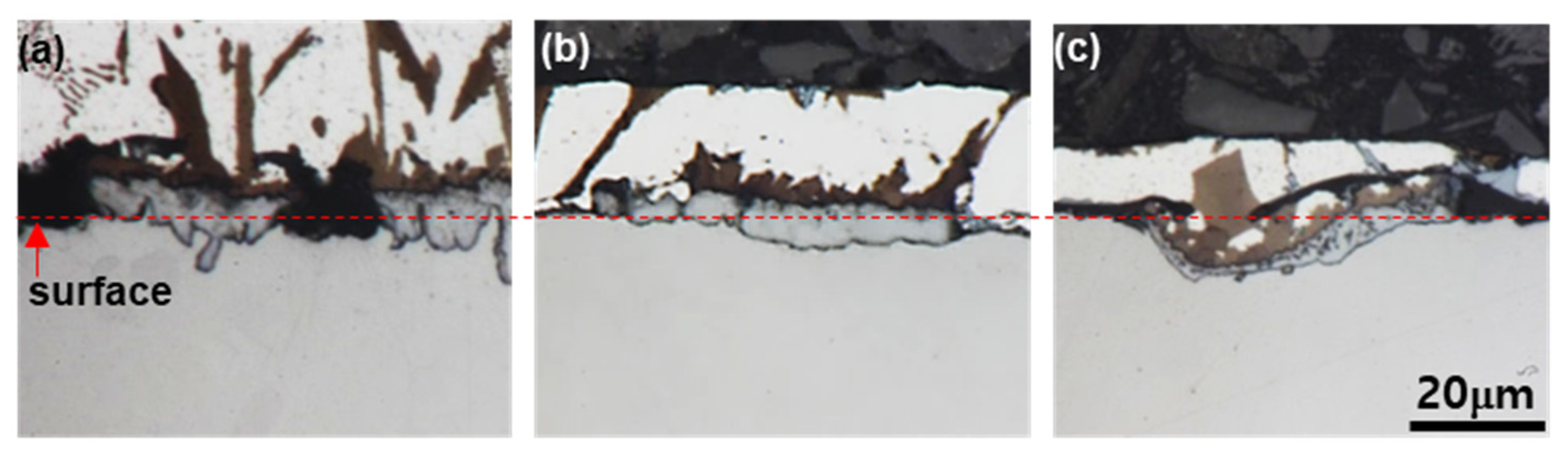
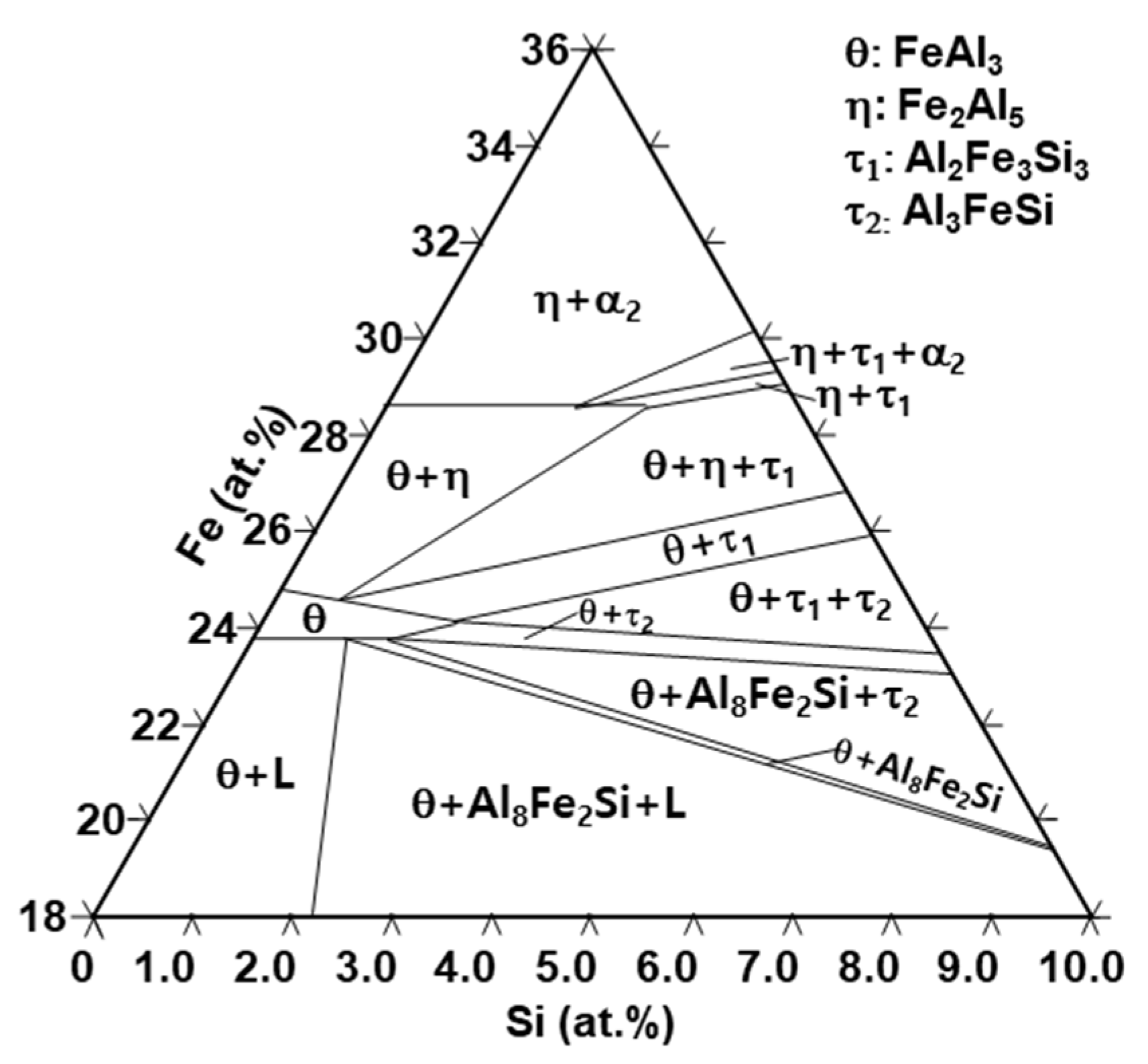
3.3. Identification of Phases on Reaction Layers Depending on Various Levels of Si Addition in Coating Composition
4. Discussion
5. Conclusions
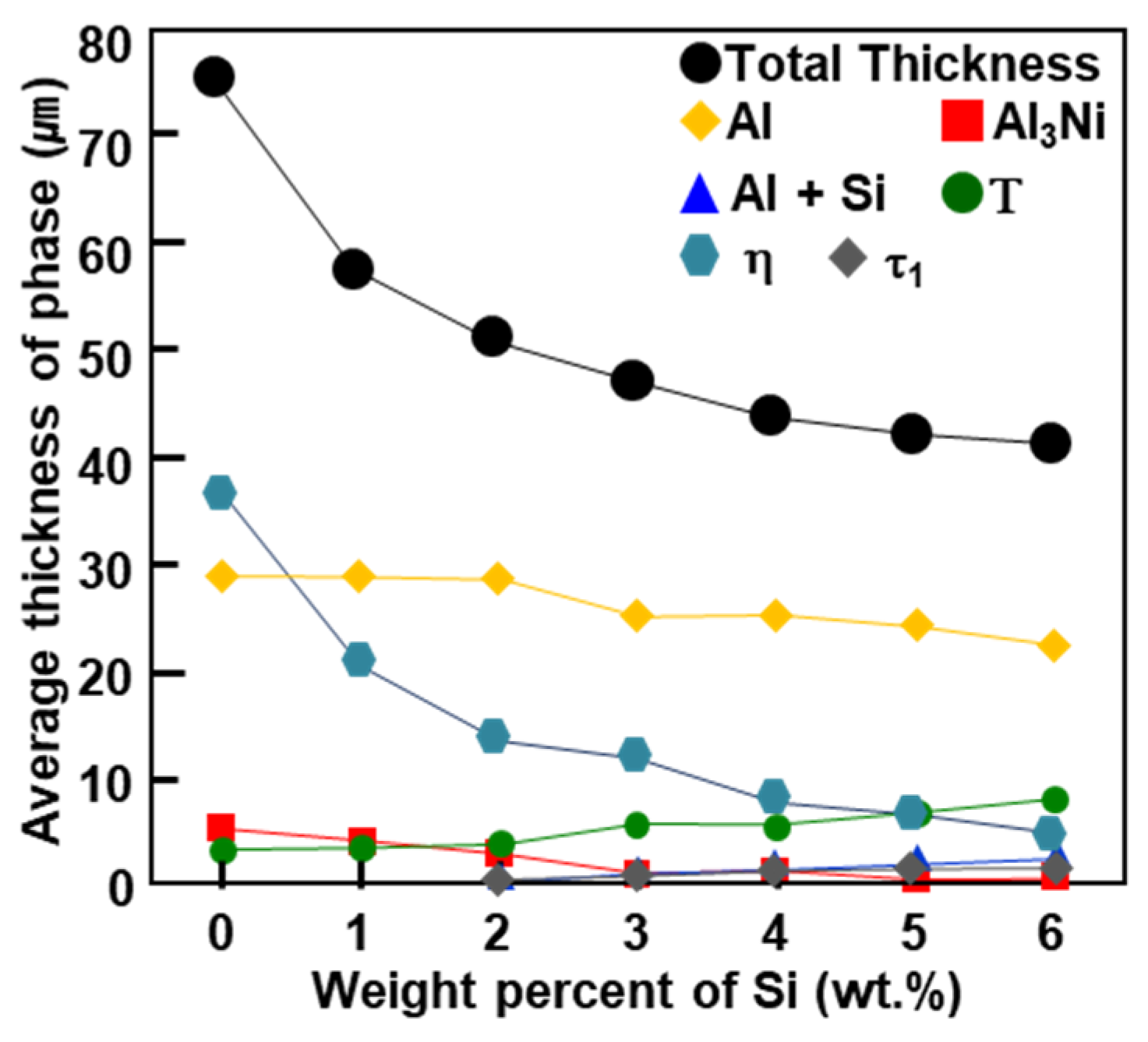
- According to the amount of Si, the phases of the pure coating layer (C) are composed of an Al–7Ni wt % coating layer and a primary Al + primary Al3Ni + eutectic structure (Al + Al3Ni). However, as the amount of Si added to the coating layer increases from 1 to 6 wt %, the size and amount of the primary Al3Ni phase increases. In addition, the crystallization of Si (Fd-3m) in the pure coating layer also increases.
- The phase (R1) of the original surface of all wt % Si added specimens is an Al9FeNi (T) phase (space group: P21/c), which is the same as for the specimen without Si. This phase increases linearly from 3.8 µm for Si-free specimens to 9.7 µm for 6 wt % Si additions. Both the previous research and the present study show that, as the Si content in the coating increases, the Fe flux from the steel interface to the liquid phase increases. The reason for the change is the amount of Τ phase formation described below.
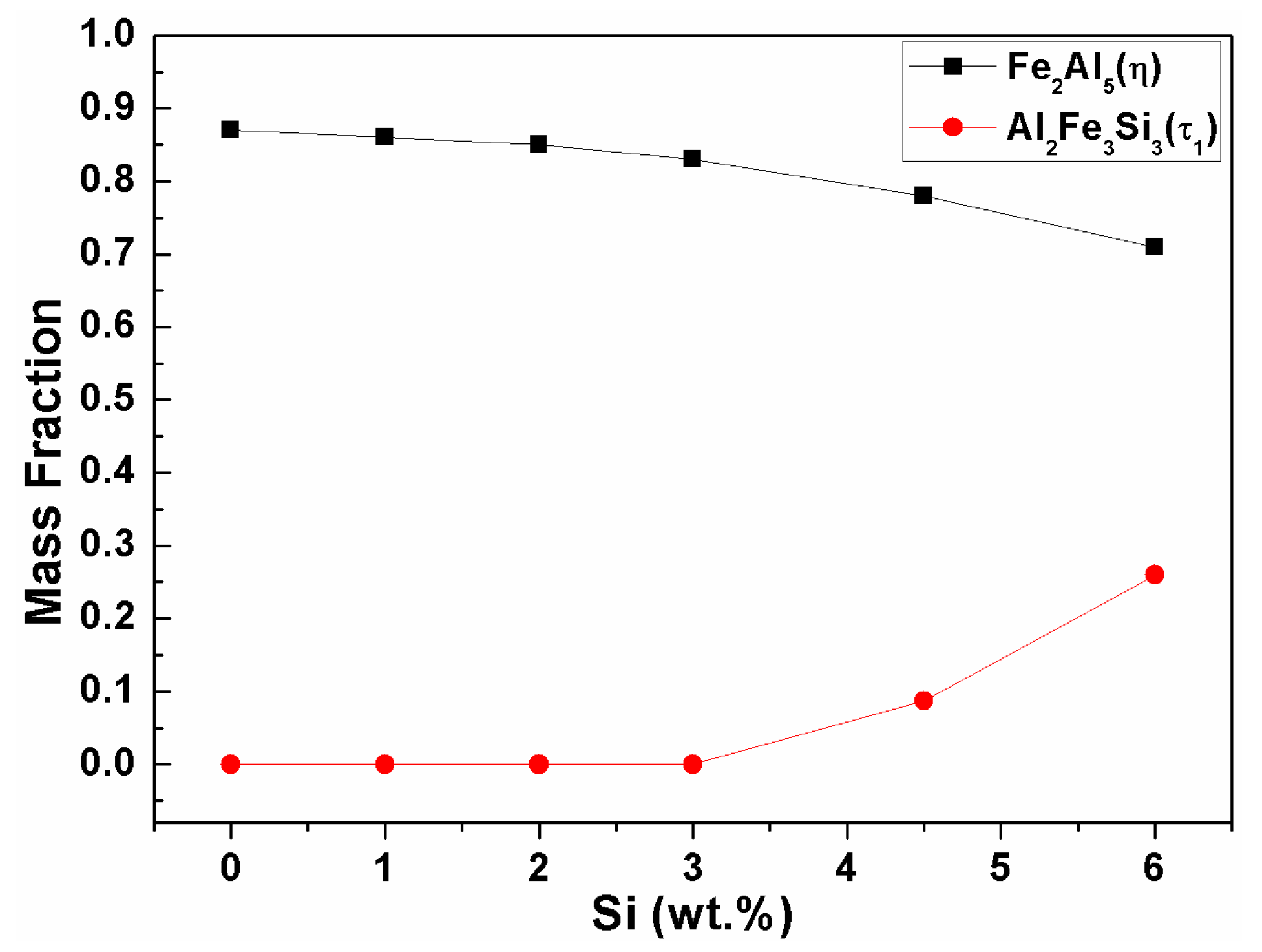
- The reaction phase (R2) formed in the reactor is an Fe2Al5 (η) phase (space group: Cmcm). The thickness variation of this phase decreases drastically because of the addition of 1 wt % Si and steadily decreases until the addition of 6 wt % Si. This is because approximately 1–2 wt % of Si solid solution in the η phase affects the vacancy inside the phase, thereby interfering with the diffusion of Al and inhibiting its phase growth.
- The Al2Fe3Si3 (τ1) phase (space group: P-1) is generated when the amount of Si addition is more than 2 wt % in the Al–7Ni wt % coating. The amount of this phase is proportional to the amount of Si added to the coating bath. In addition, the ∆G·mol−1 value obtained by Thermo-calc™ was lower than that of the η phase. Therefore, when the τ1 phase is generated, it consumes Fe of the steel material used for the η phase generation, and Al diffused from the liquid phase; therefore, the η phase growth is inhibited.
- Since the intermediate concentration layer of 19–28 at % Fe concentration between the Τ phase and the η phase is generated only in the Si-added coating, approximately 10 at % Si exists in this layer. Therefore, the θ phase is more stable than the η phase in this layer.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aranda, L.G.; Ravier, P.; Chastel, Y. Hot stamping of quenchable steels: Material data and process simulations. In Proceedings of the IDDRG, Bled, Slovenia, 11–15 May 2003; pp. 164–166. [Google Scholar]
- Kolleck, R.; Veit, R.; Merklein, M.; Lechler, J.; Geiger, M. Investigation on induction heating for hot stamping of boron alloyed steels. CIRP Ann. Manuf. Technol. 2009, 58, 275–278. [Google Scholar] [CrossRef]
- Liu, H.S.; Xing, Z.W.; Bao, J.; Song, B.Y. Investigation of the hot-stamping process for advanced high-strength steel sheet by numerical simulation. J. Mater. Eng. Perform. 2010, 19, 325–334. [Google Scholar] [CrossRef]
- Geiger, M.; Merklein, M.; Hoff, C. Basic investigations on the hot stamping steel 22MnB5. Adv. Mater. Res. 2005, 6–8, 795–804. [Google Scholar] [CrossRef]
- Neugebauer, R.; Altan, T.; Geiger, M.; Kleiner, M.; Sterzing, A. Sheet metal forming at elevated temperatures. CIRP Ann. Manuf. Technol. 2006, 55, 793–816. [Google Scholar] [CrossRef]
- Cretteur, L.; Vierstraete, R.; Yin, Q.; Ehling, W.; Pic, A. Development of a laser decoating process for fully functional Al–Si coated press hardened steel laser welded blank solutions. In Proceedings of the 5th International WLT-Conference: Lasers in Manufacturing, Munich, Germany, 15–18 June 2009; pp. 409–413. [Google Scholar]
- Jung, B.H.; Kong, J.P.; Kang, C.Y. Effect of hot-stamping heat treatment on the microstructure of Al-segregated zone in TWB laser joints of Al-Si-coated boron steel and Zn-coated DP steel. Korean J. Met. Mater. 2012, 50, 455–462. [Google Scholar]
- Oh, M.-H.; Kong, J.-P.; Kwon, M.-S.; Kang, C.-Y. Effect of hot-stamping on microstructures and tensile properties of Al-Si coated boron steel welds with laser source. J. Weld. Join. 2013, 31, 96–106. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Oh, M.-H.; Shin, H.-J.; Kang, C.-Y. Comparison of microstructure and phase transformation of laser-welded joints in Al-10 wt % Si-coated boron steel before and after hot stamping. Mater. Charact. 2017, 128, 195–202. [Google Scholar] [CrossRef]
- Yun, J.-G.; Lee, J.-H.; Kwak, S.-Y.; Kang, C.-Y. Microstructural evolution of intermetallic compound formed in boron steel hot-dipped in Al–7%Ni alloy. Metals 2017, 7, 393. [Google Scholar] [CrossRef]
- Li, Y.S.; Spiegel, M. Models describing the degradation of FeAl and NiAl alloys induced by ZnCl2–KCl melt at 400–450 °C. Corros. Sci. 2004, 46, 2009–2023. [Google Scholar] [CrossRef]
- Eggeler, G.; Auer, W.; Kaesche, H. On the influence of silicon on the growth of the alloy layer during hot dip aluminizing. J. Mater. Sci. 1986, 21, 3348–3350. [Google Scholar] [CrossRef]
- Lemmens, B.; Springer, H.; De Graeve, I.; De Strycker, J.; Raabe, D.; Verbeken, K. Effect of silicon on the microstructure and growth kinetics of intermetallic phases formed during hot-dip aluminizing of ferritic steel. Surf. Coat. Technol. 2017, 319, 104–109. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Yin, F.; Zhao, M.; Liu, Y.; Han, W.; Li, Z. Effect of Si on growth kinetics of intermetallic compounds during reaction between solid iron and molten aluminum. Trans. Nonferr. Met. Soc. China 2013, 23, 556–561. [Google Scholar] [CrossRef]
- ASTM B487-85 Test Method for Measurement of Metal and Oxide Coating Thicknesses by Microscopical Examination of a Cross Section; ASTM: West Conshohocken, PA, USA, 2013.
- Murray, J.L.; Mcalister, A.J. The Al-Si (aluminum-silicon) system. J. Phase Equilib. 1984, 5, 74–84. [Google Scholar] [CrossRef]
- Heumann, T.; Dittrich, N. Structure character of the Fe2Al5 intermetallics compound in hot dip aluminizing process. Z. Met. 1959, 50, 617–623. [Google Scholar]
- Cheng, W.-J.; Wang, C.-J. Growth of intermetallic layer in the aluminide mild steel during hot-dipping. Surf. Coat. Technol. 2009, 204, 824–828. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Crystallography of Fe2Al5 phase at the interface between solid Fe and liquid Al. Intermetallics 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Komatsu, N.; Nakamura, M.; Fujita, H. Reactions of iron with liquid aluminum. J. Jpn. Inst. Light Met. 1968, 18, 467–473. [Google Scholar] [CrossRef]
- Awan, G.H.; Faiz, U.H. The morphology of coating/substrate interface in hot-dip-aluminized steels. Mater. Sci. Eng. A 2008, 472, 157–165. [Google Scholar] [CrossRef]





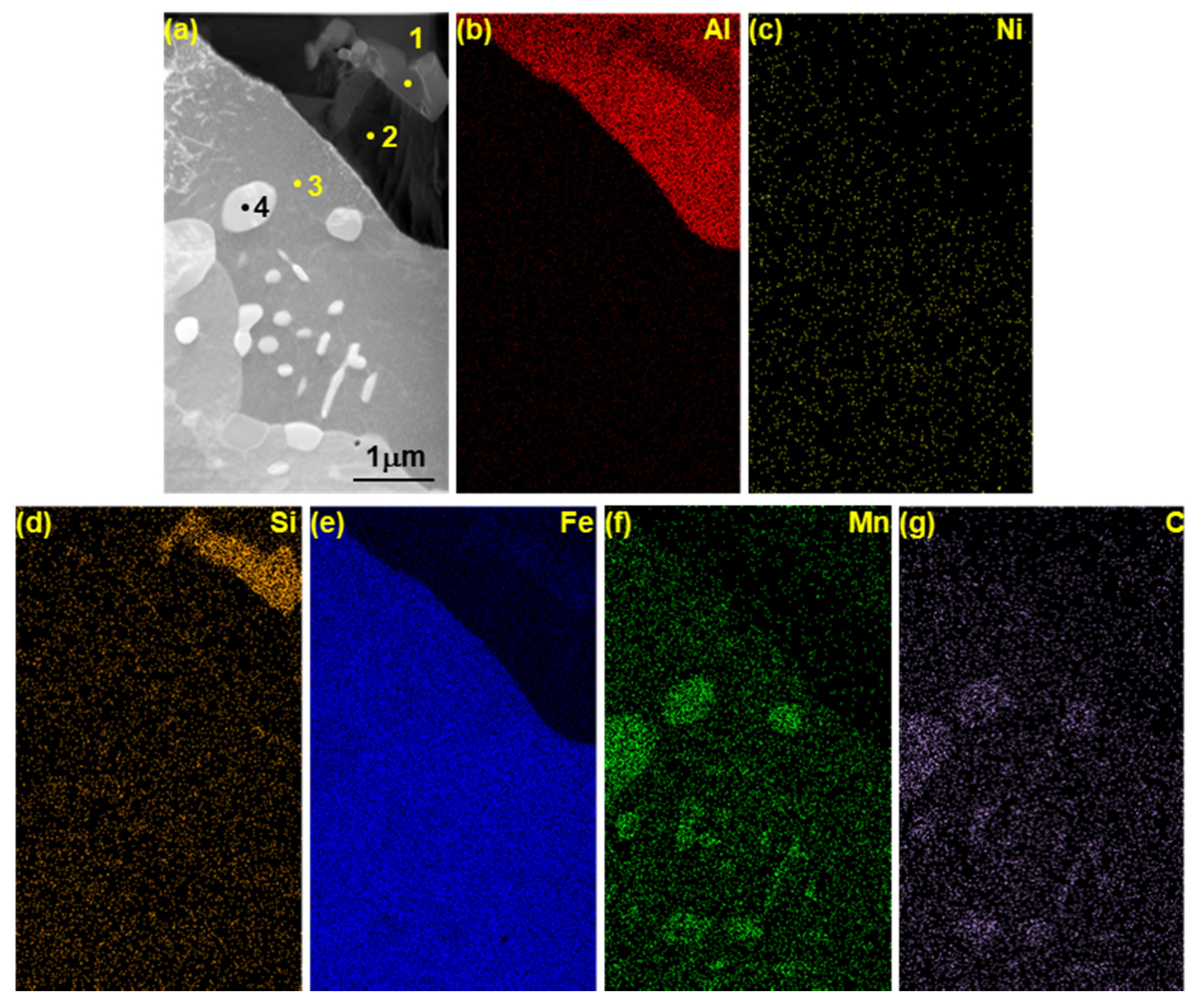

| C | Si | Mn | Cr | Nb | Ti | B | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0.229 | 0.238 | 1.189 | 0.183 | 0.004 | 0.036 | 0.002 | 0.002 | 0.002 | Bal. |
| No. | Chemical Composition (at %) | Expected Phase | |||||
|---|---|---|---|---|---|---|---|
| Al | Ni | Si | Fe | Mn | |||
| 1 | 1 | 98.33 | 0.69 | 0.60 | 0.38 | 0.49 | Al |
| 2 | 97.59 | 0.55 | 1.31 | 0.55 | 0.30 | ||
| 3 | 97.47 | 0.64 | 1.21 | 0.68 | 0.45 | ||
| 4 | 97.43 | 0.42 | 1.57 | 0.58 | 0.36 | ||
| 2 | 1 | 83.36 | 14.50 | 0.80 | 0.86 | 0.48 | Al3Ni |
| 2 | 76.51 | 22.32 | 0.27 | 0.59 | 0.32 | ||
| 3 | 74.94 | 23.23 | 0.96 | 0.58 | 0.29 | ||
| 4 | 84.43 | 13.20 | 1.06 | 0.82 | 0.49 | ||
| 3 | 1 | 86.83 | 11.27 | 0.72 | 0.81 | 0.37 | Al3Ni |
| 2 | 78.55 | 19.02 | 1.28 | 1.00 | 0.25 | ||
| 3 | 80.42 | 18.38 | 0.74 | 0.38 | 0.08 | ||
| 4 | 82.39 | 15.77 | 0.87 | 0.79 | 0.18 | ||
| 4 | 1 | 78.33 | 12.18 | 0.78 | 7.96 | 0.76 | Al9FeNi |
| 2 | 80.63 | 12.26 | 0.57 | 5.86 | 0.68 | ||
| 3 | 81.14 | 12.21 | 0.92 | 5.14 | 0.59 | ||
| 4 | 79.69 | 11.71 | 1.57 | 6.32 | 0.71 | ||
| 5 | 1 | 71.41 | 0.88 | 1.08 | 25.44 | 1.19 | Fe2Al5 |
| 2 | 75.00 | 1.08 | 0.90 | 22.09 | 0.93 | ||
| 3 | 75.44 | 0.00 | 1.45 | 22.92 | 0.20 | ||
| 4 | 71.01 | 0.36 | 2.92 | 25.45 | 0.16 | ||
| 5 | 69.90 | 0.50 | 3.65 | 25.77 | 0.18 | ||
| 6 | 1 | 74.48 | 0.59 | 24.42 | 0.5 | 0.01 | (Al,Si) |
| 2 | 50.42 | 0.56 | 48.46 | 0.54 | 0.02 | ||
| 7 | 1 | 53.69 | 0.35 | 16.98 | 28.75 | 0.23 | FeAlSi |
| 2 | 51.44 | 0.54 | 16.69 | 31.08 | 0.25 | ||
| at % | Al | Fe | Ni | Si | Mn | C | Expected Phase |
|---|---|---|---|---|---|---|---|
| 1 | 69.40 | 11.68 | 14.76 | 2.95 | 0.03 | 1.18 | Al9FeNi |
| 2 | 69.11 | 14.30 | 11.38 | 4.67 | 0.03 | 0.51 | Al9FeNi |
| 3 | 66.91 | 29.96 | 0.04 | 1.66 | 0.25 | 1.18 | Fe2Al5 |
| 4 | 61.08 | 34.42 | 0.03 | 2.98 | 0.40 | 1.09 | Fe2Al5 |
| 5 | 61.86 | 33.88 | 0.04 | 2.08 | 0.59 | 1.55 | Fe2Al5 |
| 6 | 34.52 | 41.21 | 0.15 | 19.72 | 0.73 | 3.67 | Al2Fe3Si3 |
| 7 | 62.77 | 25.27 | 0.70 | 10.11 | 0.65 | 0.50 | FeAl3 |
| at % | Al | Fe | Ni | Si | Mn | C | Expected Phase |
|---|---|---|---|---|---|---|---|
| 1 | 70.86 | 13.05 | 13.15 | 2.78 | – | 0.16 | Al9FeNi |
| 2 | 64.76 | 19.22 | 4.69 | 11.17 | – | 0.16 | FeAl3 |
| 3 | 63.80 | 30.01 | 0.07 | 3.81 | 0.41 | 1.90 | Fe2Al5 |
| 4 | 28.12 | 42.92 | 0.08 | 26.41 | 0.26 | 2.21 | Al2Fe3Si3 |
| 5 | 27.98 | 42.97 | – | 27.27 | 0.39 | 1.39 | Al2Fe3Si3 |
| 6 | 59.97 | 37.30 | 0.06 | 1.64 | 0.34 | 0.69 | Fe2Al5 |
| at % | Al | Fe | Ni | Si | Mn | C | Suggested Phase |
|---|---|---|---|---|---|---|---|
| 1 | 32.21 | 46.85 | 0.08 | 19.63 | 0.30 | 0.92 | Al2Fe3Si3 |
| 2 | 63.22 | 30.70 | – | 0.88 | 0.17 | 5.12 | Fe2Al5 |
| 3 | 0.09 | 97.74 | – | 0.68 | 1.09 | 0.40 | αFe |
| 4 | 0.05 | 77.12 | – | 0.14 | 5.38 | 17.32 | (Fe,Mn)3C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.-G.; Lee, J.-H.; Kwak, S.-Y.; Kang, C.-Y. Study on the Formation of Reaction Phase to Si Addition in Boron Steel Hot-Dipped in Al–7Ni Alloy. Coatings 2017, 7, 186. https://doi.org/10.3390/coatings7110186
Yun J-G, Lee J-H, Kwak S-Y, Kang C-Y. Study on the Formation of Reaction Phase to Si Addition in Boron Steel Hot-Dipped in Al–7Ni Alloy. Coatings. 2017; 7(11):186. https://doi.org/10.3390/coatings7110186
Chicago/Turabian StyleYun, Jung-Gil, Jae-Hyeong Lee, Sung-Yun Kwak, and Chung-Yun Kang. 2017. "Study on the Formation of Reaction Phase to Si Addition in Boron Steel Hot-Dipped in Al–7Ni Alloy" Coatings 7, no. 11: 186. https://doi.org/10.3390/coatings7110186





