Nucleation and Growth of Intermetallic Compounds Formed in Boron Steel Hot-Dipped in Al–Ni alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
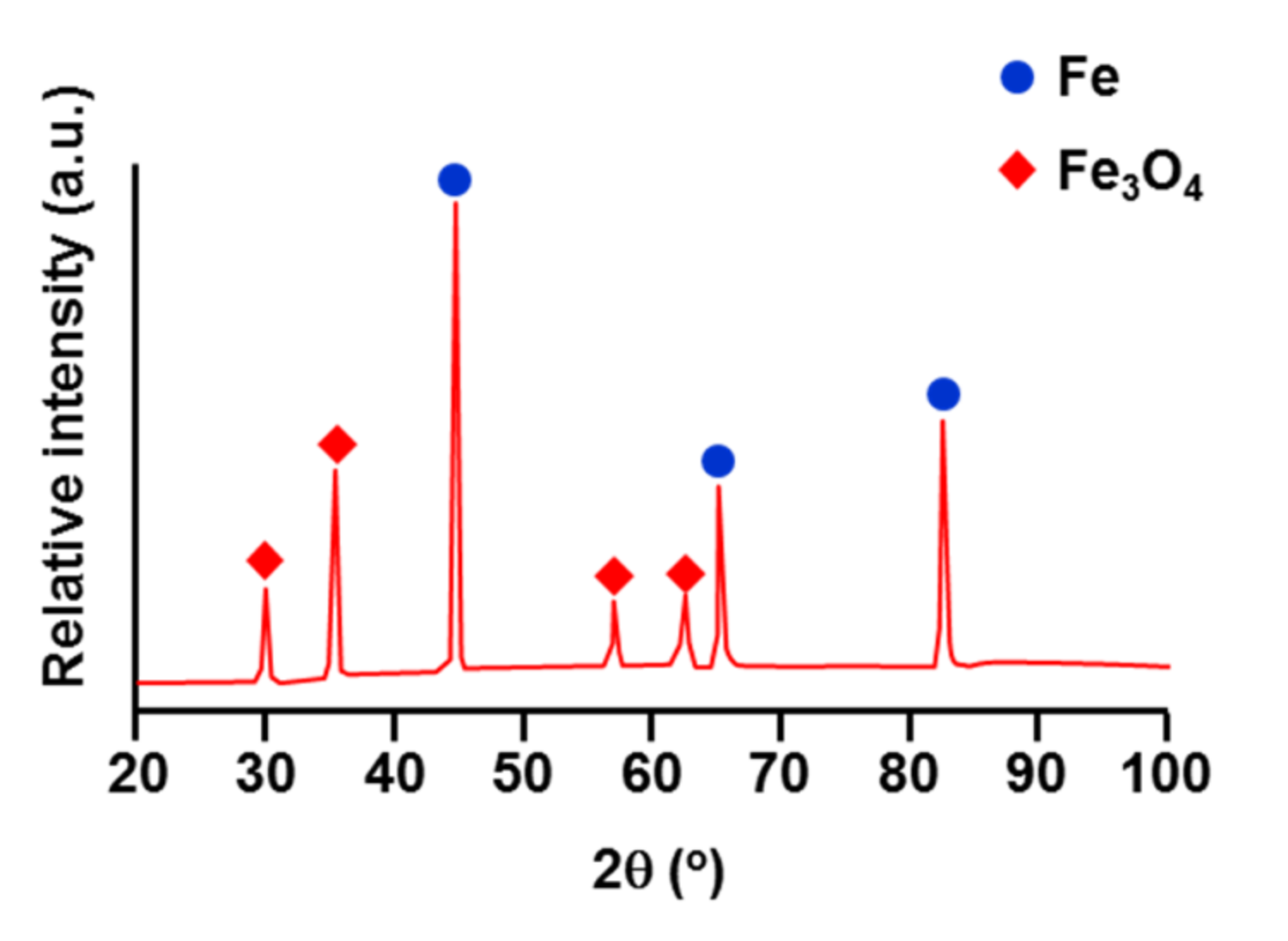
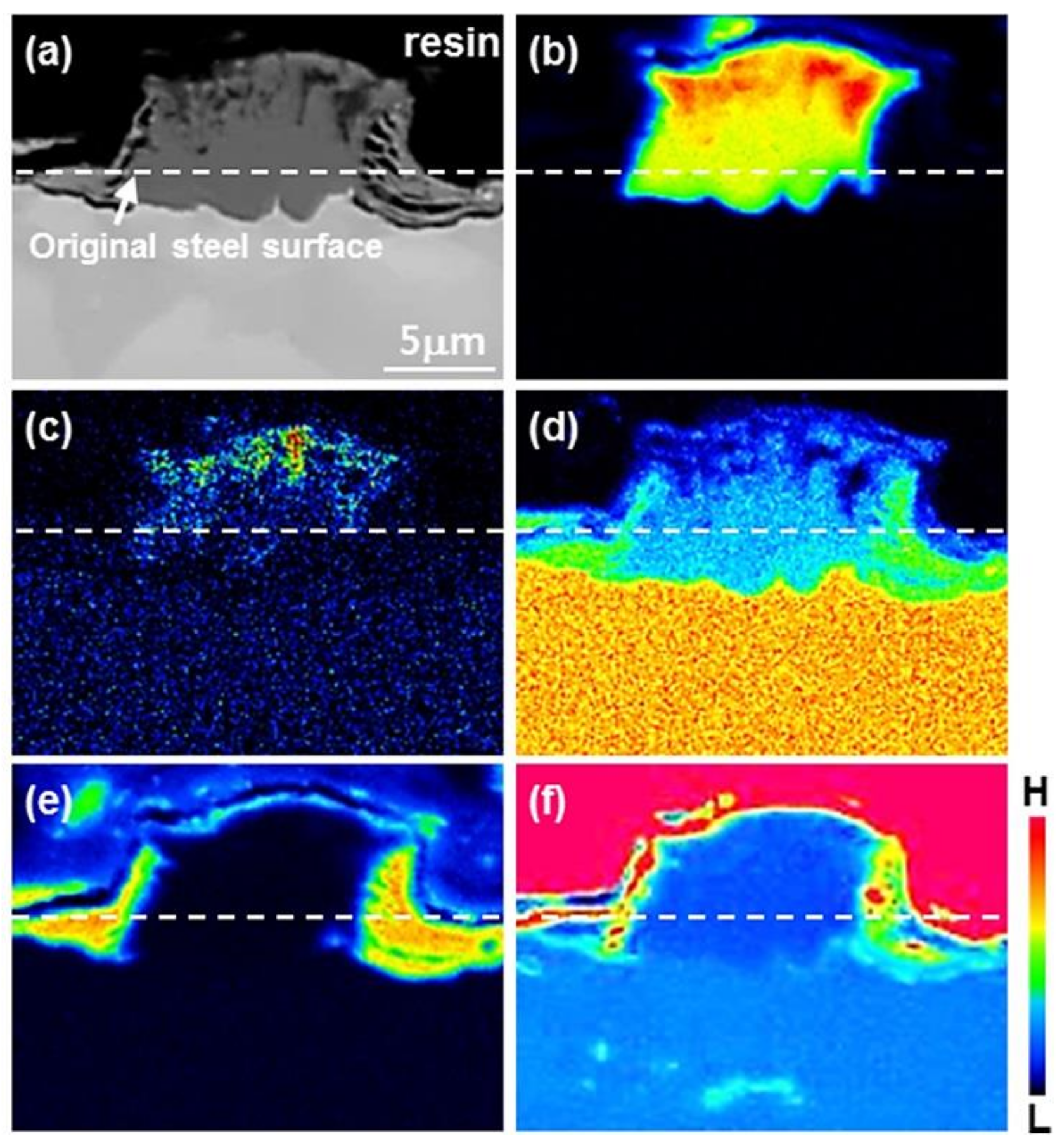
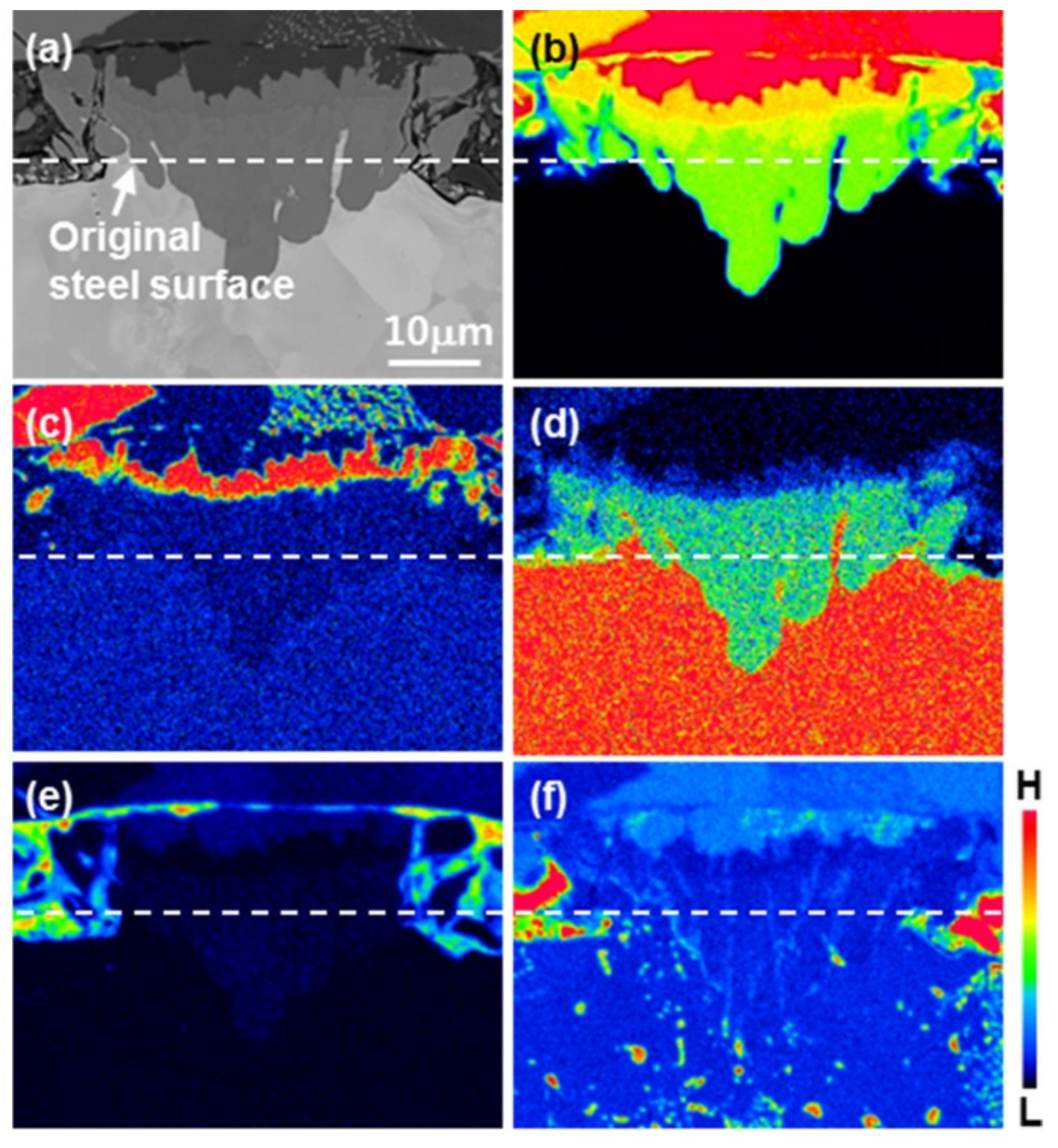
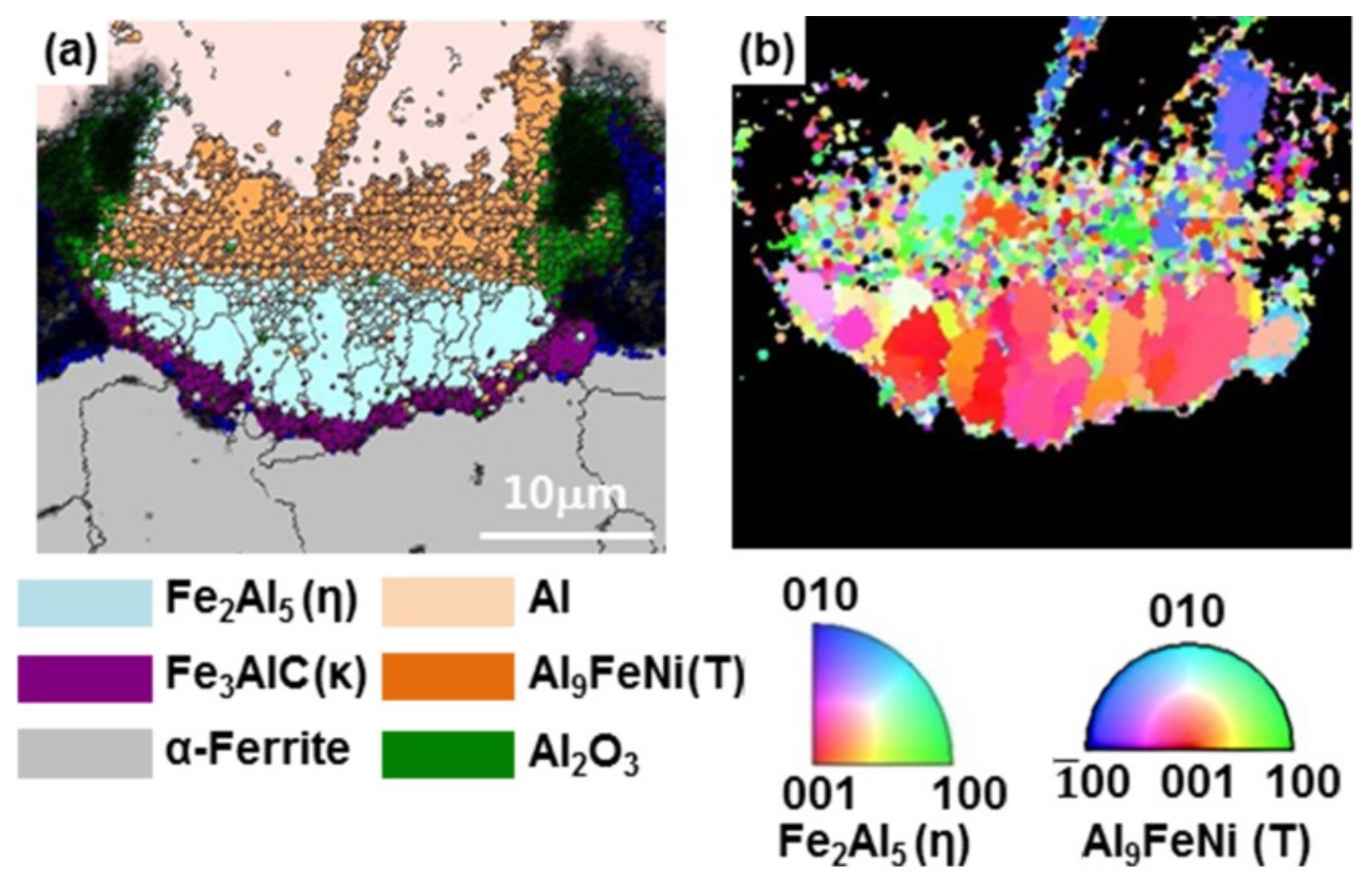
3.1. Nucleation of the Intermetallic Compounds
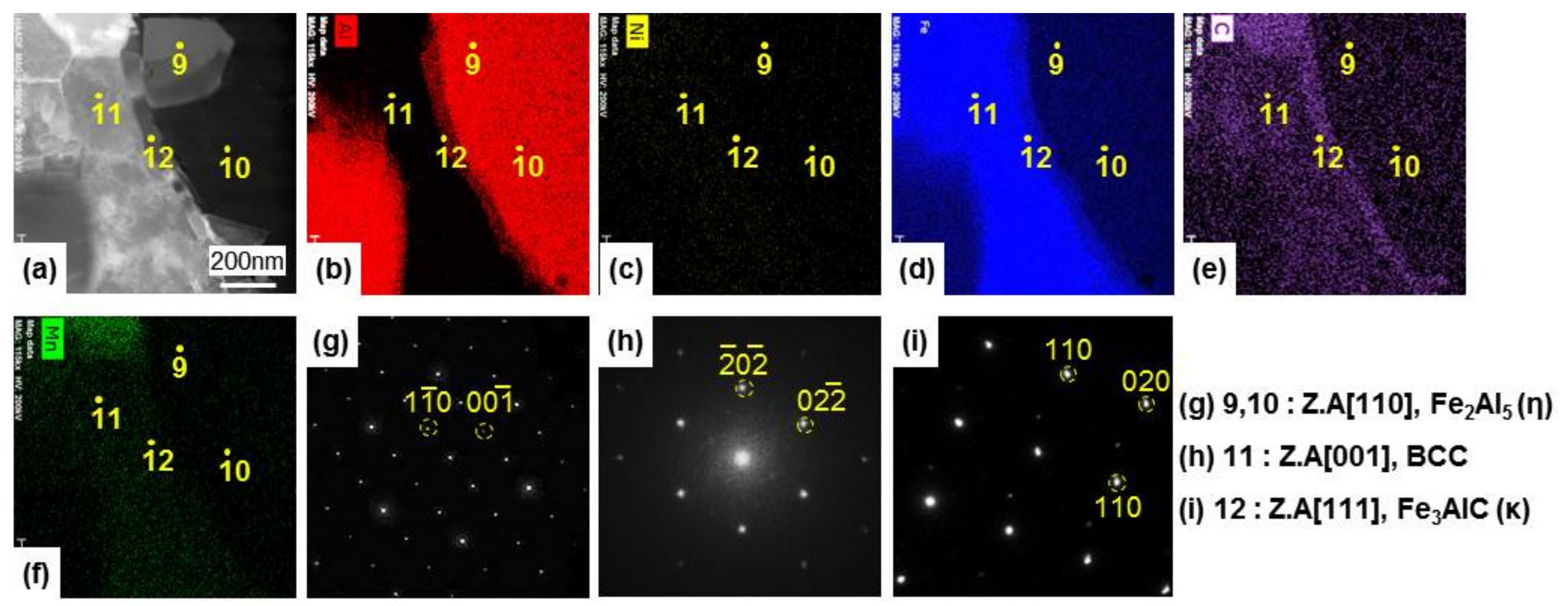
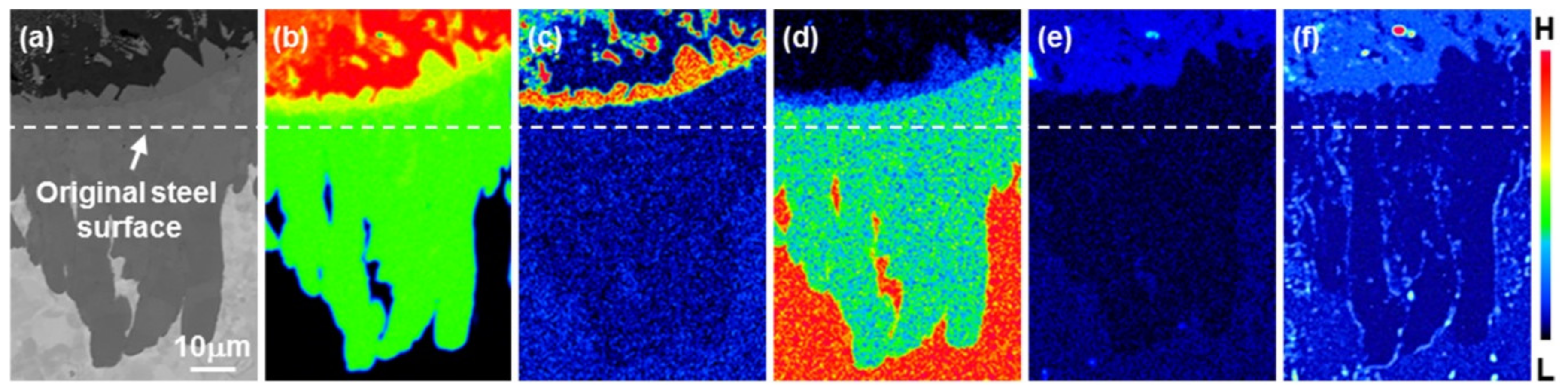
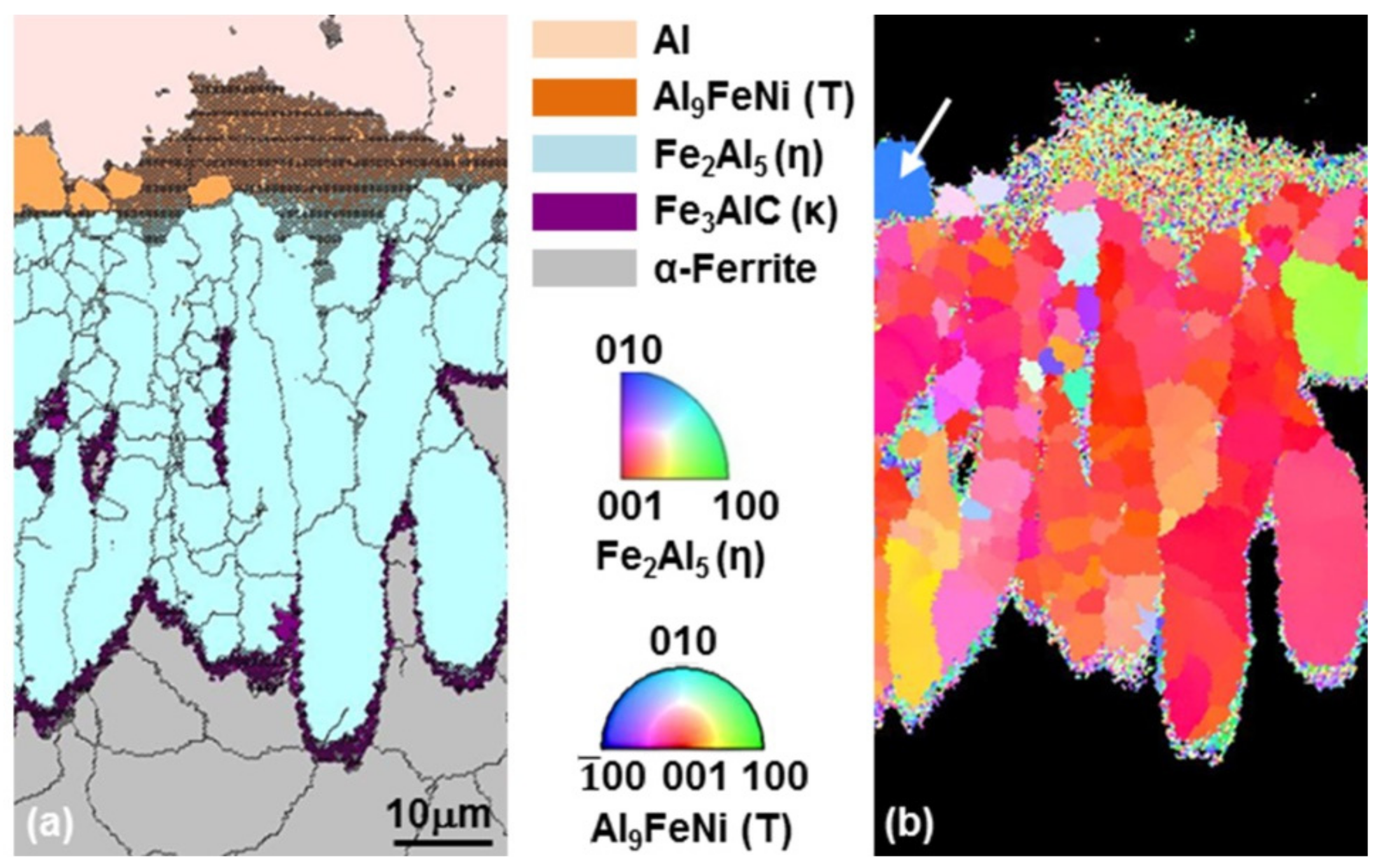
3.2. Growth of the Intermetallic Compounds
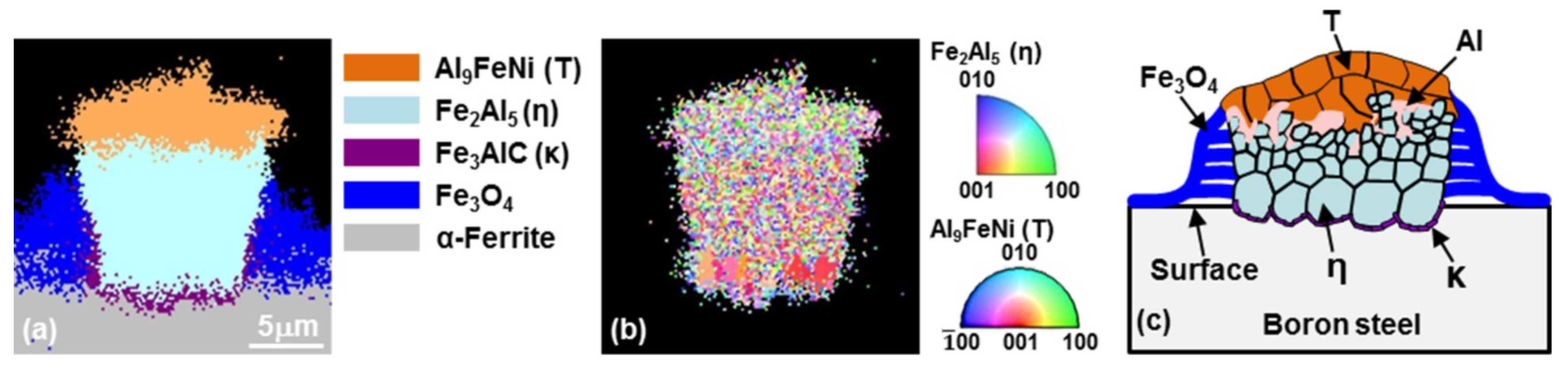
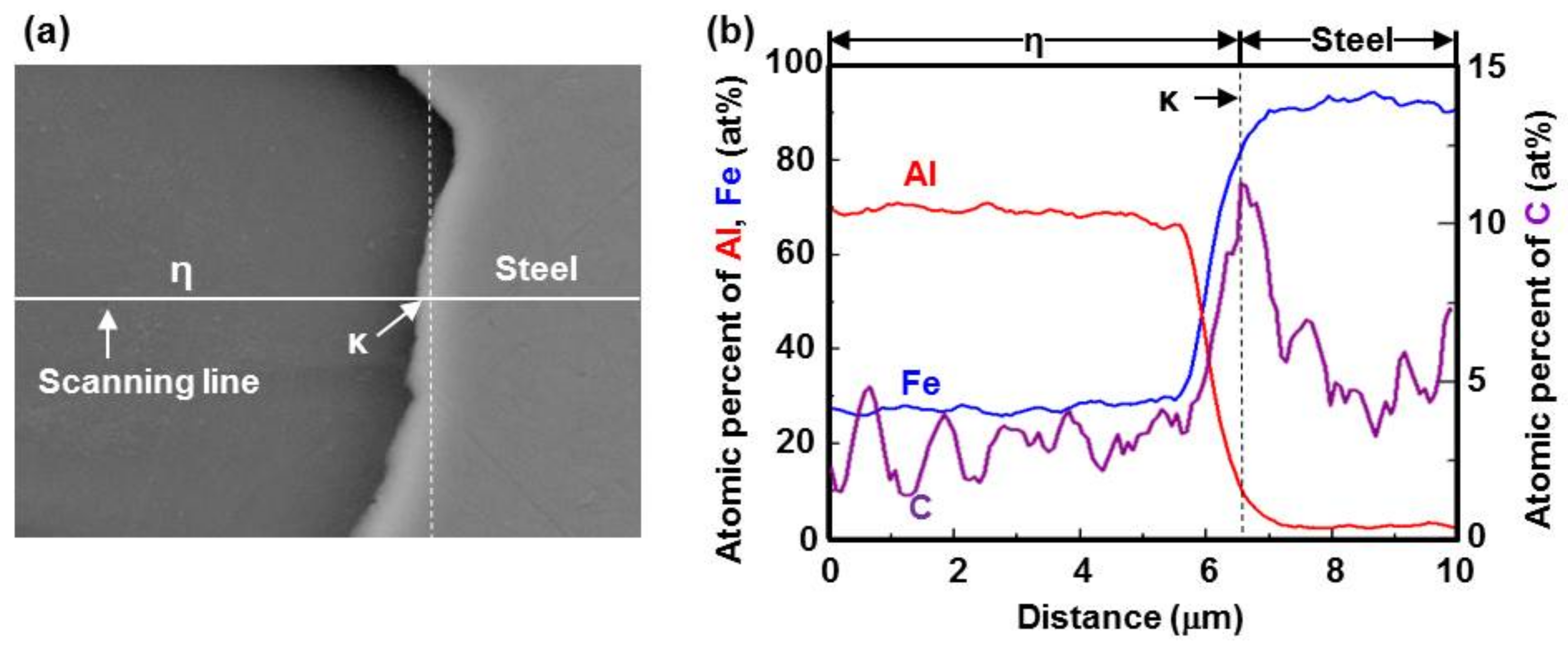
3.3. Nucleation and Growth of the Intermetallic Compounds
4. Discussion
5. Conclusions
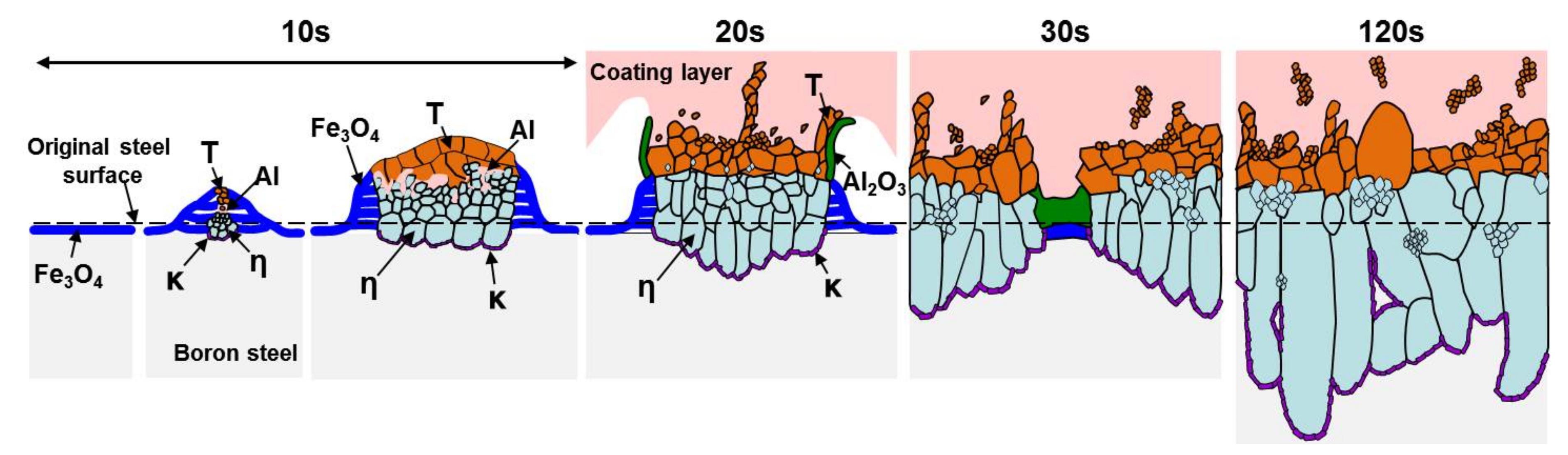
- In the early stages of the dipping process, the Fe3O4 oxide layer formed on the surfaces, then decomposed sporadically. The intermetallic compounds formed on the surfaces as the Al–Ni molten alloy permeated into the oxide layer breakdown region and reacted with the eluted Fe from the steel.
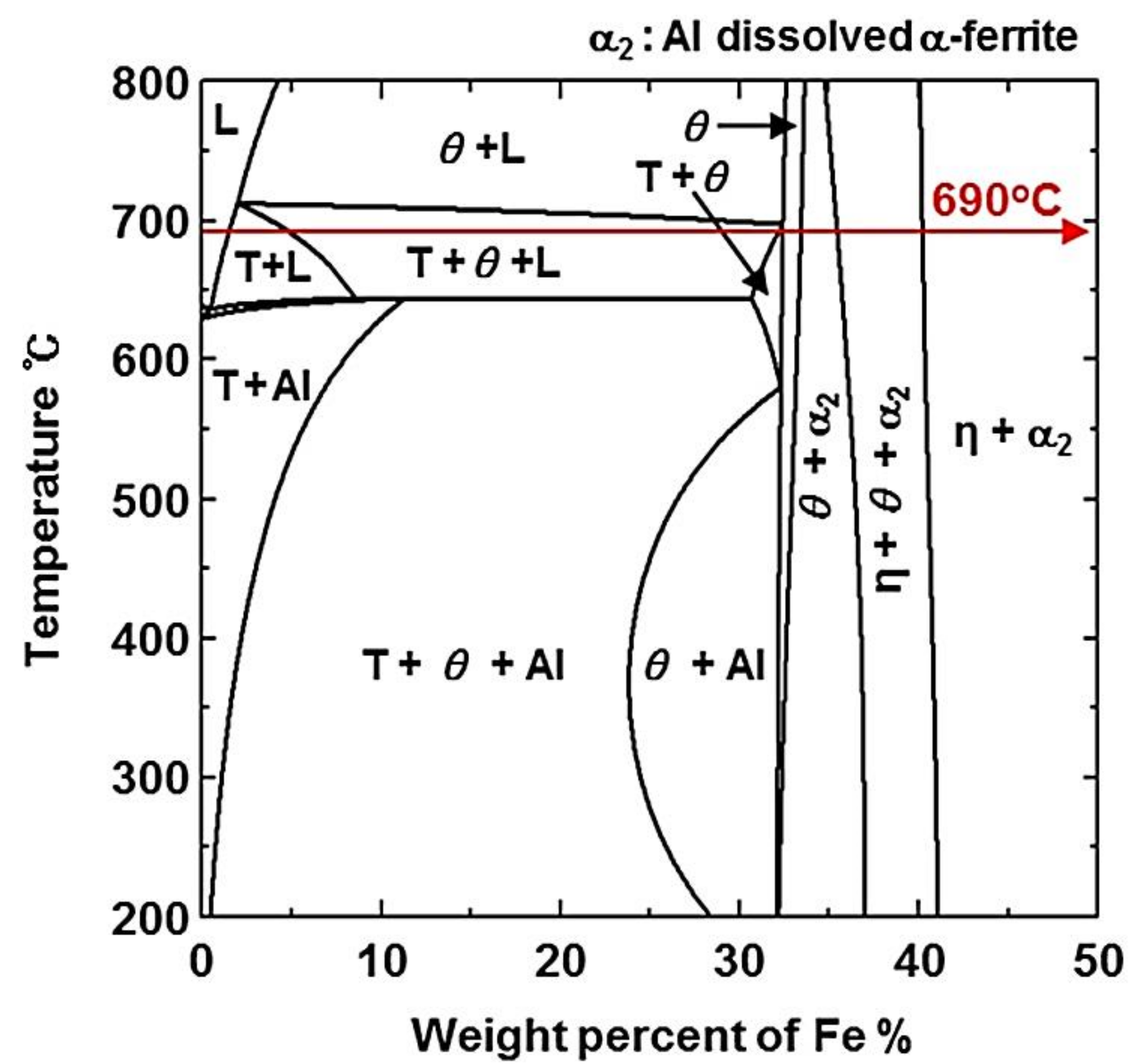
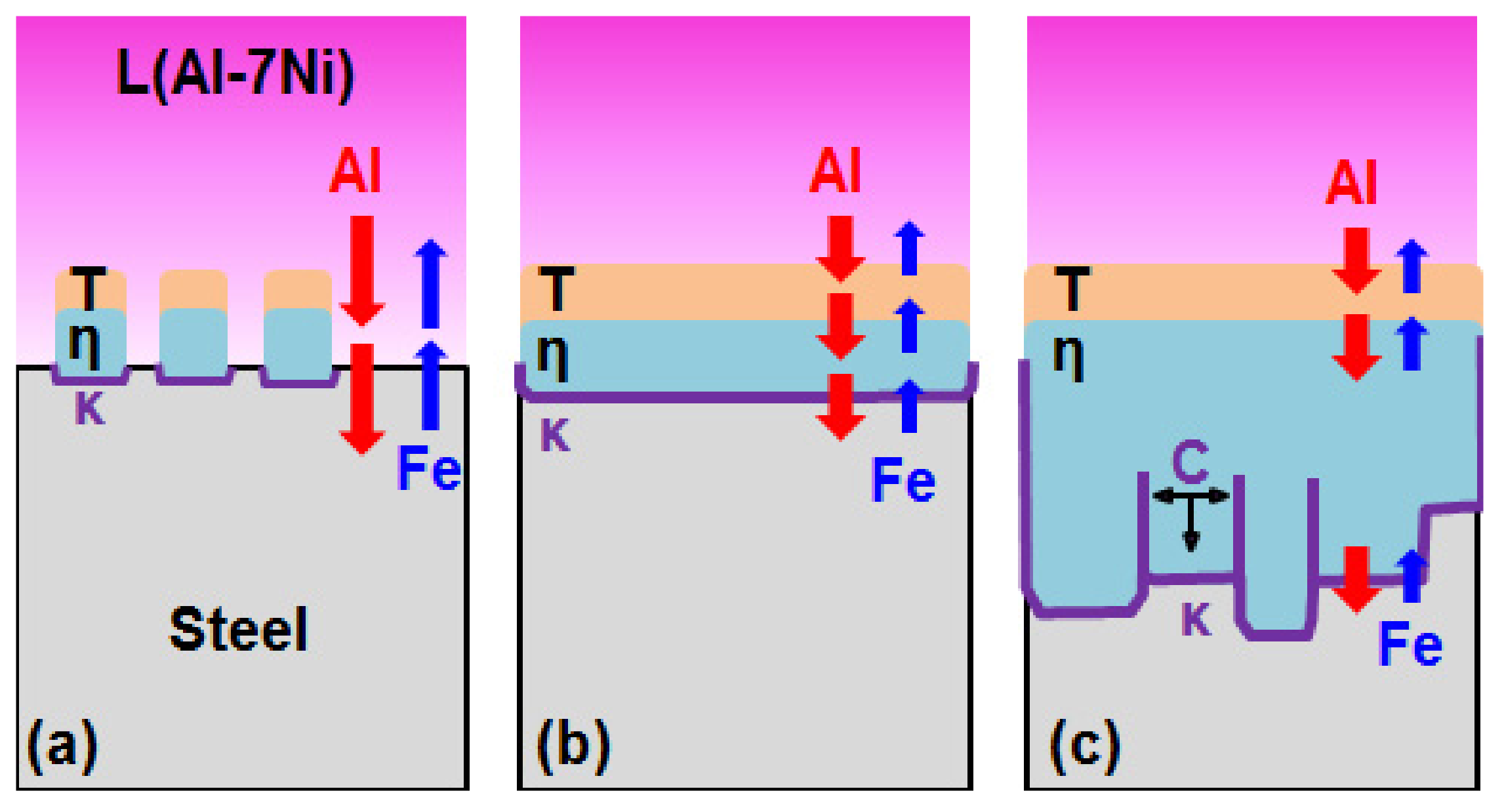
- From the (Al–7Ni)–xFe (wt %) quasi-binary phase diagram, a coexistence zone of the Al9FeNi (T) phase + liquid (Al–Ni alloy) phase was identified in the composition range of 1.5–5.0 wt % Fe. This implies that 1.5 wt % or more of Fe dissolved into the liquid phase formed the Al9FeNi (T) phase and the liquid phase at 690 °С initially, and the Al phase was formed by cooling the liquid phase. The Al9FeNi (T) phase acts as a channel for Al, but as a barrier for Fe, and facilitates only grain growth without significant change in the thickness.
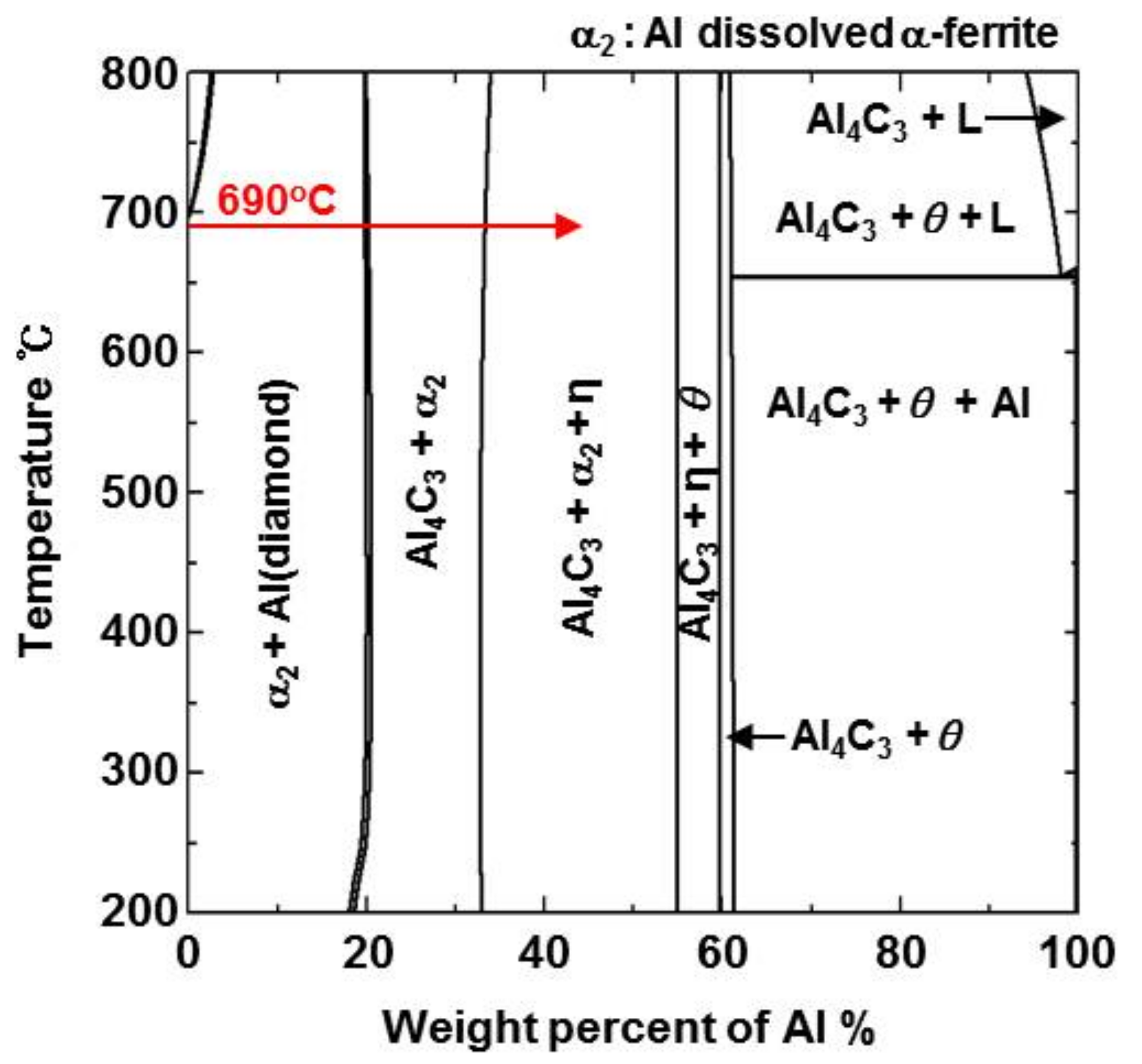
- From the Steel–xAl binary phase diagram, a coexistence zone of the α2 and Fe2Al5 (η) phase was identified in the composition range of 32–55 wt % Al. Therefore, Al diffused according to the sequence Al9FeNi (T) phase → Fe2Al5 (η) phase → steel, and the Fe2Al5 (η) phase with a polygonal structure grew in a columnar form in the [001] direction along the c-axis where Al diffused easily.
- C did not exhibit dissolution into the Fe2Al5 (η) phase and diffused toward the Fe2Al5 (η) phase/steel interface. Moreover, the concentration of Al decreased, resulting in the formation of the Fe3AlC (κ) phase at the steel interface.
- As the dipping time increased, the intermetallic compounds grew along the surface and toward the depth of the steel, and finally combined to form a single coating layer.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saha, D.C.; Biro, E.; Gerlich, A.P.; Zhou, Y.N. Fiber Laser Welding of Al–Si-Coated Press-Hardened Steel. Procedia Eng. 2016, 95, 147–156. [Google Scholar]
- Kang, C.Y.; Jung, B.H.; Kong, J.P. Effect of Hot-stamping Heat Treatment on the Microstructure of Al-Segregated Zone in TWB Laser Joints of Al–Si-coated Boron Steel and Zn-coated DP Steel. Korean J. Met. Mater. 2012, 50, 455–462. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, D.; Han, L.; Liu, D. Interfacial microstructure and mechanical property of resistance spot welded joint of high strength steel and aluminium alloy with 4047 AlSi12 interlayer. Mater. Des. 2014, 57, 186–194. [Google Scholar] [CrossRef]
- Kang, M.; Kim, C. Joining Al 5052 alloy to aluminized steel sheet using cold metal transfer process. Mater. Des. 2015, 81, 95–103. [Google Scholar] [CrossRef]
- Sun, J.; Yan, Q.; Gao, W.; Huang, J. Investigation of laser welding on butt joints of Al/steel dissimilar materials. Mater. Des. 2015, 83, 120–128. [Google Scholar] [CrossRef]
- Chen, R.; Wang, C.; Jiang, P.; Shao, X.; Zhao, Z.; Zhongmei, G.; Yue, C. Effect of axial magnetic field in the laser beam welding of stainless steel to aluminum alloy. Mater. Des. 2016, 109, 146–152. [Google Scholar] [CrossRef]
- Sun, J.; Yan, Q.; Li, Z.; Huang, J. Effect of bevel angle on microstructure and mechanical property of Al/steel butt joint using laser welding-brazing method. Mater. Des. 2016, 90, 468–477. [Google Scholar] [CrossRef]
- Bahadur, A.; Mohanty, O.N. Structural studies of hot dip aluminized coatings on mild steel. Mater. Trans. JIM 1991, 11, 1053–1061. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Characterization of intermetallic compounds formation in aluminide/nickel duplex coating on mild steel. Mater. Charact. 2012, 69, 63–70. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Crystallography of Fe2Al5 phase at the interface between solid Fe and liquid Al. Intermetallics 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Bouche, K.; Barbier, F.; Coulet, A. Intermetallic compound layer growth between solid iron and molten aluminum. Mater. Sci. Eng. A 1998, 249, 167–175. [Google Scholar] [CrossRef]
- Shahverdi, H.R.; Ghomashchi, M.R.; Shabestari, S.; Hejazi, J. Microstructural analysis of interfacial reaction between molten aluminium and solid iron. J. Mater. Process. Technol. 2002, 124, 345–352. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Morphology and formation of Fe–Al intermetallic layers on iron hot-dipped in Al–Mg–Si alloy melt. Intermetallics 2014, 54, 136–142. [Google Scholar] [CrossRef]
- Yin, F.; Zhao, M.; Liu, Y.; Han, W.; Li, Z. Effect of Si on growth kinetics of intermetallic compounds during reaction between solid iron and molten aluminum. Trans. Nonferr. Met. Soc. China 2013, 23, 556–561. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Growth of intermetallic compounds in the aluminide mild steel during hot-dipped. Surf. Coat. Technol. 2009, 204, 824–828. [Google Scholar] [CrossRef]
- Chen, S.; Yang, D.; Zhang, M.; Huang, J.; Zhao, X. Intermetallic between the growth and dissolution of intermetallic compounds in the interfacial reaction between solid iron and liquid aluminum. Metall. Mater. Trans. A 2016, 47, 5088–5100. [Google Scholar] [CrossRef]
- Sasaki, T.; Yakou, T. Features of intermetallic compounds in aluminized steels formed using aluminum foil. Surf. Coat. Technol. 2006, 201, 2131–2139. [Google Scholar] [CrossRef]
- Wang, D.; Shi, Z.; Zou, L. A liquid aluminum corrosion resistance surface in steel substrate. Appl. Surf. Sci. 2003, 214, 304–311. [Google Scholar]
- Van Alboom, A.; Lemmens, B.; Breitbach, B.; de Grave, E.; Cottenier, S.; Verbeken, K. Multi-method identification and characterization of the intermetallic surface layers of hot-dip Al-coated steel: FeAl3 or Fe4Al13 and Fe2Al5 or Fe2Al5+x. Surf. Coat. Technol. 2017, 324, 419–428. [Google Scholar] [CrossRef]
- Naoi, D.; Kajihara, M. Growth behavior of Fe2Al5 during reactive diffusion between Fe and Al at solid-state temperatures. Mater. Sci. Eng. A 2007, 459, 375–382. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Khitouni, M.; Escoda, L.; Sunol, J.J. Recovery, grain growth and recrystallization of mechanically alloyed FeAl alloy. IOP Conf. Ser. Mater. Sci. Eng. 2010, 13, 012021. [Google Scholar] [CrossRef]
- Heumann, T.; Dittrich, N. Structure character of the Fe2Al5 intermetallics compound in hot dip aluminizing process. Z. Metallk 1959, 50, 617–625. [Google Scholar]
- Burkhardt, U.; Grin, Y.; Ellner, M. Structure Refinement of the Iron-Aluminum Phase with the Approximate Composition Fe2Al5. Acta Cryst. B 1994, 50, 313–316. [Google Scholar] [CrossRef]
- Sasaki, T.; Yakou, T.; Mochiduki, K.; Ichinose, K. Effects of carbon contents in steels on alloy layer growth during hot-dip aluminum coating. ISIJ Int. 2005, 45, 1887–1892. [Google Scholar] [CrossRef]
- Pasche, G.; Scheel, M.; Schäublin, R.; Hébert, C.; Rappaz, M.; Hessler-Wyser, A. Time-resolved X-ray microtomography observation of intermetallic formation between solid Fe and liquid Al. Met. Mater. Trans. Coruña 2013, 44, 4119–4123. [Google Scholar] [CrossRef]
- Awan, G.H.; ul Hasan, F. The morphology of coating/substrate interface in hot-dip-aluminized steels. Mater. Sci. Eng. A 2008, 472, 157–165. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Microstructural evolution of intermetallic layer in hot-dipped aluminide mild steel with silicon addition. Surf. Coat. Technol. 2011, 205, 4726–4731. [Google Scholar] [CrossRef]
- Sakidja, R.; Perepezko, J.H.; Calhoun, P. Synthesis, Thermodynamic Stability and Diffusion Mechanism of Al5Fe2-Based Coatings. Oxid. Met. 2014, 81, 167–177. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Yun, J.-G.; Lee, J.-H.; Kwak, S.-Y.; Kang, C. Microstructural Evolution of Intermetallic Compound Formed in Boron Steel Hot-Dipped in Al–7%Ni Alloy. Metals 2017, 7, 393. [Google Scholar] [CrossRef]





| % | Fe | O | B | C |
|---|---|---|---|---|
| at % | 45.5 | 47.0 | 3.3 | 4.2 |
| wt % | 76.0 | 22.4 | 1.1 | 0.5 |
| No. | Chemical Composition (at %) | Expected Phase | ||
|---|---|---|---|---|
| Al | Ni | Fe | ||
| 1 | 80.1 | 8.30 | 11.6 | Al9FeNi (T) |
| 2 | 74.5 | 1.2 | 24.3 | Fe2Al5 (η) |
| No. | Chemical Composition (at %) | Expected Phase | ||
|---|---|---|---|---|
| Al | Ni | Fe | ||
| 1 | 81.4 | 7.2 | 11.5 | Al9FeNi (T) |
| 2 | 82.5 | 8.6 | 8.9 | Al9FeNi (T) |
| 3 | 76.8 | 2.3 | 20.7 | Fe2Al5 (η) |
| 4 | 77.7 | – | 22.3 | Fe2Al5 (η) |
| 5 | 100 | – | – | Al |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Yun, J.-G.; Kwak, S.-Y.; Kang, C.-Y. Nucleation and Growth of Intermetallic Compounds Formed in Boron Steel Hot-Dipped in Al–Ni alloy. Coatings 2017, 7, 195. https://doi.org/10.3390/coatings7110195
Lee J-H, Yun J-G, Kwak S-Y, Kang C-Y. Nucleation and Growth of Intermetallic Compounds Formed in Boron Steel Hot-Dipped in Al–Ni alloy. Coatings. 2017; 7(11):195. https://doi.org/10.3390/coatings7110195
Chicago/Turabian StyleLee, Jae-Hyeong, Jung-Gil Yun, Sung-Yun Kwak, and Chung-Yun Kang. 2017. "Nucleation and Growth of Intermetallic Compounds Formed in Boron Steel Hot-Dipped in Al–Ni alloy" Coatings 7, no. 11: 195. https://doi.org/10.3390/coatings7110195





