Four IPN specimens were taken as representative among the many (the investigations were performed using more than 100 preparations [
17] so as to cover many parameter effects); they were named as S4, S6, SF1, and SF2, respectively (
Table 1). S4 and S6 are composed of a mixture of three resins (silicone, epoxy, and carboxylic acrylic) together to pigment black, functional fillers, and additives. A phenyl silicone resin is present in S4, while a methyl-phenyl silicone resin is present S6, with the same weight fraction. In SF1 and SF2 IPNs, the same S6 formulation was maintained, except for partial substitution of the pigment concentration by graphene nano-platelets, with different weight fractions. Functional fillers and additives were added to decrease the amount of pyrolytic decomposition of the organic binder and to avoid defect formation during the film-forming process, respectively [
19].
3.1. Adhesion
In order to satisfactorily prevent substrate oxidation in thermal oxidative conditions, coatings must adhere to the surface on which they are applied [
4,
5]. Cross-cut tests were carried out to assess film adhesion before and after heat treatment.
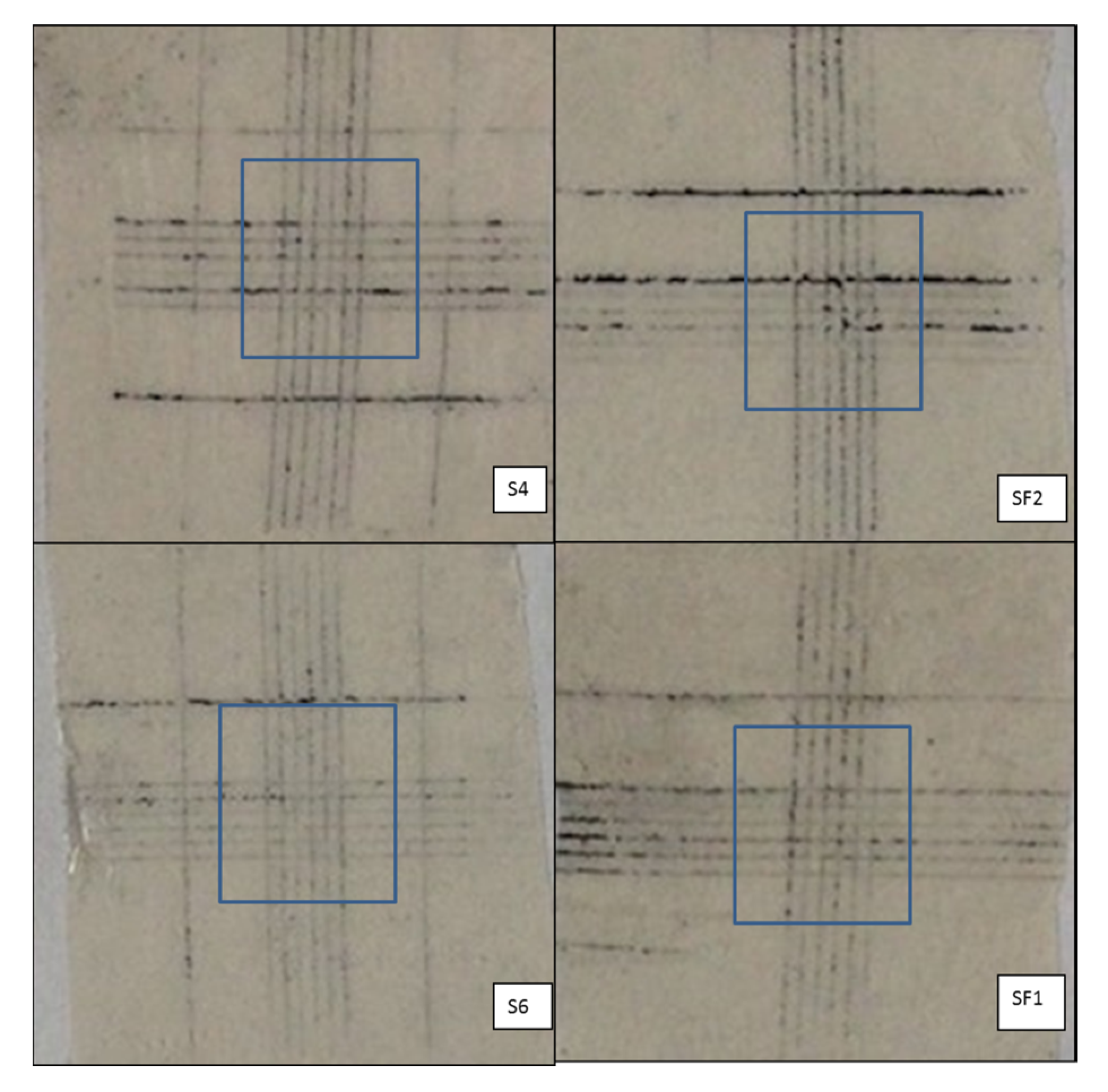
The images of the coated surfaces printed on the tapes, removed from the cross-cut areas, are presented in
Figure 1 and
Figure 2 in order to provide evidence of the flaking (powdering) degree. Optical microscopy has been used to obtain the high-definition images for a reliable evaluation. S6 and SF1 paint formulations show the best adhesion before heat treatment and their rating is consequently classified as 0 (
Table 3 according to ISO 2409:2007 (E)); a different intensity of failures was found for the S4 and SF2 samples.
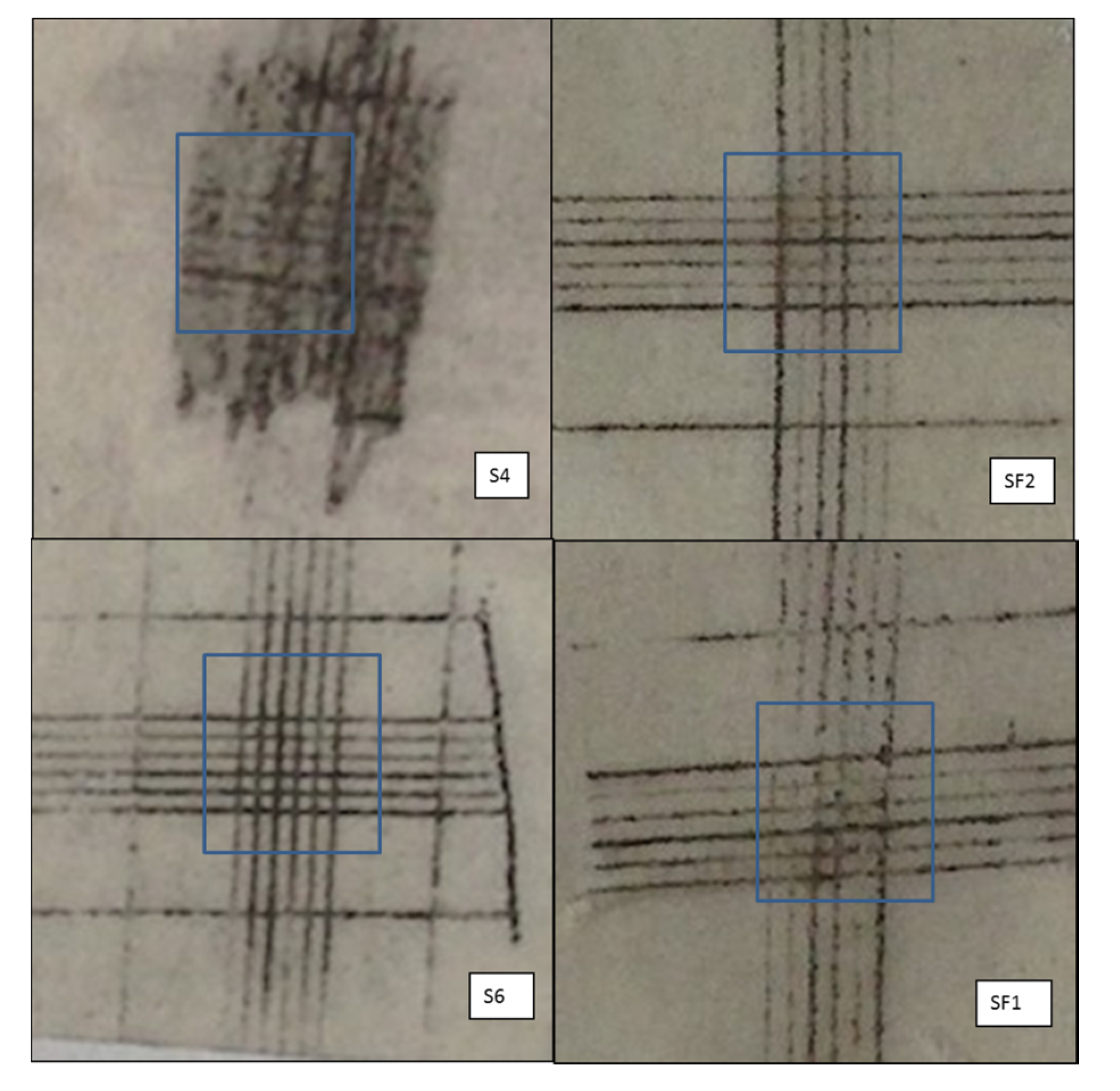
After thermal treatment (the tape images, after heat treatment, are reported in
Figure 2), a strong pyrolytic decomposition of the IPN organic part was expected. Indeed, although silicone resin backbone has high thermal stability, its organic substituents suffer strong degradation in air at
T > 300 °C [
20,
21,
22,
23,
24]. According to [
20], the oxygen catalyses the silicone weight loss, the residual part of it (the backbone) undergoes the formation of a thermally-stable and tightly-reticulated, more inorganic than organic, network. Furthermore, the adhesion on the substrate may be promoted by the high temperature due to element inter-phase diffusion. Thus, cross-cut tests were carried out one more time to check the substrate coating adhesion and the silicone effect. The adhesion classification, before and after HT, is reported in
Table 3.
The S4 samples have the weakest adhesion with a worsening after HT, while the S6 and SF1 coatings remain almost stable and the SF2 samples seems to show a small increase of the adhesion after heat treatment. Adhesion tests suggest that the phenyl amount on silicone chains have to be reduced: phenyl silicone S4 sample indeed shows a bad result on cross-cut test after heat treatment. In an attempt to explain the behaviour, we could hypothesize that carbon and carbonaceous residues would remain for a longer time in the silicon oxide network and interfere with the formation of bonds to the metal during pyrolysis due to slow combustion kinetic of aromatic substituent at 450 °C.
Indications of chalking can be inferred from the images of the adhesion tests; firstly, referring to the high degradation degree of the S4 sample (
Figure 2), the tape surface does not present any transparency over the whole cutting area caused by the de-structured binder. In this case, the phenyl silicone resin shows a strong and intense chalking, so the cross-cut area is much darker in S4 tape in comparison with the same area of methyl-phenyl silicone-containing samples.
For the sake of completeness, in
Figure 3 we report the images of S6 and SF1 panels after heat treatment. At the same adhesion, no shadows are present on the SF1 surface, both in and out of the cutting area, indicating a more limited chalking.
The presence of graphene probably increases adhesion after thermal treatment as the SF2 sample assessment is two steps before HT (
Table 3, change from two to one). As expected, the chalking increases after heating as supported by the bad transparency of the tapes, as reported in
Figure 2. However, from the visual exam of the panel instead of the tapes after HT (
Figure 3), SF1 presents fewer shadows than S6 indicating a less chalking degree. Investigating the filler amount effect, SF1 and SF2 present little and no discernible cleanliness difference on tapes.
The superior resistance to chalking produced by graphene addition may be related to enhanced properties of polymer-graphene nanocomposites [
10]. The network of graphene nanoplatelets inside the binder matrix is supposed to increase mechanical and thermal performances at high temperatures [
9,
11]. Finally, thermal conductivity of the graphene sheet may favor the homogeneous degradation of the organic part of the IPNs binder, avoiding high-temperature peaks [
4].
3.2. Thermal Analysis
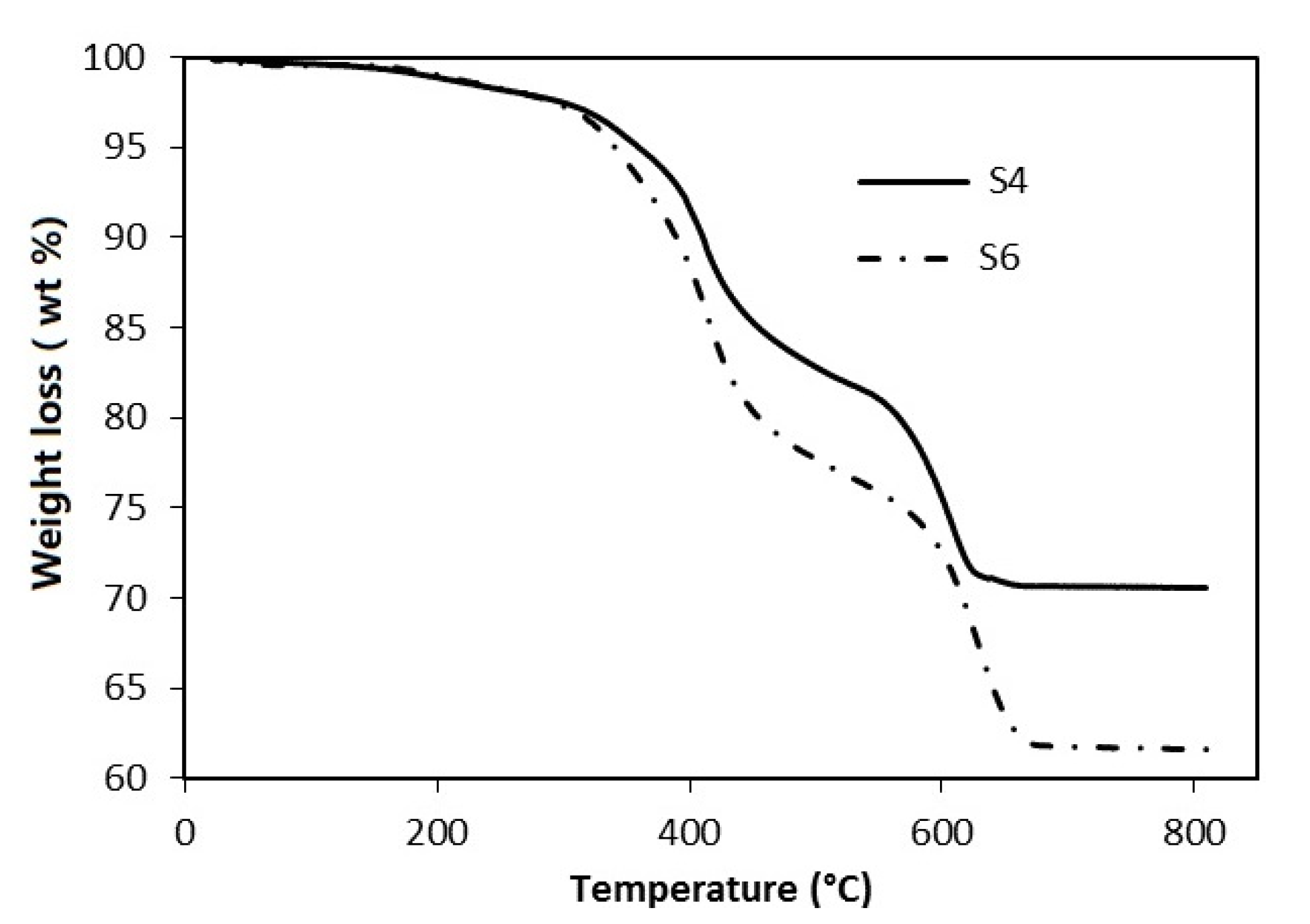
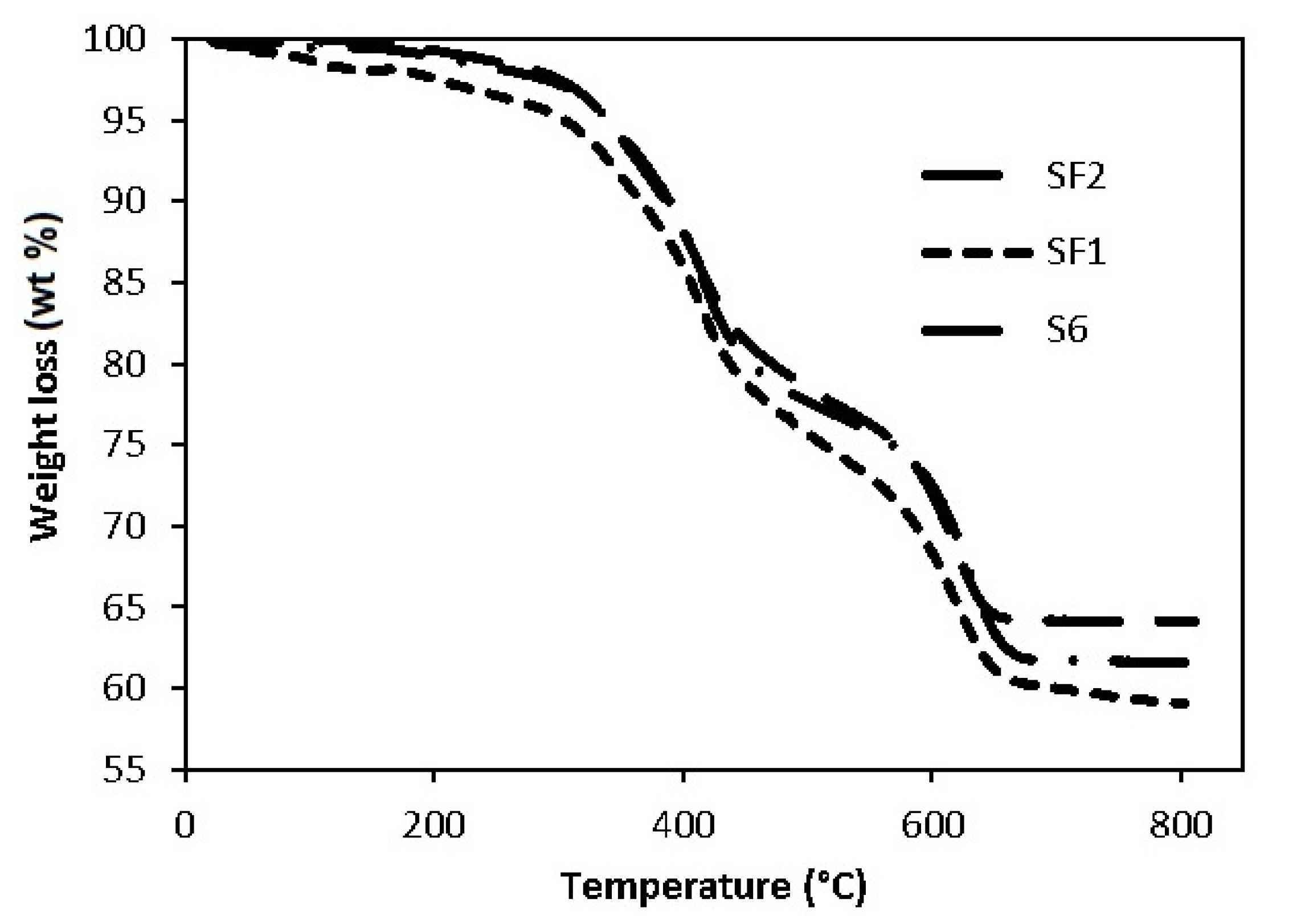
The thermo-gravimetric analysis (TG) provides information on thermal stability (weight loss at increasing temperature) of coatings after heat curing (210 °C) and suggests hypotheses about the degradation mechanism. All curves are characterized by two weight losses (
Figure 4) according to the cited literature with different rates of degradation.
The first and the second weight loss (wt %) are reported in
Table 4 relative to the four examined samples: the total losses are in the 26–37.3% range at high temperatures (680 °C, max). The result is related not only to the presence of a high amount of functional fillers in each formulation, as reported in
Table 1, but also to the presence of silicone polymers that have high residual masses after thermal degradation (up to 55% for methyl-phenyl [
24]). Nevertheless, the oxidative cross-linking reaction rearranges all the residual organosilanes to silica. The reaction mechanism is probably triggered by the formation of radicals on side groups that react with oxygen and produce peroxide functions which accelerate the rearrangement. Finally, in an oxidative environment, some silica may be obtained from the competition between volatilization of oligomers and oxidative cross-linking [
20,
21,
22,
25].
The S4 sample shows the lowest total wt %, mainly concentrated at low temperatures. S6 and SF1 are characterized by higher weight losses than S4, while SF2 presents an intermediate total wt %. The similarity of the second step wt % between S6 and SF1 allowed us to conclude that weight loss does not depend on graphene. We have to note that, from the thermogravimetric point of view, the SF2 losses are less than both S6 and SF1, and are probably due to a different formulation (more pigment is present, as reported in
Table 1).
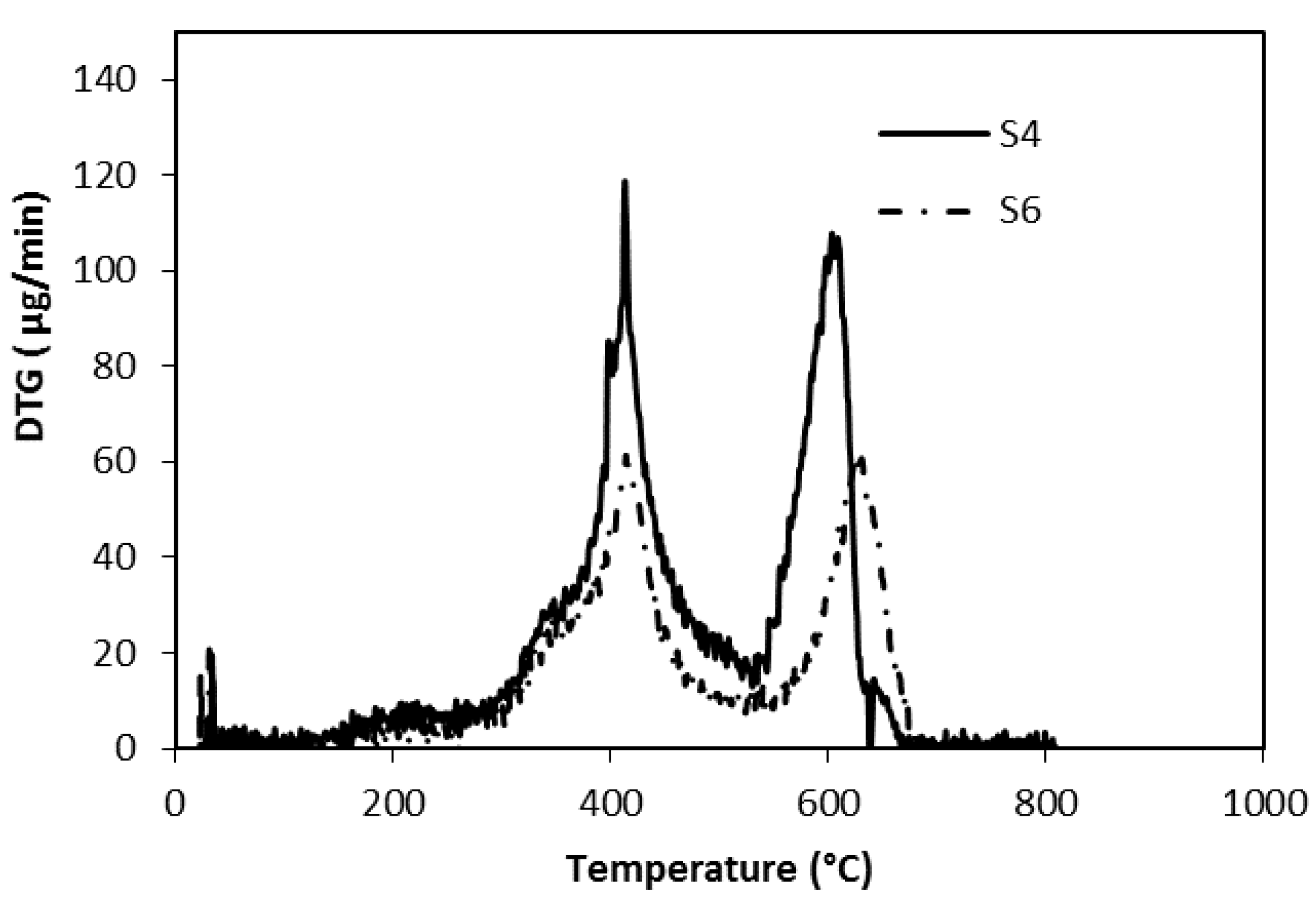
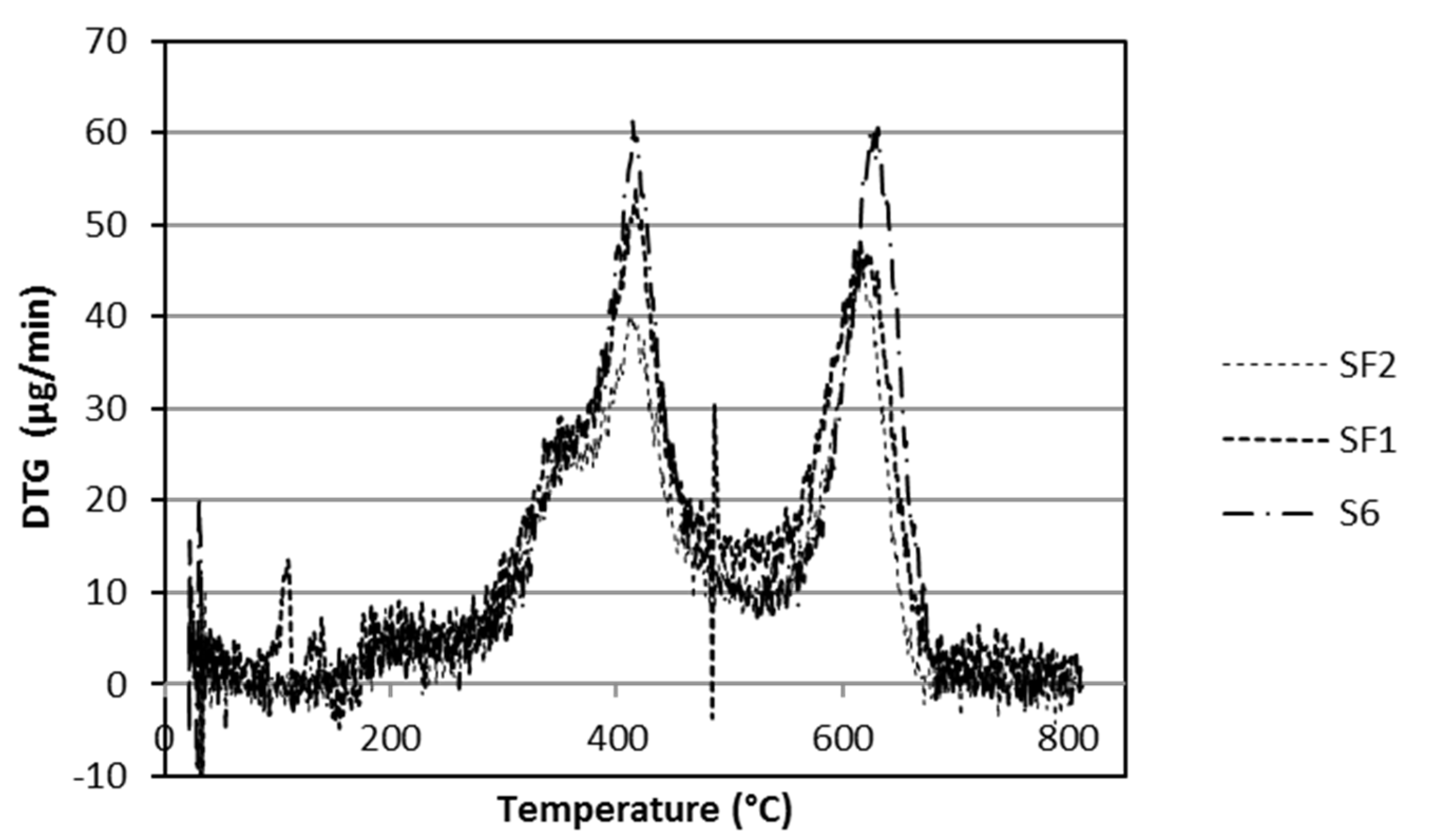
The presence of two strong weight losses were highlighted by time differentiating the TG curves of S4 and S6 samples, as reported in
Figure 5.
For both of these samples, two maxima appear at 413 °C and 630 °C, respectively. The first ones have a similar shape (with a shoulder before the peak) and the same maximum temperatures at 413 °C, suggesting the same thermal effect characterized by a beginning lower than 300 °C that could be attributed to the depolymerisation and bond breakings of epoxy-acrylic organic binder fraction. Therefore, DTG analysis revealed that thermal behaviour of epoxy-acrylic binder is similar, notwithstanding the different substituents (phenyl, instead of methyl groups). The only difference is the shoulder that has a different intensity is probably due to the phenyl group fraction.
Once again, the phenyl group seems responsible for the significant temperature differences attributed to the oxidation of organic substituents of silicones, highlighted for the second peak: this appears 27 °C before in the S4 formulation than in S6. Thermal analysis is in agreement with the literature for PDMS stability [
20,
21,
22,
23,
24], even when considering the presence of other components inside formulations. The temperature difference between the two high-temperature DTG peaks may be attributed to different organic parts, as S4 silicon resin has phenyl groups only, while S6 has methyl and phenyl substituents. The introduction of phenyl substituents on silicone chains has been found to increase the onset temperature of degradation [
21,
22]. According to [
23], the thermal stability of siloxane polymers increases on decreasing the phenyl content and this trend is confirmed by our analyses (the maximum rate of the weight loss on S4 sample appears at ca. 580 °C instead of ca. 600 °C for the S6 sample). Analysing the thermal behaviour at higher temperatures, during polydiphenyl-dimethyl siloxane pyrolysis, evolution of free benzene and volatile cyclic siloxanes occurs, as reported in [
20,
22]. As seen in
Figure 4, the carbon removal, during silicone thermal degradation appears more pronounced at the 280–530 °C weight loss because of a decrease in the phenyl content (a difference of 5.9%); this effect may be due both to the different steric hindrances and to the layout of silicone chains due to methyl of phenyl groups [
23]. Accordingly, the inspections of residues of polydiphenyl-dimethyl siloxanes thermally degraded in air revealed a greyish white powder constituted mainly of white silica and black silicon-oxycarbide [
23,
25] in which Si atoms are simultaneously bonded to carbon and oxygen [
26]. Moreover, from the elemental analysis, the weight percent of C concentrations inside residues has been found to be three times higher in the case of a phenyl polysiloxane with respect to a fully-methylated one [
23]. Hence, as the phenyl content decreases, a more stable and protective Si–O–C layer is formed without aromatic ring interferences, with low carbon content [
24]. As a conclusion, this hypothesis is in accordance with thermogravimetric analysis, which clearly indicates that the S6, SF1, and SF2 samples, composed by a methyl-phenyl silicone, show a greater weight loss than S4. Specifically, thermal analyses indicate that S4 has a lower weight loss than S6, in agreement with the difficulty of carbon residues’ oxidation/separation, as hypothesized above.
Finally, the S4 residue was supposed to present high C content and low ceramic yield. This hypothesis is strongly supported by the lower adhesion and stronger chalking exhibited by the S4 coating with respect to S6, as discussed above [
23,
24]. Moreover, the second DTG peak is about 30 °C before for the phenyl substituted silicon chain, probably meaning that the oxidized fragments are placed in the upper part of the film, in a more favourable position for chalking to occur.
S6, SF1, and SF2 formulations are compared in
Figure 6; the shape of the curves is the same for the three coatings. From the DTG plot, reported in
Figure 7, it is evident that the amount of graphene is not relevant as the weight losses occur at approximately the same temperatures. In comparison with graphene containing formulations, the shape of the DTG curves of S6 are almost the same as SF1 and SF2, with the exception that the rates of weight losses of SF1 and SF2 (these curves are superimposed in
Figure 7), graphene-containing formulations, occur 15 °C before those of the S6 sample, probably due to a different material thermal conductivity.
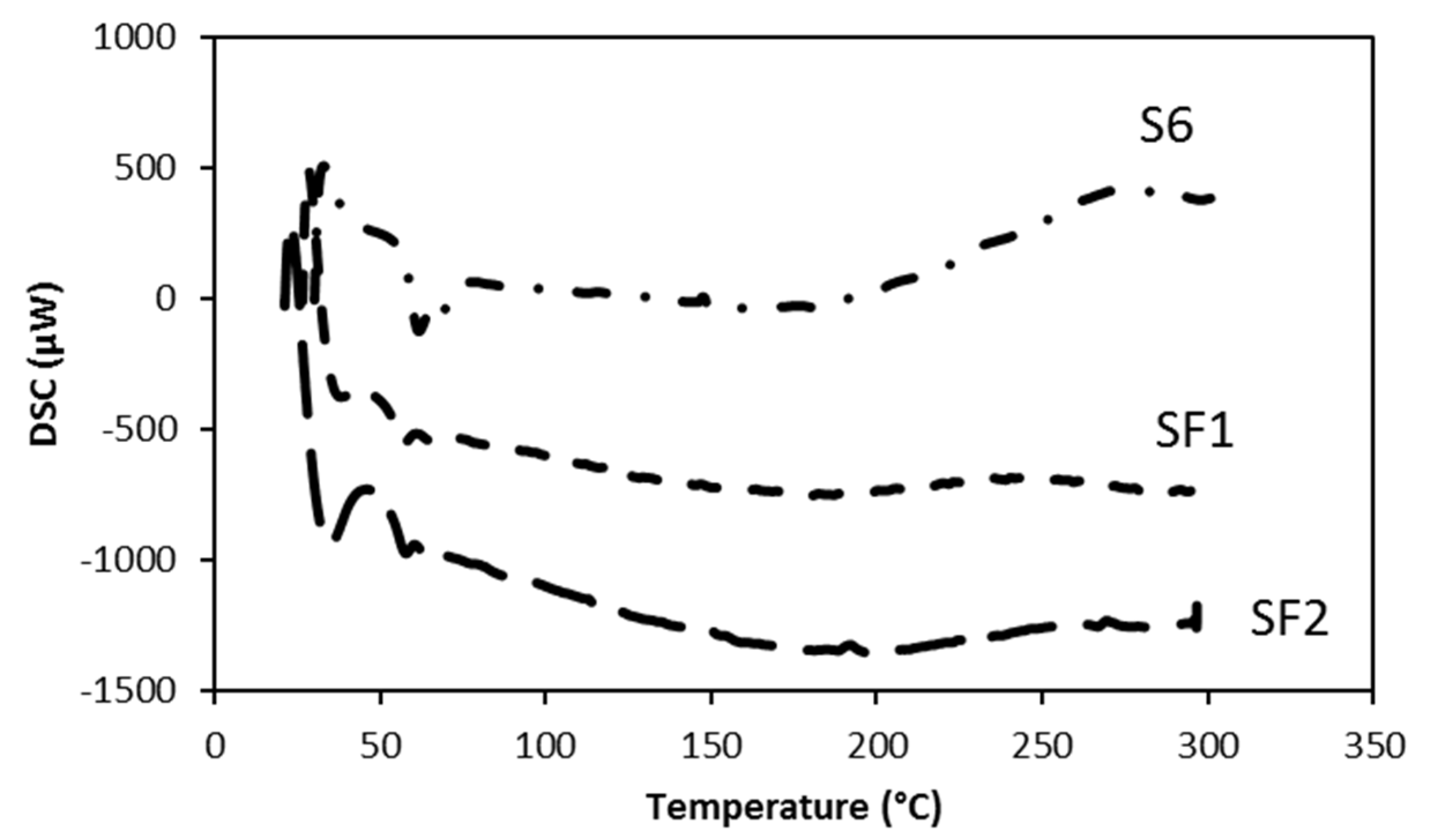
Differential scanning calorimetry (DSC) analyses for the same samples, performed until 300 °C, are reported in
Figure 8. The curves are placed at different μW level and have a similar outline, probably related to the graphene amount. In fact, SF1 and SF2 curves appear flatter mainly after 200 °C, meaning a stability of the specific heat, probably related to graphene amount because of the slope of the curve, decreases in the order: S6 (0% graphene) > SF2 (0.3% graphene) > SF1 (0.5% graphene).
3.3. Film Chemical Composition
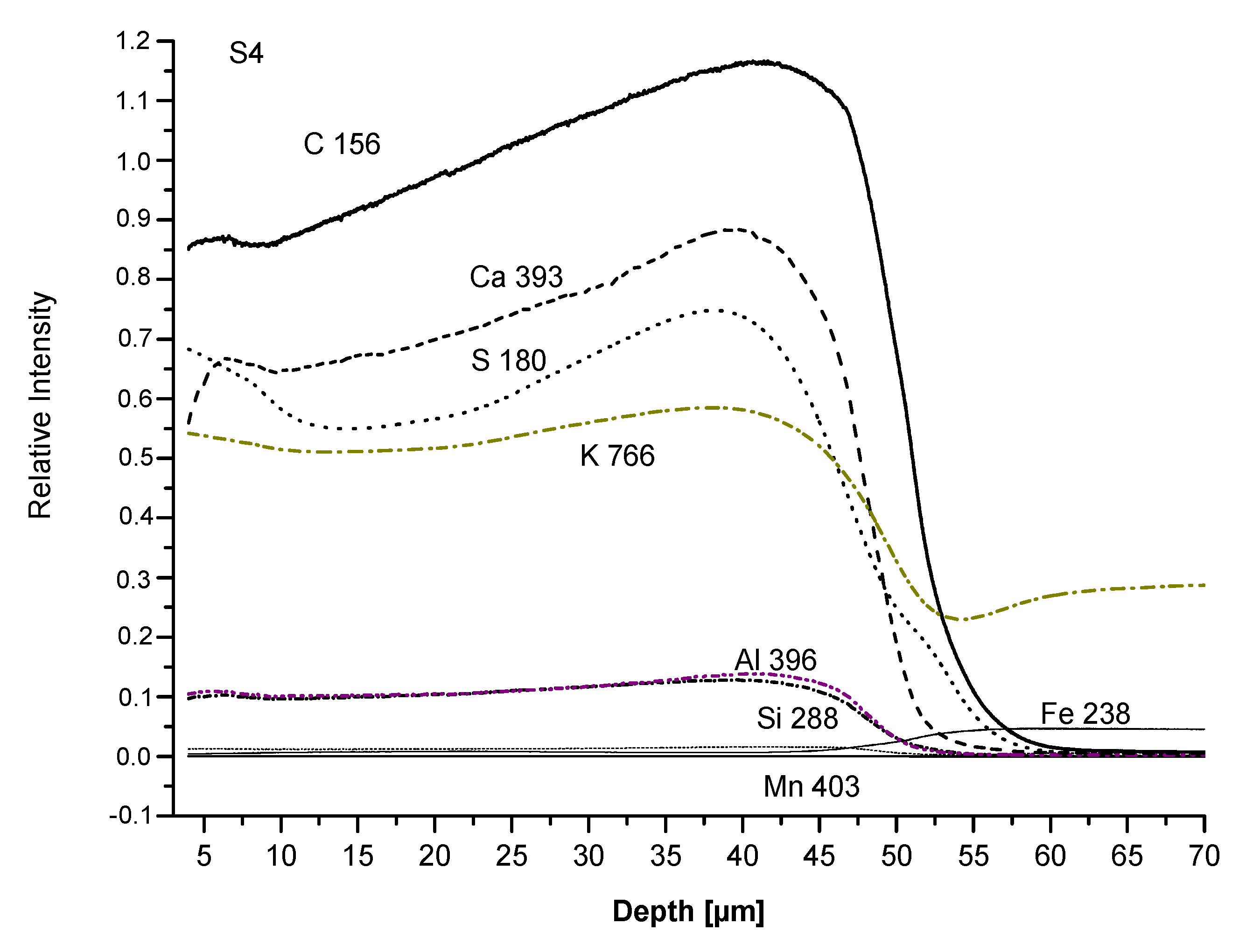
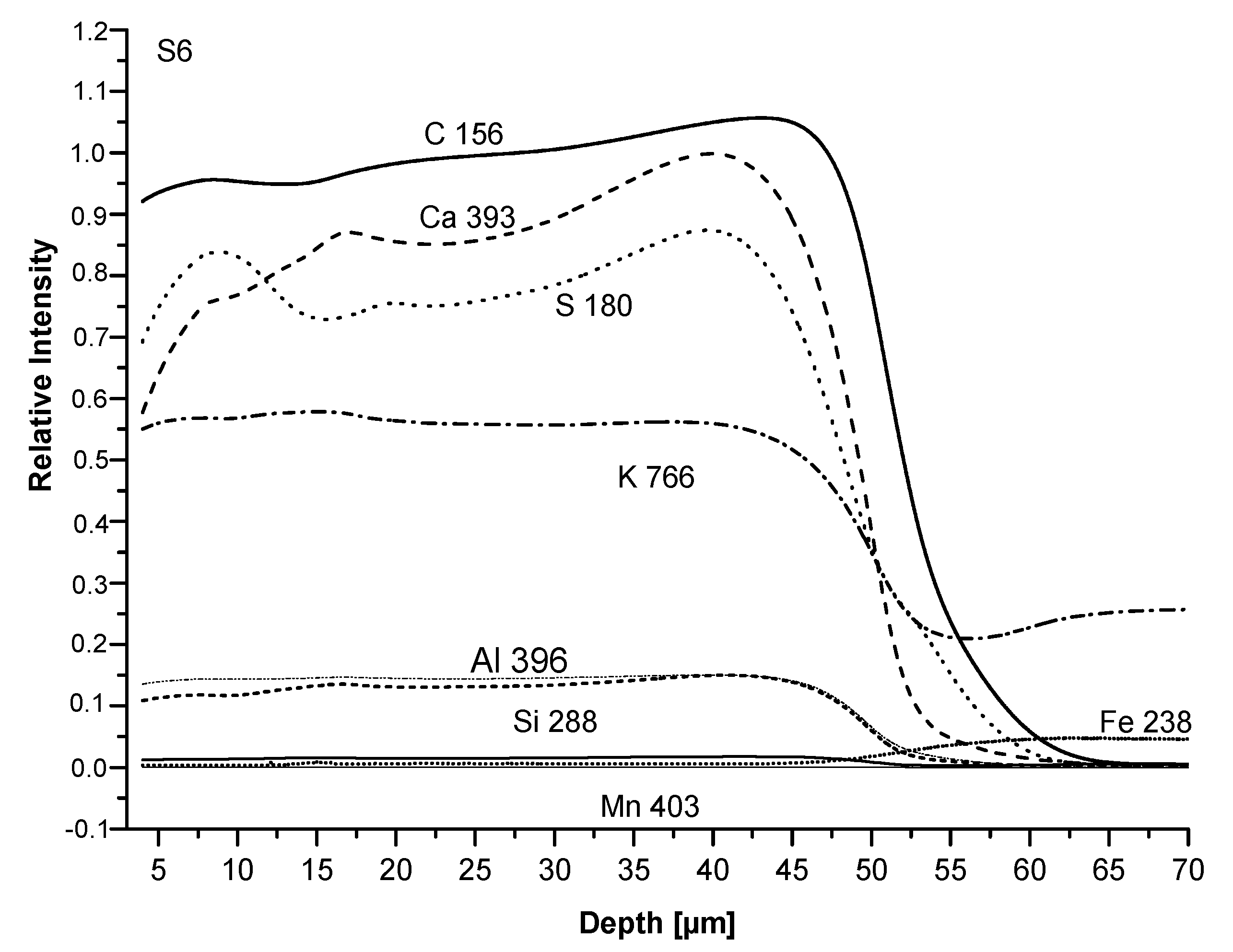
We analysed the S4 and S6 coatings with the Glow discharge optical emission spectroscopy (GDOES). The difference between the two samples is the organic part of silicone binder: methyl (S4) and phenyl-methyl (S6), respectively (
Table 1). The analysis allows checking if some layering had taken place due to the inhomogeneous distribution of pigments and fillers all along the coating thickness. Results are plotted in
Figure 9 and
Figure 10.
Firstly, the film thickness appears properly produced, between 40 and 60 μm. Moreover, no relevant layering is observed in both coatings as the concentration of all elements from the film surface to the substrate both slightly change at the same ratio (C, Ca, S) or remain unchanged (K, Al, Si, Mn). The first group of elements is characterized by concentration gradients; C (from the binder) and Ca appear more densely packed near the substrate, particularly in the S4 than in the S6 sample. Otherwise, the S line, related to the baryte concentration, appears more elevated both at the surface and at the bottom of the film than in the middle. Inversely, S4 and S6 analyses show that the curves related to Mn are nearly horizontal, confirming the homogeneous dispersion of pigment inside the coatings. Similarly, K, Si, and Al concentrations, related to micro mica, appear quite stable. To conclude, the comparison between S4 and S6 coatings indicates a greater homogeneity and lack of important gradients of concentration, this is true of S4 in the S6 composition. For chalking, the GDOES analysis seems to confirm that it depends on the release of pigment particles or extenders (particularly baryte) after the thermal degradation of the binder; no relevant layering of pigment and/or filler appears inside the coating to justify the difference in the chalking performance. From the other site, the increasing binder concentration from the surface towards the substrate, which is very pronounced in the S4 sample, could have caused a deeper degradation and an increase of the particle amount released at a constant concentration of all the other elements.
3.4. SEM Analysis
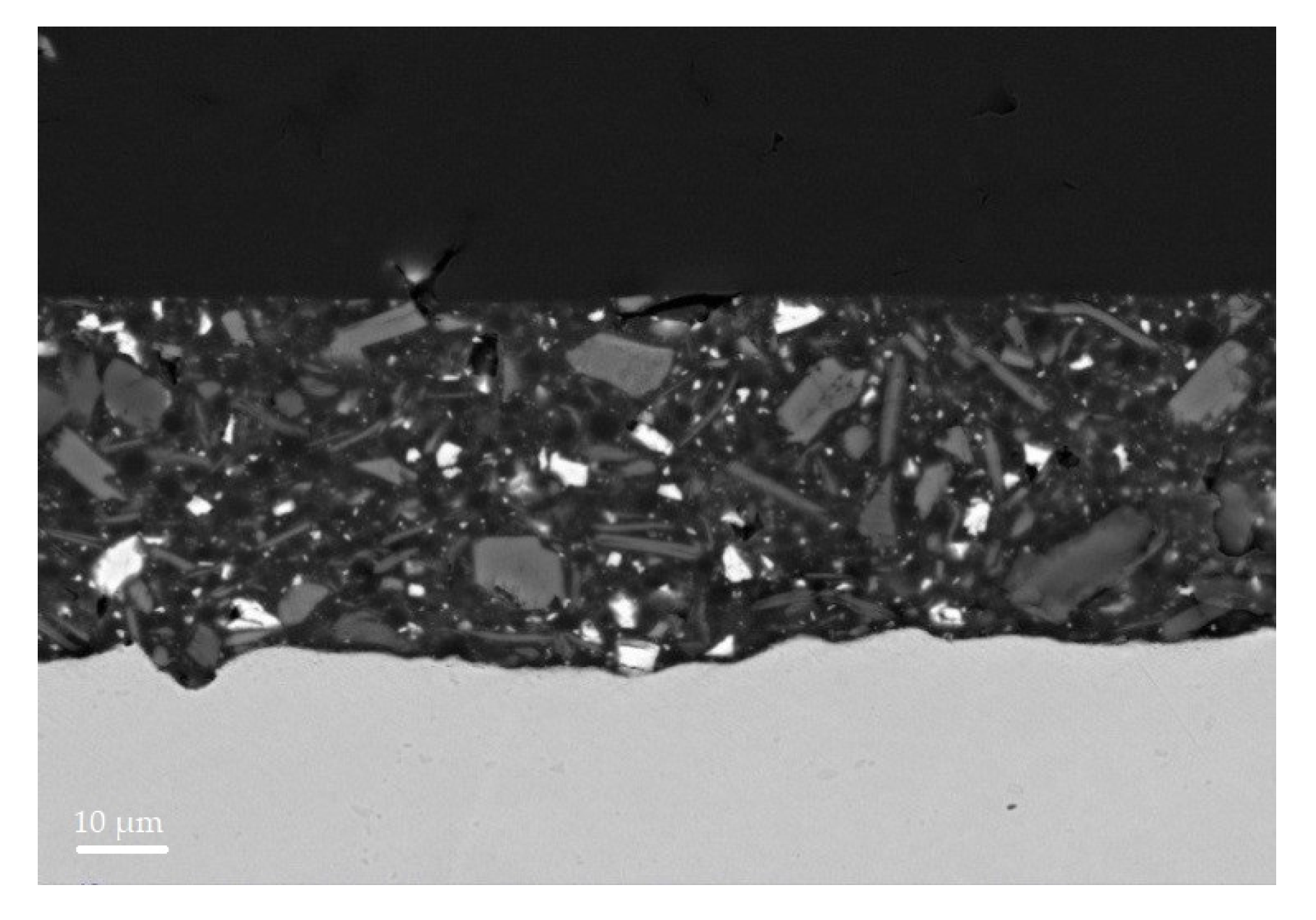
Coating morphology was analysed by scanning electron microscope (SEM) and energy dispersive spectroscopy (EDS). S4 and S6 SEM micrographs, before HT, are reported in
Figure 11 and
Figure 12.
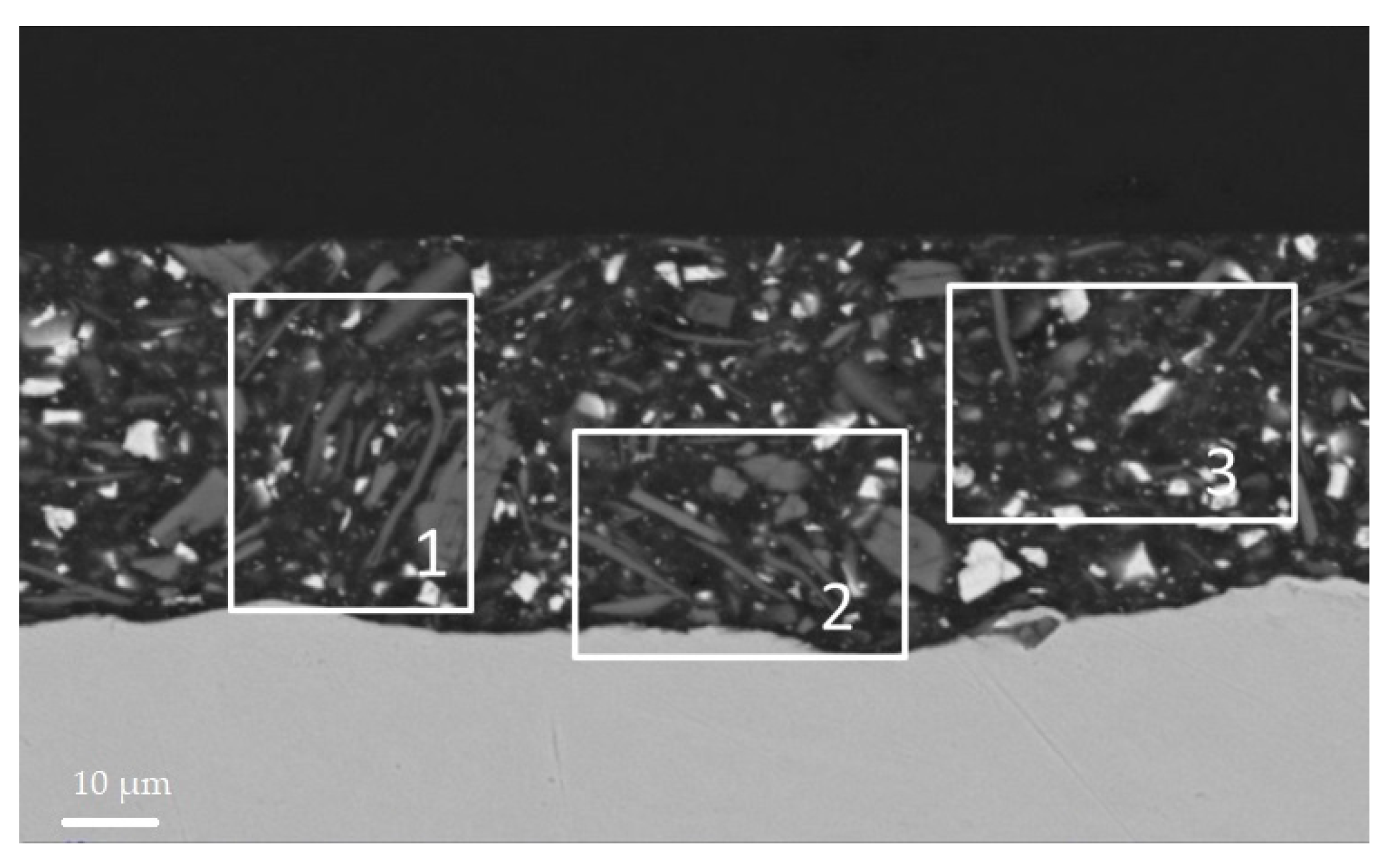
The SEM micrographs of the coating section before the heat treatment help to analyse the distribution of fillers in the film. White spots were found to be baryte, large light grey ones’, wollastonite, and dark grey ones micro mica. By comparing the distribution of the large wollastonite particles it appears that the filler distribution is more homogeneous in the S6 section (
Figure 12). All the particles, however, are (a) more homogeneously distributed in the S6 film than in the S4 one supporting the chalking results; and (b) denser and not homogeneously packed in S4 sample near the substrate surface than in the S6 one, validating the GDOES results.
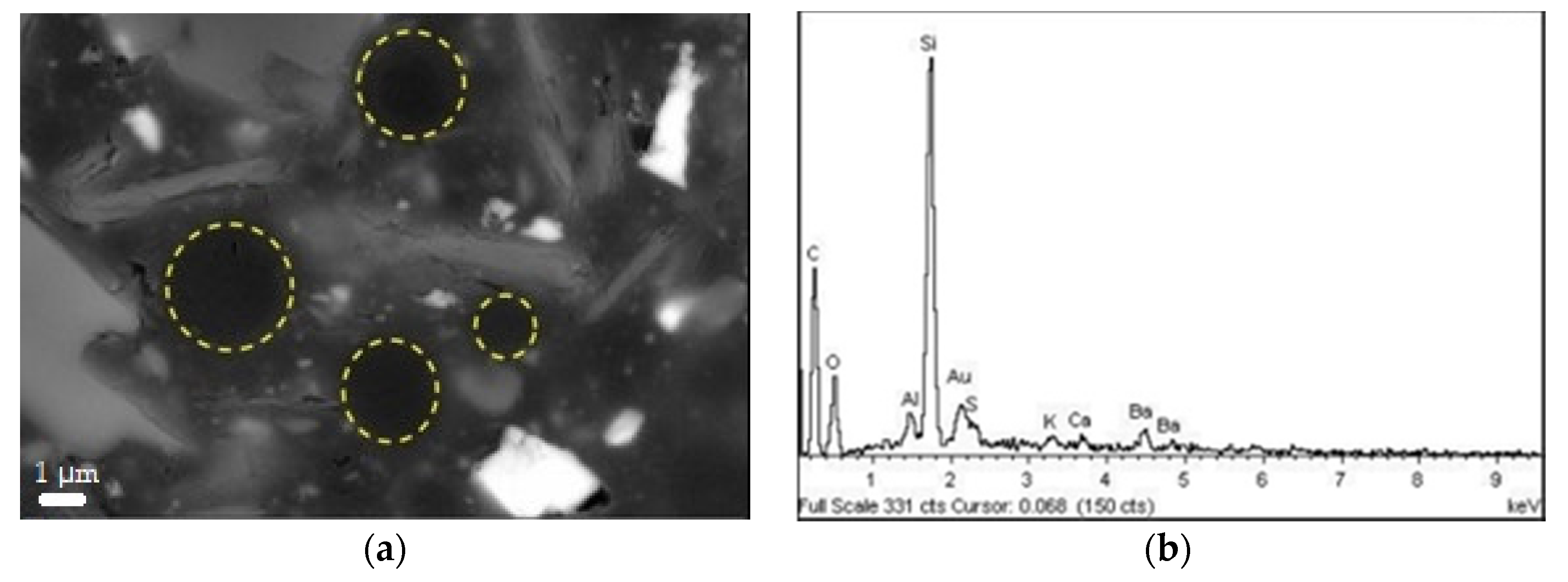
Polymeric domains, with a resembling sphere geometry, were identified in the S6 and not in the S4 coating. Images are reported in
Figure 13. From EDS analysis, the domains are rich of silicone inside IPNs. According to [
27], the uniformly dispersed dark spherical agglomerates might be formed by condensed polyhedral oligomeric 3D siloxane structures. These may be promoted by the presence of low steric hindrance methyl groups, allowing rearrangements of macromolecules. These domains are reinforcing agents and they can properly affect properties of organic-inorganic hybrid composites. Hence, by heat treatment, agglomerates may be converted into thermally-stable silica particles, enhancing substrate adhesion and film cohesiveness. Additionally, the complexity of paint formulations prevents a more precise understanding of the phenomena. In fact, as reported in [
23], the presence of acidic or basic impurities of fillers and residual catalysts might influence the polysiloxane thermo-oxidative degradation mechanism. The choice of the most performant coatings was, however, aimed to link the chalking effect to the film surface morphology after thermal degradation.
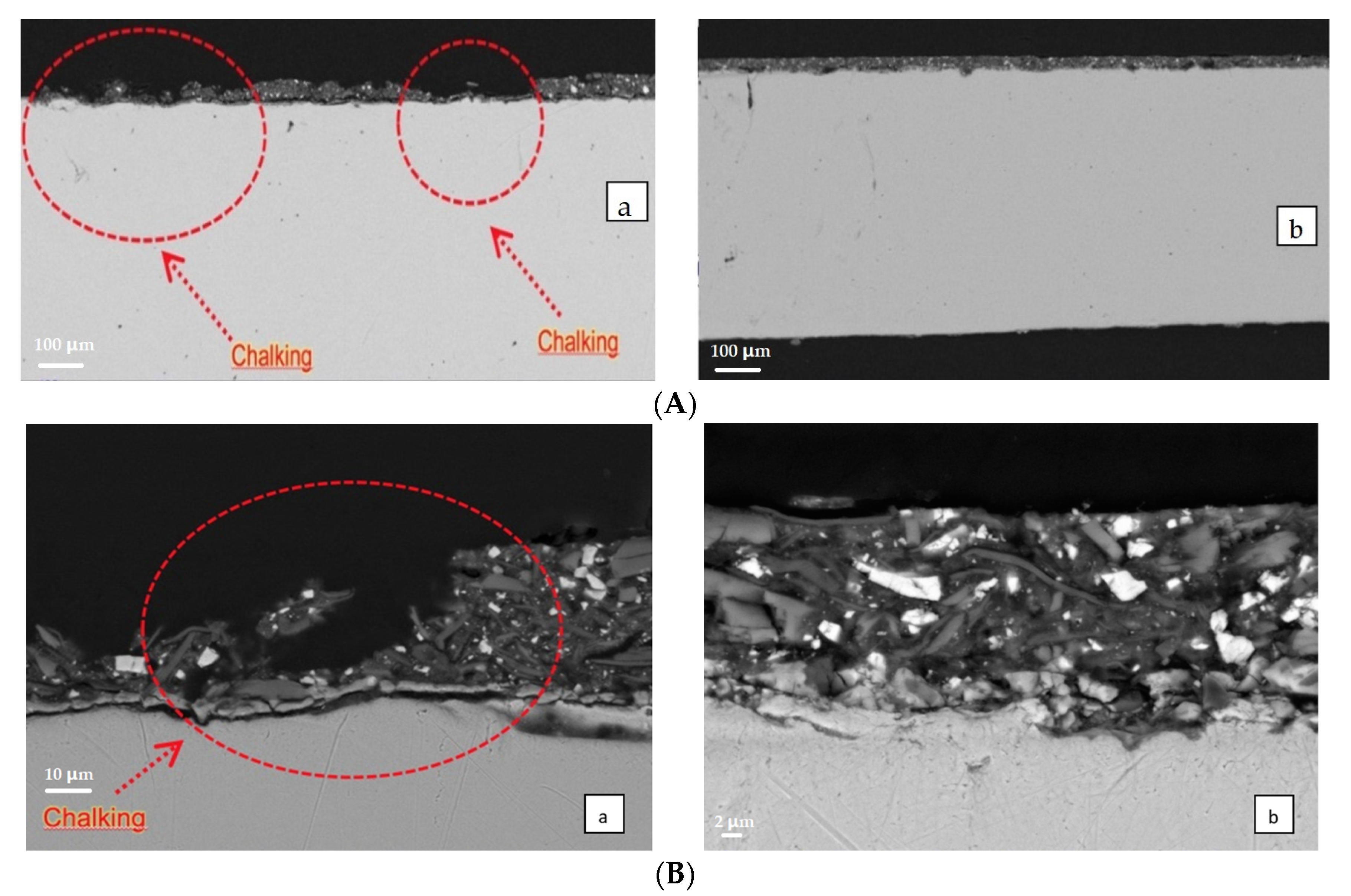
After 12 h at 450 °C, we examined S6 and SF1 samples by SEM-EDS; the low-magnification images are reported in
Figure 14.
The two images at low magnification give a large sampling area and they clearly show large coating failures. It is evident that SF1 has a superior resistance to chalking in comparison with the S6 sample as the integrity of the coating is preserved in the SF1 sample, but not in the S6 one. Examining more deeply the images of
Figure 14, the SF1 coating presents no delamination, blistering, and void formation, while the S6 sample is broken in several points of the coating.
At the end of our investigation, SEM images clearly indicate a greater resistance to deformation and breaking of the graphene containing coatings with respect to the S-type formulations. Superior properties of polymer-graphene nanocomposites are reported in literature [
9,
10,
11,
13]. Moreover, during thermal degradation of polysiloxanes, evolution of free benzene and volatile cyclic oligomers occurs [
19,
20,
22].
According to [
22], the importance of mass transfer of degradation products away from the coating is significant and it controls the degradation process. Hence, being that graphene has low permeability to all gases [
13], it may promote the formation of a nano-structured ceramic residue, upon thermal conversion [
26], leading to higher surface integrity, as revealed in
Figure 14.