Polymerization of PEDOT/PSS/Chitosan-Coated Electrodes for Electrochemical Bio-Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Electrochemical Polymerization of PEDOT/PSS/Chitosan Coatings on Pt Electrodes
2.3. Electrical Properties of PEDOT/PSS/Chitosan-Coated Pt Electrodes Characterized by Electrochemical Cyclic Voltammetry (CV)
2.4. Electrical Properties of PEDOT/PSS/Chitosan-Coated Pt Electrodes Characterized by Electrochemical Impedance Spectroscopy (EIS)
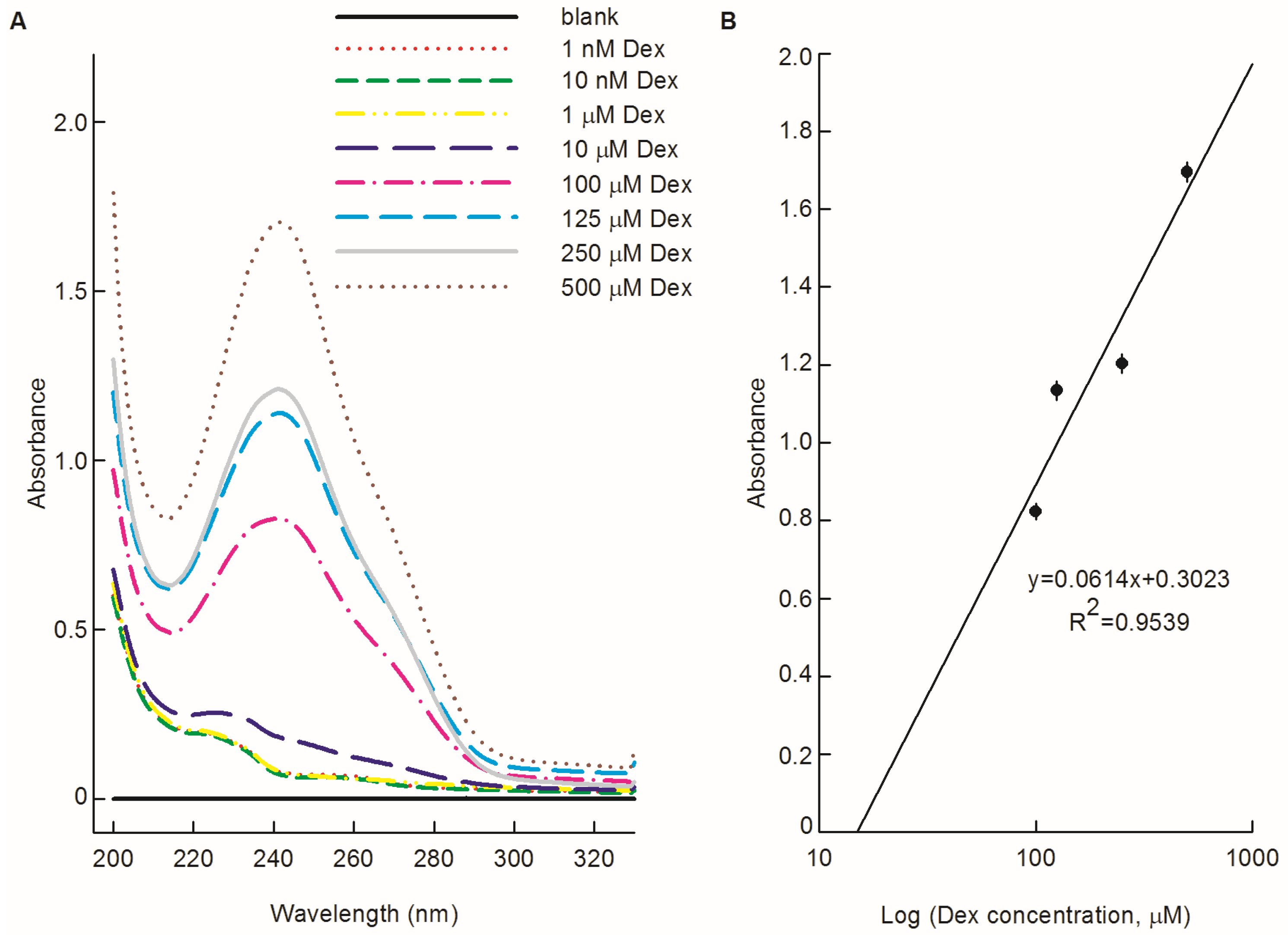
2.5. Ultraviolet (UV) Spectroscopy
2.6. Statistical Analysis
3. Results
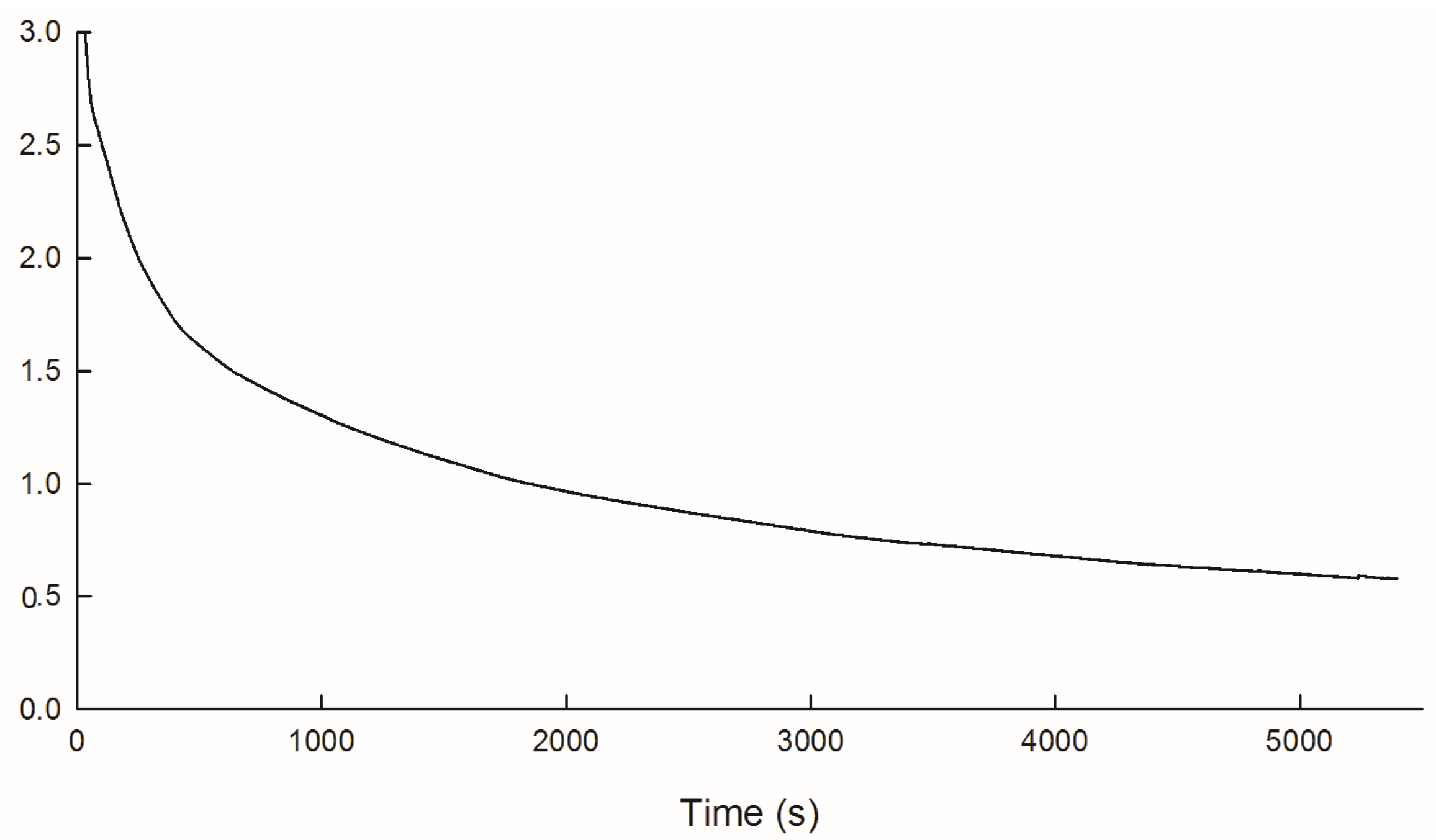
3.1. Electrochemical Polymerization of PEDOT/PSS/Chitosan Coatings on Pt Electrodes
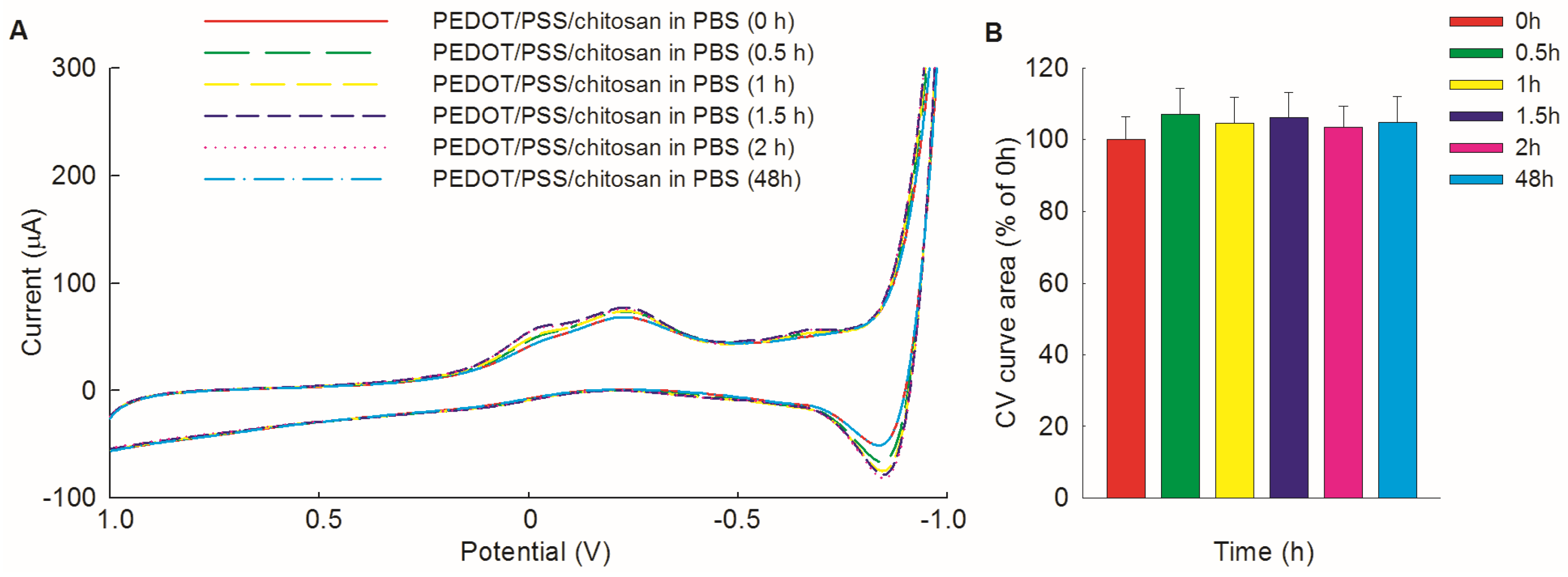
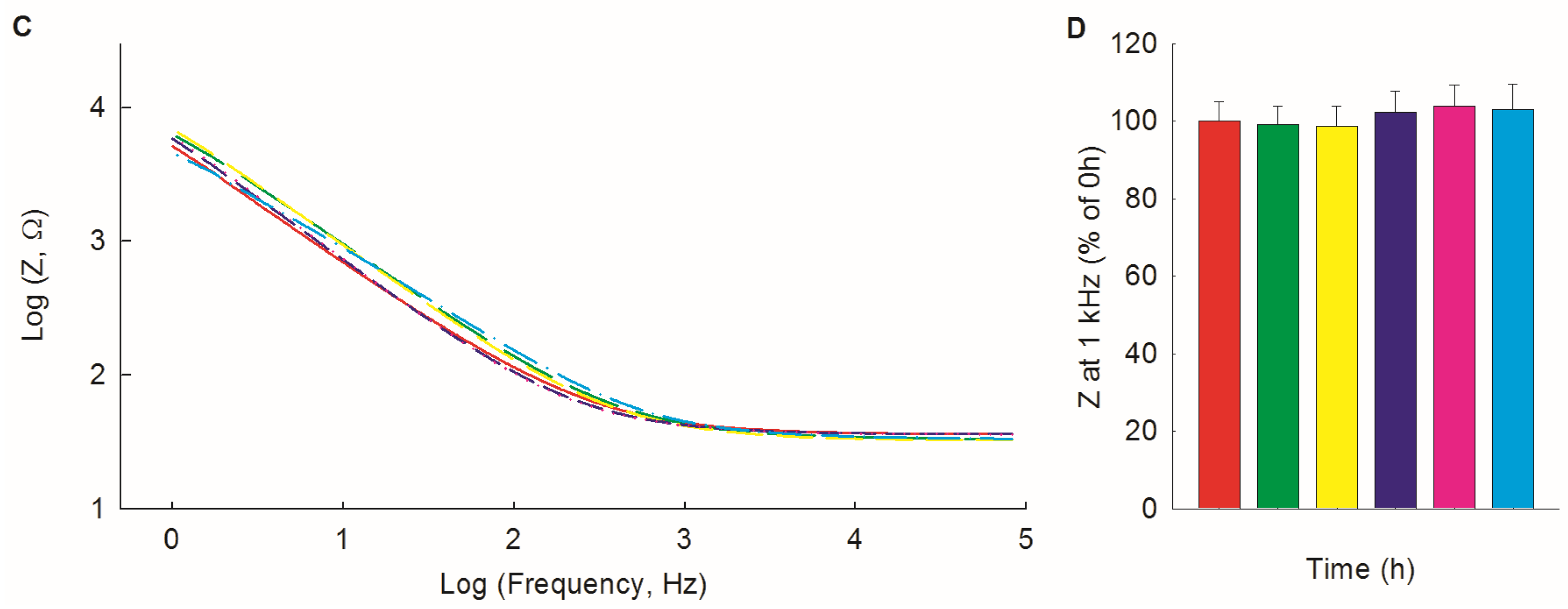
3.2. Electrochemical Stability of PEDOT/PSS/Chitosan-Coated Pt Electrodes in PBS
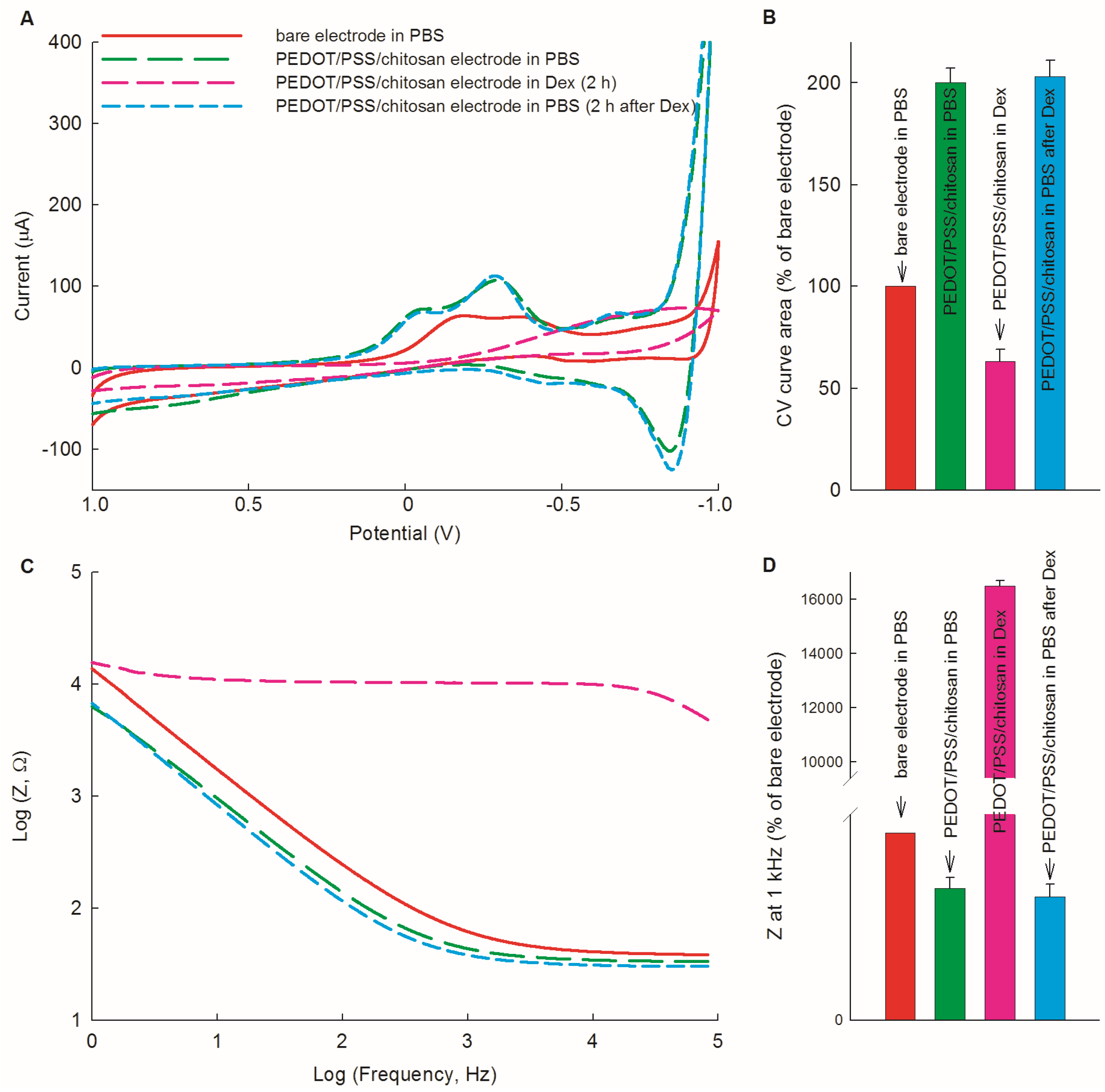
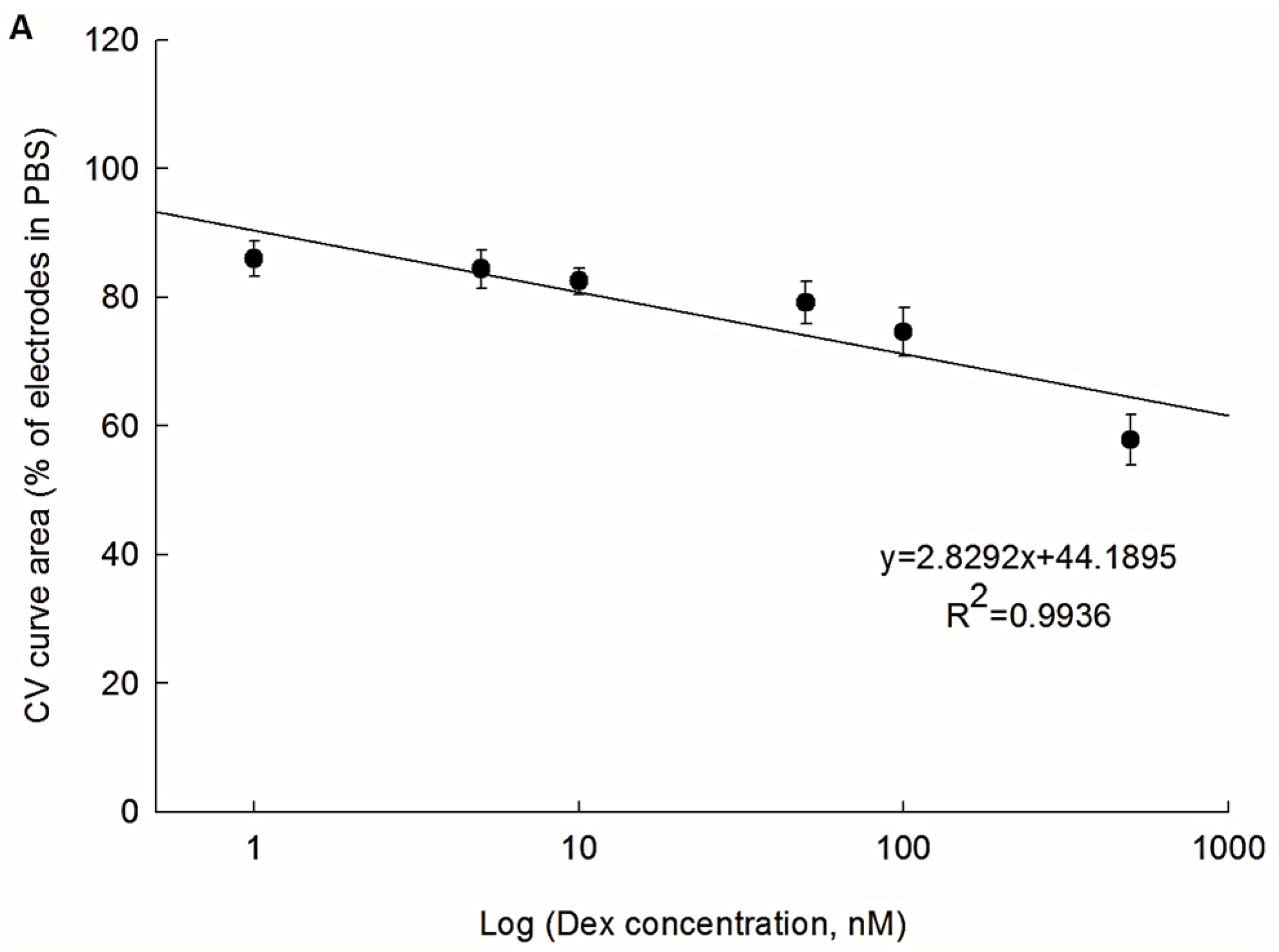
3.3. Possibility of the PEDOT/PSS/Chitosan-Coated Pt Electrodes for Dex Bio-Sensing
3.4. Electrochemical Evaluation of PEDOT/PSS/Chitosan-Coated Pt Electrodes
3.5. Sensitivity of PEDOT/PSS/Chitosan-Coated Pt Electrodes for Dex Bio-Sensiing
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Petraki, F.; Kennou, S.; Nespurek, S.; Biler, M. A spectroscopic study for the application of a PEDOT-type material as buffer layer in electronic devices. Org. Electron. 2010, 11, 1423–1431. [Google Scholar] [CrossRef]
- Denneulin, A.; Blayo, A.; Bras, J.; Neuman, C. PEDOT: PSS coating on specialty papers: Process optimization and effects of surface properties on electrical performances. Prog. Org. Coat. 2008, 63, 87–91. [Google Scholar] [CrossRef]
- Green, R.A.; Matteucci, P.B.; Hassarati, R.T.; Giraud, B.; Dodds, C.W.; Chen, S.; Byrnes-Preston, P.J.; Suaning, G.J.; Poole-Warren, L.A.; Lovell, N.H. Performance of conducting polymer electrodes for stimulating neuroprosthetics. J. Neural Eng. 2013, 10, 016009. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Hendricks, J.; King, Z.A.; Sereno, A.J.; Richardson-Burns, S.; Martin, D.; Carmena, J.M. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Chikar, J.A.; Hendricks, J.L.; Richardson-Burns, S.M.; Raphael, Y.; Pfingst, B.E.; Martin, D.C. The use of a dual PEDOT and RGD-functionalized alginate hydrogel coating to provide sustained drug delivery and improved cochlear implant function. Biomaterials 2012, 33, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Song, X.J.; Ren, J.; Cai, W.J.; Ju, L.H.; Wang, Y.; Wang, L.Y.; Chen, M. In vitro and in vivo evaluation of poly(3,4-ethylenedioxythiophene)/poly(styrene sulfonate)/dopamine-coated electrodes for dopamine delivery. J. Biomed. Mater. Res. A 2014, 102, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Nikolou, M.; Malliaras, G.G. Applications of poly(3,4-ethylenedioxythiophene) doped with poly(styrene sulfonic acid) transistors in chemical and biological sensors. Chem. Rec. 2008, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rozlosnik, N. New directions in medical biosensors employing poly(3,4-ethylenedioxy thiophene) derivative-based electrodes. Anal. Bioanal. Chem. 2009, 395, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Abidian, M.R.; Daneshvar, E.D.; Egeland, B.M.; Kipke, D.R.; Cederna, P.S.; Urbanchek, M.G. Hybrid conducting polymer-hydrogel conduits for axonal growth and neural tissue engineering. Adv. Healthc. Mater. 2012, 1, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.C.; Mohamed Ali, E.; Tansil, N.C.; Yu, H.H.; Gao, S.; Kantchev, E.A.; Ying, J.Y. Poly(3,4-ethylenedioxythiophene) (PEDOT) nanobiointerfaces: Thin, ultrasmooth, and functionalized PEDOT films with in vitro and in vivo biocompatibility. Langmuir 2008, 24, 8071–8077. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.C.; Yu, H.H.; Wan, A.C.; Han, Y.; Ying, J.Y. A general synthesis for PEDOT-coated nonconductive materials and PEDOT hollow particles by aqueous chemical polymerization. Small 2008, 4, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Povlich, L.K.; Cho, J.C.; Leach, M.K.; Corey, J.M.; Kim, J.; Martin, D.C. Synthesis, copolymerization and peptide-modification of carboxylic acid-functionalized 3,4-ethylenedioxythiophene (EDOTacid) for neural electrode interfaces. Biochim. Biophys. Acta 2013, 1830, 4288–4293. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Lovell, N.H.; Poole-Warren, L.A. Impact of co-incorporating laminin peptide dopants and neurotrophic growth factors on conducting polymer properties. Acta Biomater. 2010, 6, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N. Chitosan in nanostructured thin films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Wang, W.; Qi, J.; Huang, S. A DNA biosensor based on graphene paste electrode modified with Prussian blue and chitosan. Analyst 2011, 136, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- Junbo, B.; Cunxian, S. Chitosan chip and application to evaluate DNA loading on the surface of the metal. Biomed. Mater. 2009, 4, 011002. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.M.; Abdullah, L.C.; Sadrolhosseini, A.R.; Mat Yunus, W.M.; Moksin, M.M.; Tahir, P.M. Surface plasmon resonance sensing detection of mercury and lead ions based on conducting polymer composite. PLoS ONE 2011, 6, e24578. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, A.G.; Uygun, A.; Kaplan Can, H. The effect of synthesis media on the properties of substituted polyaniline/chitosan composites. Carbohydr. Res. 2011, 346, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Gong, S. Electrochemical detection of a breast cancer susceptible gene using cDNA immobilized chitosan-co-polyaniline electrode. Talanta 2009, 77, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ni, Y.; Zhang, G.; Mao, C.; Huang, X.; Shen, J. Biocompatibility of CS-PPy nanocomposites and their application to glucose biosensor. Bioelectrochemistry 2012, 88, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.; Stoytcheva, M.; Zlatev, R.; Gochev, V. Some clinical applications of the electrochemical biosensors. Mini Rev. Med. Chem. 2012, 12, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Batchelor-McAuley, C.; Dickinson, E.J.; Rees, N.V.; Toghill, K.E.; Compton, R.G. New electrochemical methods. Anal. Chem. 2012, 84, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Ates, M. A review study of (bio)sensor systems based on conducting polymers. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Park, S.M. Electrochemical impedance spectroscopy. Annu. Rev. Anal. Chem. 2010, 3, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Asplund, M.; Thaning, E.; Lundberg, J.; Sandberg-Nordqvist, A.C.; Kostyszyn, B.; Inganäs, O.; von Holst, H. Toxicity evaluation of PEDOT/biomolecular composites intended for neural communication electrodes. Biomed. Mater. 2009, 4, 045009. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, C.M.; Wang, S.; Shi, J.; Ooi, C.P. Incorporation of collagen in poly(3,4-ethylenedioxythiophene) for a bifunctional film with high bio- and electrochemical activity. J. Biomed. Mater. Res. A 2010, 92, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Dhayal, M. Chitosan/polyaniline hybrid conducting biopolymer base impedimetric immunosensor to detect Ochratoxin-A. Biosens. Bioelectron. 2009, 24, 1700–1705. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, L.; Zhang, B.; Wang, J.; Cai, A. Polymerization of PEDOT/PSS/Chitosan-Coated Electrodes for Electrochemical Bio-Sensing. Coatings 2017, 7, 96. https://doi.org/10.3390/coatings7070096
Sui L, Zhang B, Wang J, Cai A. Polymerization of PEDOT/PSS/Chitosan-Coated Electrodes for Electrochemical Bio-Sensing. Coatings. 2017; 7(7):96. https://doi.org/10.3390/coatings7070096
Chicago/Turabian StyleSui, Li, Bingshu Zhang, Jun Wang, and Ainan Cai. 2017. "Polymerization of PEDOT/PSS/Chitosan-Coated Electrodes for Electrochemical Bio-Sensing" Coatings 7, no. 7: 96. https://doi.org/10.3390/coatings7070096



