Highly-Conductive and Well-Adhered Cu Thin Film Fabricated on Quartz Glass by Heat Treatment of a Precursor Film Obtained Via Spray-Coating of an Aqueous Solution Involving Cu(II) Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Coating Solutions
2.3. Thin Film Fabrication
2.3.1. Spray-Coating
2.3.2. Heat-Treatment of the Spray-Coated Film
2.4. Measurements
2.4.1. Crystal Structure of the Films
2.4.2. Surface Morphologies of F’’edta and F’’mix and Adhesion Strength of F’’mix
2.4.3. Reflectance Spectra of F’’edta and F’’mix and Electrical Resistivity of F’’mix
2.4.4. Chemical Composition of F’’mix
3. Results
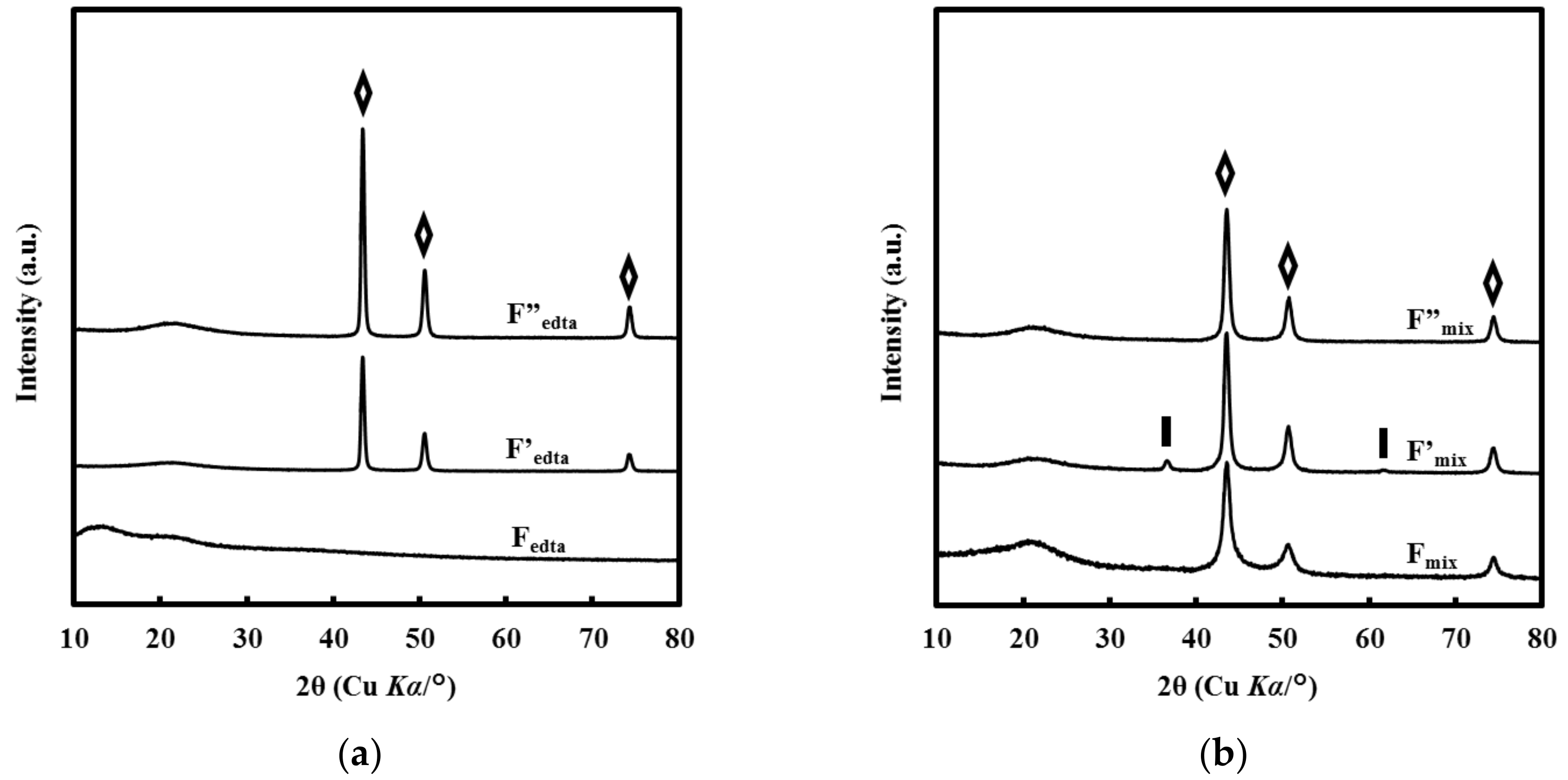
3.1. Crystal Structures of the Films
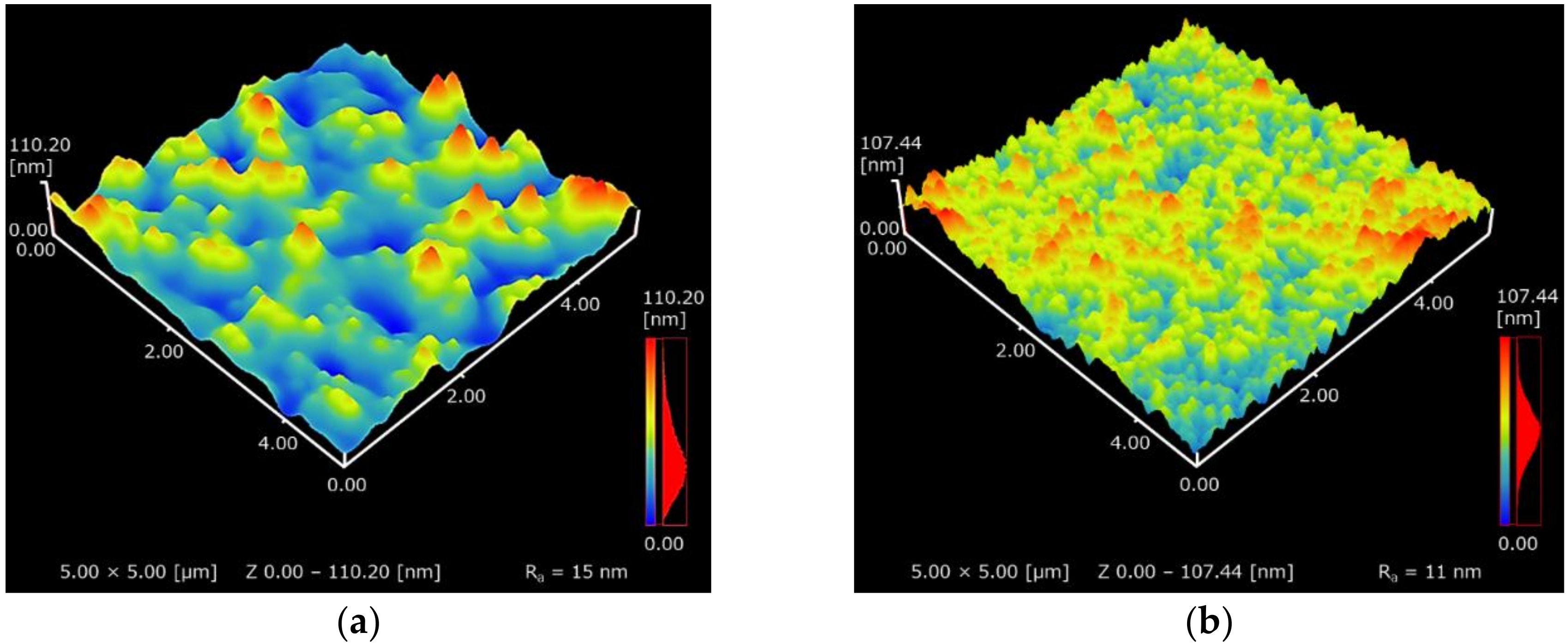
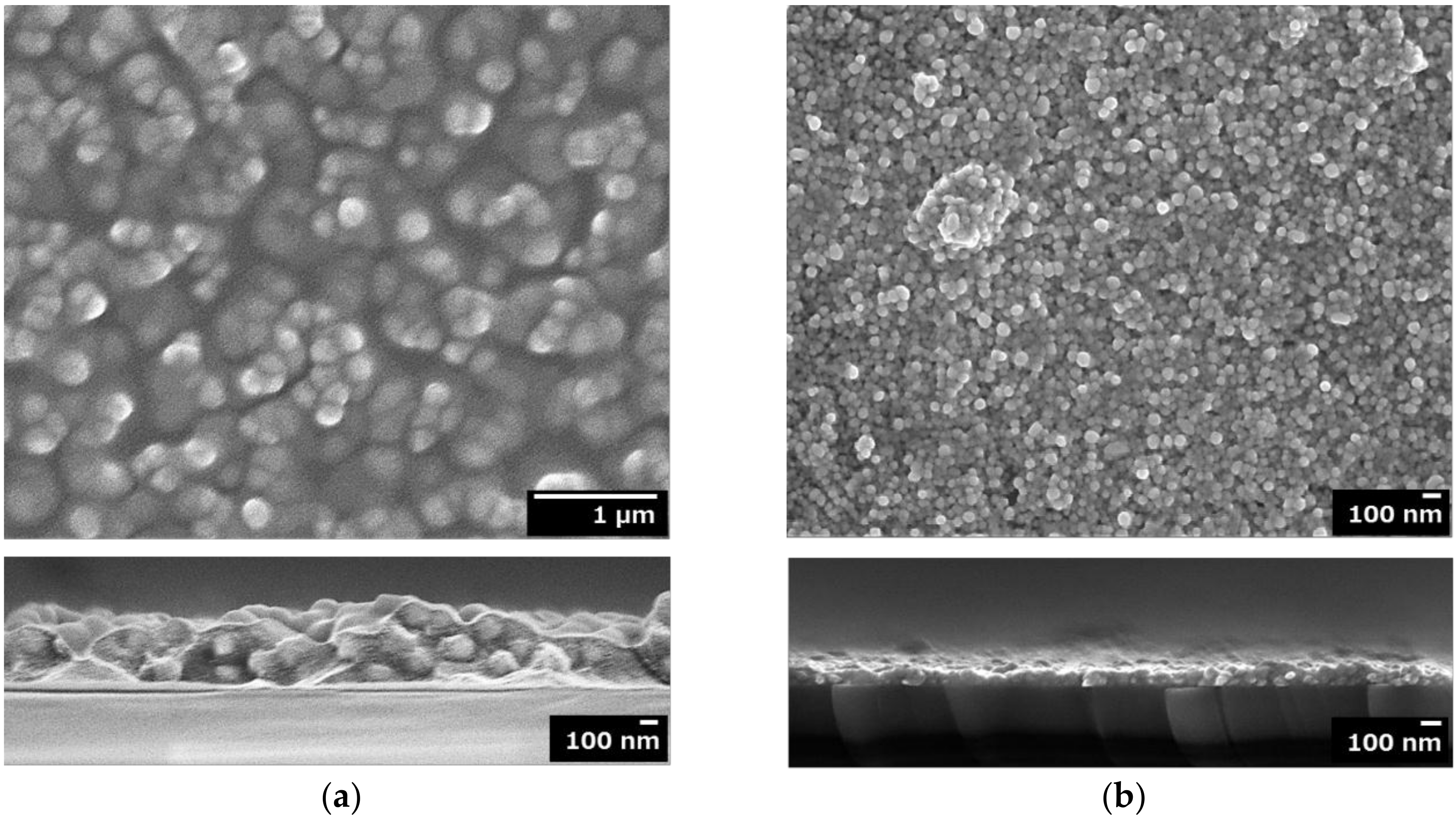
3.2. Surface Morphologies of F’’edta and F’’mix and Adhesion Strength of F’’mix
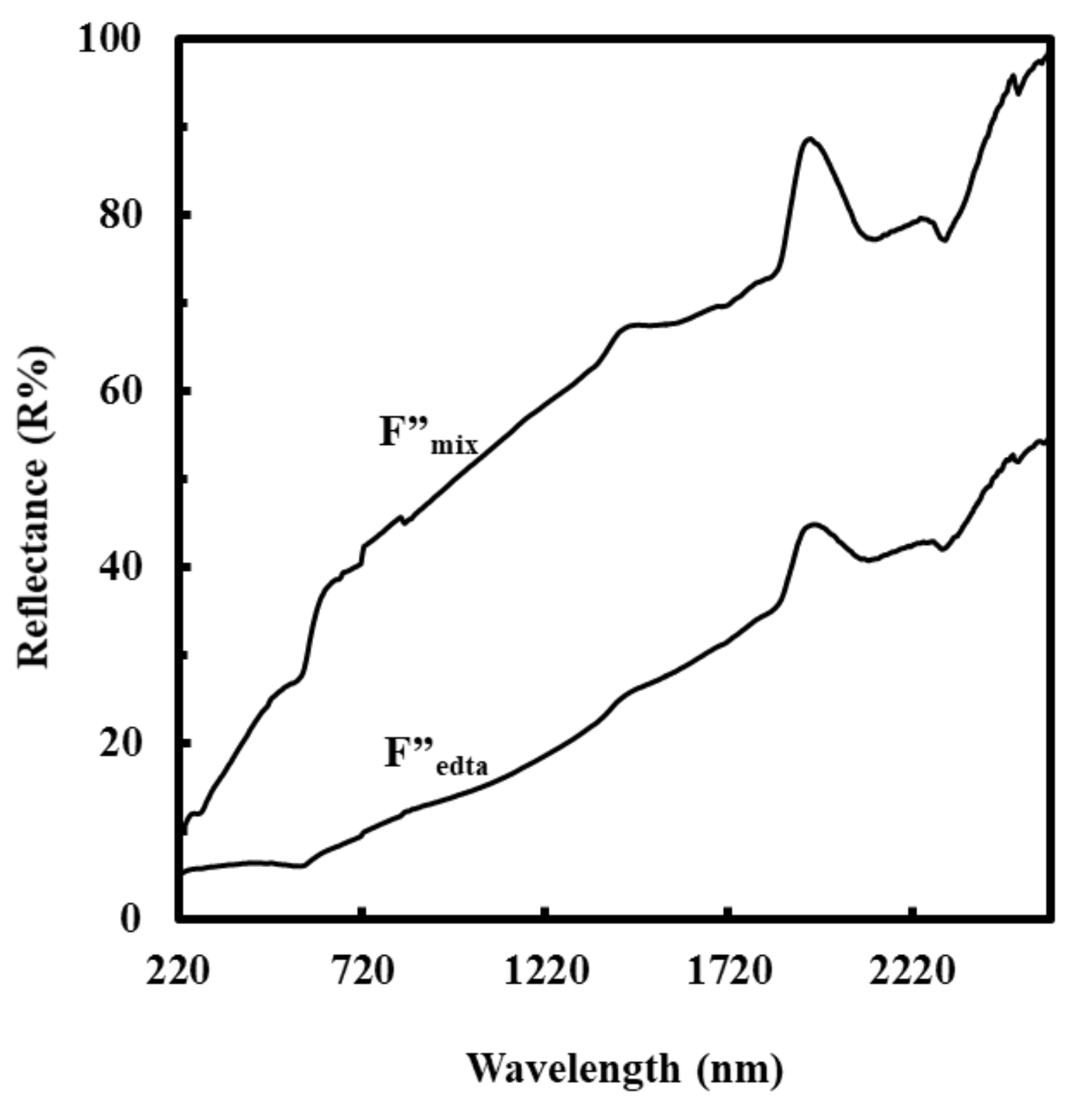
3.3. Reflectance Spectra of F’’edta and F’’mix and Electrical Resistivity of F’’mix
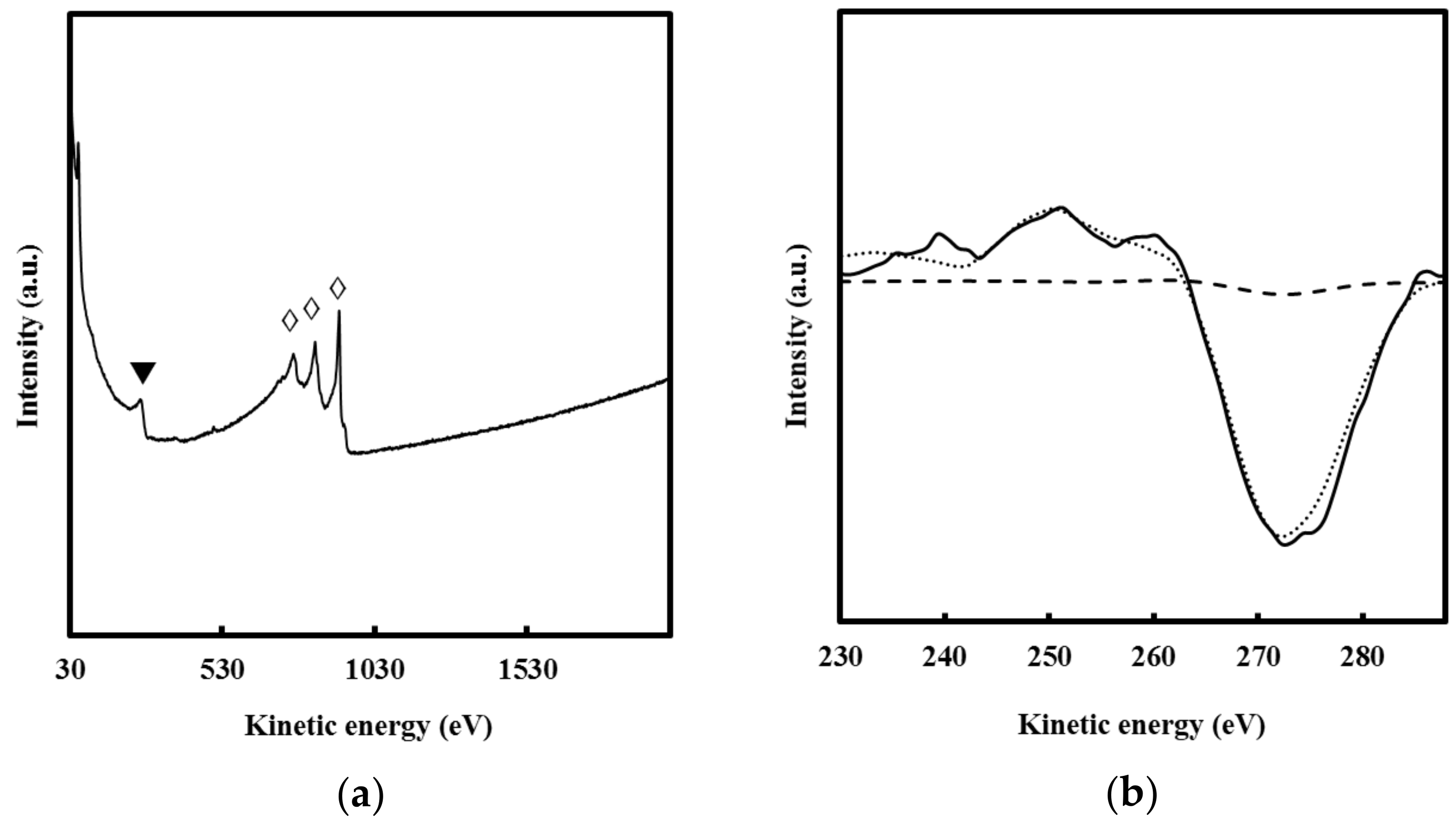
3.4. Chemical Composition of F’’mix
4. Discussion
4.1. Preparation and Properties of the Precursor Solution
4.2. Influence of the Cu(II) Complexes in the Coating Solution on the Crystal Structure of the Resultant Films
4.3. Influence of the Cu(II) Complexes in the Coating Solution on the Surface Morphologies, Electrical Conductivities and Reflectance of the Resultant Films
4.4. Chemical Composition and Adhesion-Strength of F’’mix
4.5. Versatility of MPM in Cu Thin Film Fabrication
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghotbi, M.Y.; Rahmati, Z. Nanostructured copper and copper oxide thin films fabricated by hydrothermal treatment of copper hydroxide nitrate. Mater. Des. 2015, 85, 719–723. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.Y.; Lee, T.-W.; Kim, W.-K.; Kim, B.-S.; Park, J.H.; Bae, J.-S.; Cho, Y.C.; Kim, J.; Oh, M.-W.; et al. Fabrication of high-quality single-crystal Cu thin films using radio-frequency sputtering. Sci. Rep. 2015, 4, 6230. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.G.; Kurek, A.; Barry, S.T. Trends in Copper Precursor Development for CVD and ALD Applications. ECS J. Solid State Sci. Technol. 2015, 4, N3188–N3197. [Google Scholar] [CrossRef]
- Kaelin, M.; Zogg, H.; Tiwari, A.N.; Wilhelm, O.; Pratsinis, S.E.; Meyer, T.; Meyer, A. Electrosprayed and selenized Cu/In metal particle films. Thin Solid Films 2004, 457, 391–396. [Google Scholar] [CrossRef]
- Han, S.; Lee, T.L.; Yang, C.J.; Shih, H.C. Trench gap-filling copper by ion beam sputter deposition. Mater. Chem. Phys. 2006, 97, 19–22. [Google Scholar] [CrossRef]
- Abhinav, K.V.; Rao, R.V.K.; Karthik, P.S.; Singh, S.P. Copper conductive inks: Synthesis and utilization in flexible electronics. RSC Adv. 2015, 5, 63985–64030. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, D.; Jeong, S.; Moon, J.; Kim, J.S. Direct writing of copper conductive patterns by ink-jet printing. Thin Solid Films 2007, 515, 7706–7711. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper Nanoparticles for Printed Electronics: Routes towards Achieving Oxidation Stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Sato, M. Heat Treatment in Molecular Precursor Method for Fabricating Metal Oxide Thin Films. In Heat Treatment: Conventional and Novel Applications; Czerwinski, F., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [Green Version]
- Nagai, H.; Mita, S.; Takano, I.; Honda, T.; Sato, M. Conductive and semi-transparent Cu thin film fabricated using molecular precursor solutions. Mater. Lett. 2015, 141, 235–237. [Google Scholar] [CrossRef]
- Nagai, H.; Suzuki, T.; Nakano, T.; Sato, M. Embedding of copper into submicrometer trenches in a silicon substrate using the molecular precursor solutions with copper. Mater. Lett. 2016, 182, 206–209. [Google Scholar] [CrossRef]
- Mochizuki, C.; Hara, H.; Takano, I.; Hayakawa, T.; Sato, M. Application of carbonated apatite coating on a Ti substrate by aqueous spray method. Mater. Sci. Eng. C 2013, 33, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Bedi, R.K. Studies and correlation among the structural, electrical and gas response properties of aerosol spray deposited self assembled nanocrystalline CuO. Appl. Surf. Sci. 2011, 257, 7592–7599. [Google Scholar] [CrossRef]
- Girotto, C.; Rand, B.P.; Genoe, J.; Heremans, P. Exploring spray coating as a deposition technique for the fabrication of solution-processed solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 454–458. [Google Scholar] [CrossRef]
- Abdellah, A.; Baierl, D.; Fabel, B.; Lugli, P.; Scarpa, G.; München, T.U. Spray-coating deposition for large area organic thin-film devices. NSTI-Nanotech 2009, 2, 447–450. [Google Scholar]
- Muhibbullah, M.; Hakim, M.O.; Choudhury, M.G.M. Studies on Seebeck effect in spray deposited CuO thin film on glass substrate. Thin Solid Films 2003, 423, 103–107. [Google Scholar] [CrossRef]
- Yamamoto, A.; Nakamura, M.; Seki, A.; Li, E.L. Pyrite (FeS2) thin films prepared by spray method. Sol. Energy Mater. Sol. Cells 2003, 75, 451–456. [Google Scholar] [CrossRef]
- Khim, D.; Baeg, K.; Yu, B.; Kang, S.; Kang, M. Spray-printed organic field-effect transistors and complementary inverters. J. Mater. Chem. C 2013, 1, 1500–1506. [Google Scholar] [CrossRef]
- Eslamian, M. Spray-on Thin Film PV Solar Cells: Advances, Potentials and Challenges. Coatings 2014, 4, 60–84. [Google Scholar] [CrossRef] [Green Version]
- Nagai, H.; Suzuki, T.; Hara, H.; Mochizuki, C.; Takano, I.; Honda, T.; Sato, M. Chemical fabrication of p-type Cu2O transparent thin film using molecular precursor method. Mater. Chem. Phys. 2012, 137, 252–257. [Google Scholar] [CrossRef]
- Kosugi, T.; Kaneko, S. Novel spray-pyrolysis deposition of cuprous oxide thin films. J. Am. Ceram. Soc. 1998, 81, 3117–3124. [Google Scholar] [CrossRef]
- Pattanasattayavong, P.; Thomas, S.; Adamopoulos, G.; McLachlan, M.A.; Anthopoulos, T.D. p-channel thin-film transistors based on spray-coated Cu2O films. Appl. Phys. Lett. 2013, 102, 163505. [Google Scholar] [CrossRef] [Green Version]
- Axelevitch, A.; Gorenstein, B.; Golan, G. Investigation of Optical Transmission in Thin Metal Films. Phys. Procedia 2012, 32, 1–13. [Google Scholar] [CrossRef]
- Knisley, T.J.; Ariyasena, T.C.; Sajavaara, T.; Saly, M.J.; Winter, C.H. Low Temperature Growth of High Purity, Low Resistivity Copper Films by Atomic Layer Deposition. Chem. Mater. 2011, 23, 4417–4419. [Google Scholar] [CrossRef]
- Kalutarage, L.C.; Clendenning, S.B.; Winter, C.H. Low-Temperature Atomic Layer Deposition of Copper Films Using Borane Dimethylamine as the Reducing Co-reagent. Chem. Mater. 2014, 26, 3731–3738. [Google Scholar] [CrossRef]
- Nagai, H.; Suzuki, T.; Mochizuki, C.; Honda, I.T.T.; Sato, M. Formation Mechanism of p-Type Cu2O Thin Films via Intermediate Cu0 Species Derived from Cu(II) Complex of Ethylenediamine-N,N,N′,N′-Tetraacetic Acid. Sci. Adv. Mater. 2014, 6, 603–611. [Google Scholar] [CrossRef]
- Mittal, K.L. Adhesion Measurement of Thin Films. Electrocompon. Sci. Technol. 1976, 3, 21–42. [Google Scholar] [CrossRef]
- Lim, J.D.; Lee, P.M.; Rhee, D.M.W.; Leong, K.C.; Chen, Z. Effect of surface treatment on adhesion strength between magnetron sputtered copper thin films and alumina substrate. Appl. Surf. Sci. 2015, 355, 509–515. [Google Scholar] [CrossRef]
- Lukaszkowicz, K.; Kriz, A.; Sondor, J. Structure and adhesion of thin coatings deposited by PVD technology on the X6CrNiMoTi17-12-2 and X40CrMoV5-1 steel substrates. Arch. Mater. Sci. Eng. 2011, 51, 40–47. [Google Scholar]
- Nagai, H.; Sato, M. Molecular Precursor Method for Fabricating p-Type Cu2O and Metallic Cu Thin Films. Mod. Technol. Creat. Thin-Film Syst. Coat. 2017, 3–20. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hishimone, P.N.; Nagai, H.; Morita, M.; Sakamoto, T.; Sato, M. Highly-Conductive and Well-Adhered Cu Thin Film Fabricated on Quartz Glass by Heat Treatment of a Precursor Film Obtained Via Spray-Coating of an Aqueous Solution Involving Cu(II) Complexes. Coatings 2018, 8, 352. https://doi.org/10.3390/coatings8100352
Hishimone PN, Nagai H, Morita M, Sakamoto T, Sato M. Highly-Conductive and Well-Adhered Cu Thin Film Fabricated on Quartz Glass by Heat Treatment of a Precursor Film Obtained Via Spray-Coating of an Aqueous Solution Involving Cu(II) Complexes. Coatings. 2018; 8(10):352. https://doi.org/10.3390/coatings8100352
Chicago/Turabian StyleHishimone, Philipus N., Hiroki Nagai, Masato Morita, Tetsuo Sakamoto, and Mitsunobu Sato. 2018. "Highly-Conductive and Well-Adhered Cu Thin Film Fabricated on Quartz Glass by Heat Treatment of a Precursor Film Obtained Via Spray-Coating of an Aqueous Solution Involving Cu(II) Complexes" Coatings 8, no. 10: 352. https://doi.org/10.3390/coatings8100352






