Preparation and Characterization of Sprayed-Yttrium Oxyfluoride Corrosion Protective Coating for Plasma Process Chambers
Abstract
:1. Introduction
2. Materials and Methods
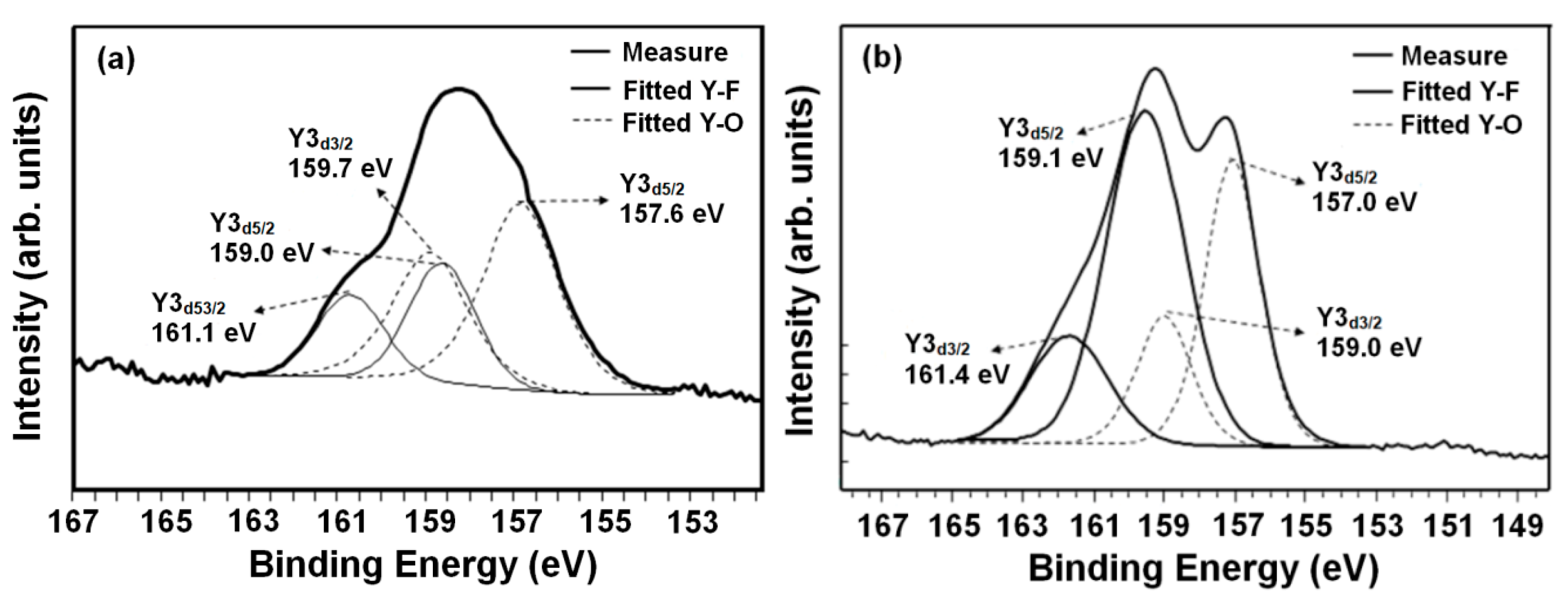
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hou, S.Y.; Chen, W.C.; Hu, C.; Chiu, C.; Ting, K.C.; Lin, T.S.; Wei, W.H.; Chiou, W.C.; Lin, V.J.C.; Chang, V.C.Y.; et al. Wafer-level integration of an advanced logic-memory system through the second-generation CoWoS technology. IEEE Trans. Electron. Devices 2017, 64, 4071–4077. [Google Scholar] [CrossRef]
- Ito, N.; Moriya, T.; Uesugi, F.; Matsumoto, M.; Liu, S.; Kitayama, Y. Reduction of particle contamination in plasma-etching equipment by dehydration of chamber wall. Jpn. J. Appl. Phys. 2008, 47, 3630–3634. [Google Scholar] [CrossRef]
- Yoo, S.W.; Hwang, N.M.; You, S.J.; Kim, J.H.; Seong, D.J. Control of nanoparticle size and amount by using the mesh grid and applying DC-bias to the substrate in silane ICP-CVD process. J. Nanopart. Res. 2017, 19, 374. [Google Scholar] [CrossRef]
- Sato, N.; Uchida, G.; Kaneko, T.; Shimizu, S.; Iizuka, S. Dynamics of fine particles in magnetized plasmas. Phys. Plasmas 2001, 8, 1786–1790. [Google Scholar] [CrossRef]
- Kim, D.M.; Kim, K.B.; Yoon, S.Y.; Oh, S.Y.; Kim, H.T.; Lee, S.M. Effects of artificial pores and purity on the erosion behaviors of polycrystalline Al2O3 ceramics under fluorine plasma. J. Ceram. Soc. 2009, 117, 863–867. [Google Scholar] [CrossRef]
- Lim, K.Y.; Kim, W.Y.; Kim, K.J. Mechanical properties of electrically conductive silicon carbide ceramics. Ceram. Int. 2014, 40, 10577–10582. [Google Scholar] [CrossRef]
- Lim, K.Y.; Blain, M.G.; Tipton, G.D.; Holber, W.M.; Selwyn, G.S.; Westerfield, P.L.; Maxwell, K.L. Particle behavior in an electron cyclotron resonance plasma etch tool. Plasma Source Sci. Technol. 1994, 3, 325–333. [Google Scholar]
- Fukumoto, H.; Fujikake, I.; Takao, Y.; Takao, K.; Ono, K. Plasma chemical behaviour of reactants and reaction products during inductively coupled CF4 plasma etching of SiO2. Plasma Sources Sci. Technol. 2009, 18, 045027. [Google Scholar] [CrossRef]
- Tezani, L.L.; Pessoa, R.S.; Maciel, H.S.; Petraconi, G. Chemistry studies of SF6/CF4, SF6/O2 and CF4/O2 gas phase during hollow cathode reactive ion etching plasma. Vacuum 2014, 106, 64–68. [Google Scholar] [CrossRef]
- Kim, D.P.; Yeo, J.W.; Kim, C.I. Etching properties of Al2O3 films in inductively coupled plasma. Thin Solid Films 2004, 459, 122–126. [Google Scholar] [CrossRef]
- Miwa, K.; Takada, N.; Sasaki, K. Fluorination mechanisms of Al2O3 and Y2O3 surfaces irradiated by high-density CF4/O2 and SF6/O2 plasma. J. Vac. Sci. Technol. A 2009, 27, 831–835. [Google Scholar] [CrossRef]
- Cao, Y.C.; Zhao, L.; Luo, J.; Wang, K.; Zhang, B.P.; Yokota, H.; Ito, Y.; Li, J.F. Plasma etching behavior of Y2O3 ceramics: Comparative study with Al2O3. Appl. Sur. Sci. 2016, 366, 304–309. [Google Scholar] [CrossRef]
- Mun, S.Y.; Shin, K.C.; Lee, S.S.; Kwak, J.S.; Jeong, J.Y.; Jeong, Y.H. Etch defect reduction using SF6/O2 plasma cleaning and optimizing etching recipe in photo resist masked gate poly silicon etch process. Jpn. J. Appl. Phys. 2005, 44, 4891. [Google Scholar] [CrossRef]
- Kim, D.M.; Jang, M.R.; Oh, Y.S.; Kim, S.; Lee, S.M.; Lee, S.H. Relative sputtering rates of oxides and fluorides of aluminum and yttrium. Surf. Coat. Technol. 2017, 309, 694–697. [Google Scholar] [CrossRef]
- Kim, D.M.; Oh, Y.S.; Kim, S.; Kim, H.T.; Lim, D.S.; Lee, S.M. The erosion behaviors of Y2O3 and YF3 coatings under fluorocarbon plasma. Thin Solid Films 2011, 519, 6698–6702. [Google Scholar] [CrossRef]
- Lin, T.K.; Wang, W.K.; Huang, S.Y.; Tasi, C.T.; Wuu, D.S. Comparison of erosion behavior and particle contamination in mass-production CF4/O2 plasma chambers using Y2O3 and YF3 protective coatings. Nanomaterials 2017, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Wuu, D.S.; Huang, S.Y.; Wang, W.K. Characteristics of yttrium fluoride and yttrium oxide coatings for plasma process equipment prepared by atmospheric plasma spraying. Jpn. J. Appl. Phys. 2016, 55, 126201. [Google Scholar] [CrossRef]
- ASTM D412-98a Standard Test Methods for Vulcanized Rubber and Thermoplastic Rubbers and Thermoplastic Elastomers-Tension; ASTM International: West Conshohocken, PA, USA, 1998.
- ASTM C1327-99 Standard Test Method for Vickers Indentation Hardness of Advanced Ceramics; ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM D419-09 Standard Test Method for Dielectric Breakdown Voltage and Dielectric Strength of Solid Electrical Insulating Materials at Commercial Power Frequencies; ASTM International: West Conshohocken, PA, USA, 2013.
- Kitamura, T.; Mizuno, H.; Kato, N.; Aoki, I. Plasma-erosion properties of ceramic coating prepared by plasma spraying. Mater. Trans. 2006, 47, 1677–1683. [Google Scholar] [CrossRef]
- Chai, G.D.; Dong, G.P.; Qiu, J.R.; Zhang, Q.Y.; Yang, Z.M. Phase transformation and intense 2.7 mm emission from Er3+ oped YF3/YOF submicron-crystals. Sci. Rep. 2013, 3, 1598. [Google Scholar] [CrossRef] [PubMed]
- Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication, characterization, and fluorine-plasma exposure behavior of dense yttrium oxyfluoride ceramics. Jpn. J. Appl. Phys. 2017, 56, 06HC02. [Google Scholar] [CrossRef]
- Chakravarthy, Y.; Bhandari, S.; Chaturvedi, V.; Pragatheeswaran, A.; Nagraj, A.; Thiyagarajan, T.K.; Ananthapadmanaban, P.V.; Das, A.K. Plasma spray deposition of yttrium oxide on graphite, coating characterization and interaction with molten uranium. J. Eur. Ceram. Soc. 2015, 35, 781–794. [Google Scholar] [CrossRef]
- Pei, L.; Jiapi, Z.; Yuankun, Z.; Jiecai, H. Preparation and optical properties of sputtered-deposition yttrium fluoride film. Nucl. Instrum. Methods Phys. Res. Sect. B 2013, 307, 429–433. [Google Scholar] [CrossRef]
- Zhong, H.X.; Hong, J.M.; Cao, X.F.; Chen, X.T.; Xue, Z.L. Ionic-liquid-assisted synthesis of YF3 with different crystalline phases and morphologies. Mater. Res. Bull. 2009, 44, 623–628. [Google Scholar] [CrossRef]
- Yu, P.F.; Zhang, K.; Huang, H.; Wen, M.; Li, Q.; Zhang, W.; Hu, C.Q.; Zhang, W.T. Oxygen vacancies dependent phase transition of Y2O3 films. Appl. Sur. Sci. 2017, 410, 470–478. [Google Scholar] [CrossRef]
- Kotlan, J.; Seshadri, R.C.; Sarnpath, S.; Ctibor, P.; Pala, Z.; Musalek, R. On the dielectric strengths of atmospheric plasma sprayed Al2O3, Y2O3, ZrO2-7% Y2O3 and (Ba,Sr)TiO3 coatings. Ceram. Int. 2015, 41, 11169–11176. [Google Scholar] [CrossRef]
- Sogard, M.R.; Mikkelson, A.R.; Nataraju, M.; Turner, K.T.; Engelstad, R.L. Analysis of Coulomb and Johnsen-Rahbek electrostatic chuck performance for extreme ultraviolet lithography. J. Vac. Sci. Technol. B 2007, 25, 2155–2161. [Google Scholar] [CrossRef]
- Tahara, R.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, U. Fabrication of dense yttrium oxyfluoride ceramics by hot pressing and their mechanical, thermal, and electrical properties. Jpn. J. Appl. Phys. 2018, 57, 06JF04. [Google Scholar] [CrossRef]
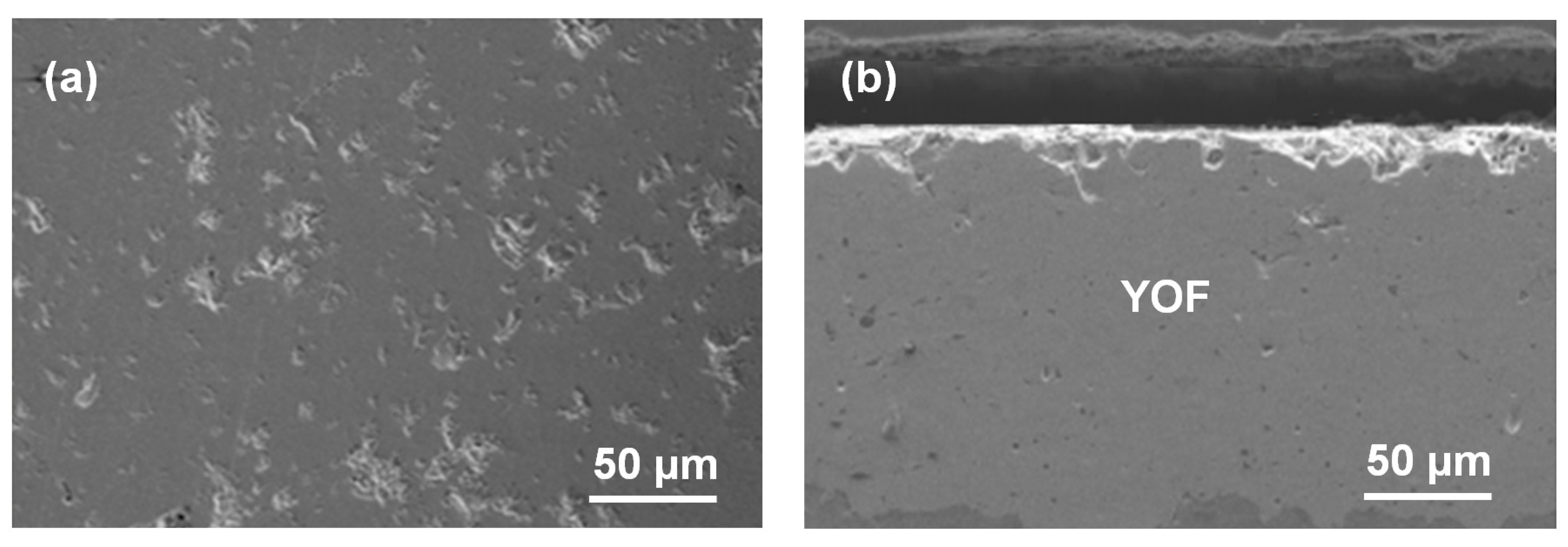

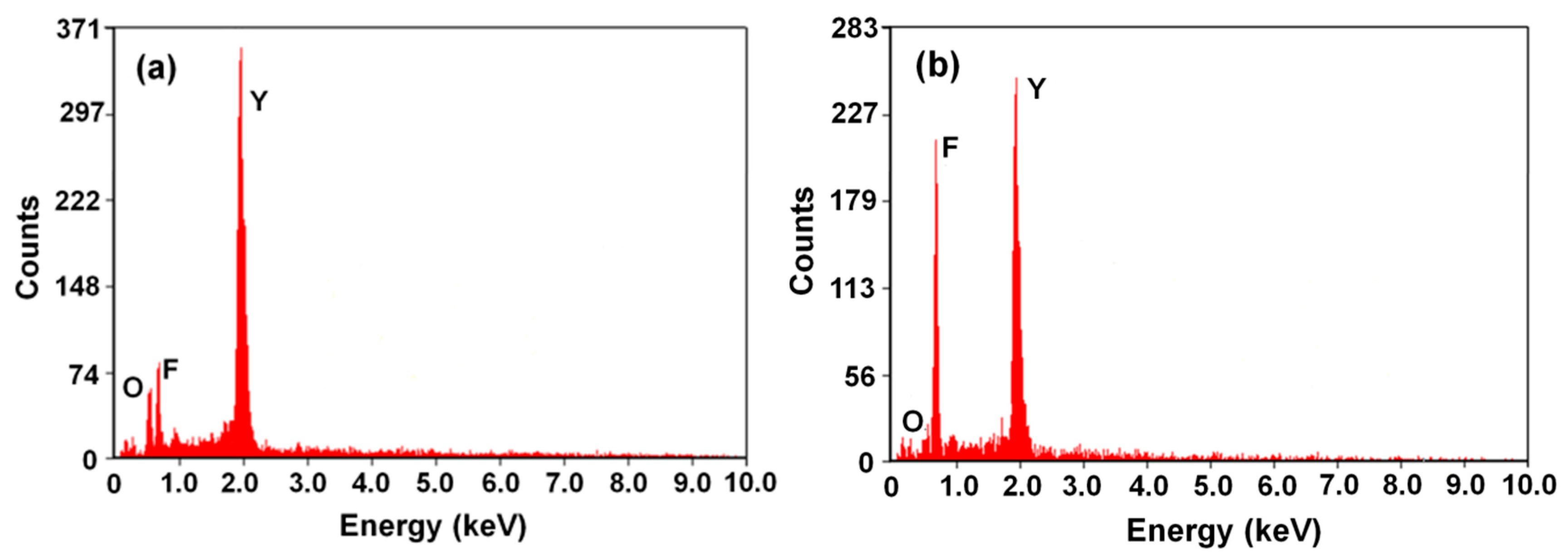
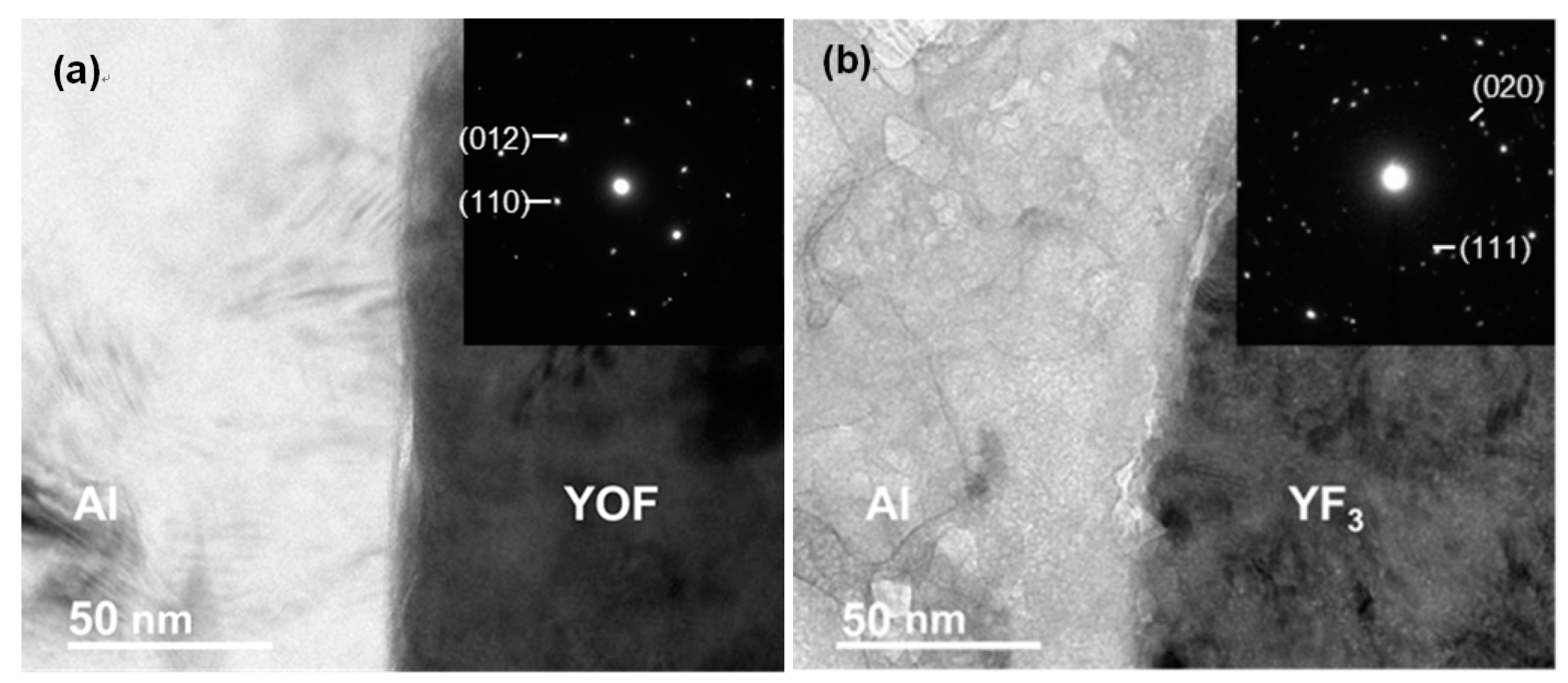
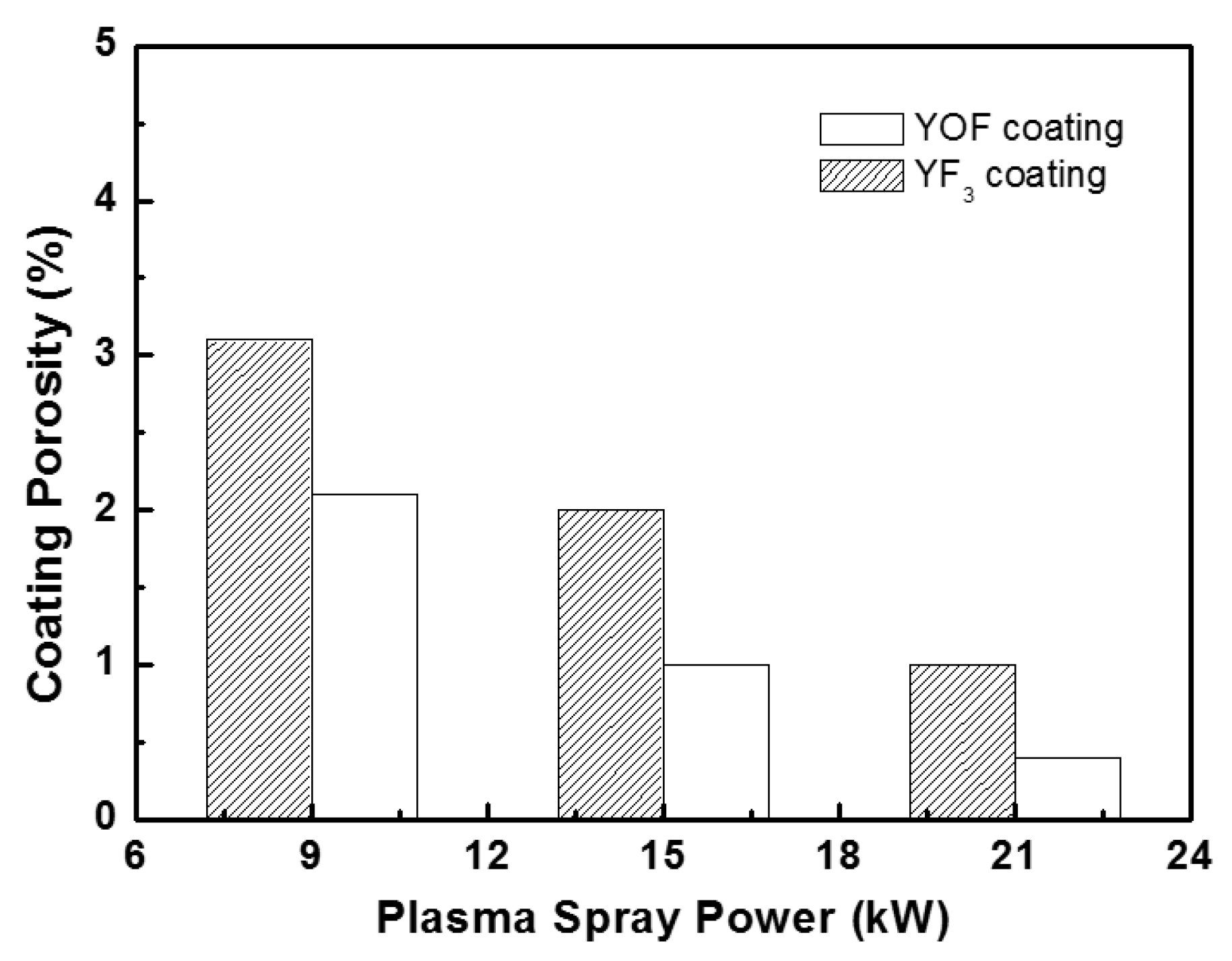
| Sparaying Parameters | YOF | YF3 |
|---|---|---|
| Gun power (kW) | 15 | 15 |
| Gun moving rate (cm) | 10 | 10 |
| Argon flow rate (L/min) | 45 | 45 |
| Hydrogen flow rate (L/min) | 6 | 6 |
| Target to substrate distance (cm) | 10 | 10 |
| Atoms | YOF | YF3 | ||
|---|---|---|---|---|
| (at.%) | (wt.%) | (at.%) | (wt.%) | |
| Fluoride atom | 26.02 | 11.67 | 64.62 | 01.68 |
| Yttrium atom | 36.01 | 75.27 | 27.01 | 32.78 |
| Oxygen atom | 25.27 | 09.51 | 03.93 | 64.11 |
| Mechanical Properties | YOF | YF3 |
|---|---|---|
| Vickers hardness (HV) | 290 ± 30 | 260 ± 25 |
| Adhesion strength (MPa) | 7.35 | 8.56 |
| Dielectric strength (kV/mm) | 24.67 | 22.65 |
| Breakdown voltage (kV) | 5.57 | 4.87 |
| Volume resistivity (Ω⋅cm) | 1016 | 1014 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-K.; Wuu, D.-S.; Huang, S.-Y.; Wang, W.-K. Preparation and Characterization of Sprayed-Yttrium Oxyfluoride Corrosion Protective Coating for Plasma Process Chambers. Coatings 2018, 8, 373. https://doi.org/10.3390/coatings8100373
Lin T-K, Wuu D-S, Huang S-Y, Wang W-K. Preparation and Characterization of Sprayed-Yttrium Oxyfluoride Corrosion Protective Coating for Plasma Process Chambers. Coatings. 2018; 8(10):373. https://doi.org/10.3390/coatings8100373
Chicago/Turabian StyleLin, Tzu-Ken, Dong-Sing Wuu, Shih-Yung Huang, and Wei-Kai Wang. 2018. "Preparation and Characterization of Sprayed-Yttrium Oxyfluoride Corrosion Protective Coating for Plasma Process Chambers" Coatings 8, no. 10: 373. https://doi.org/10.3390/coatings8100373





