Improving Corrosion and Corrosion-Fatigue Resistance of AZ31B Cast Mg Alloy Using Combined Cold Spray and Top Coatings
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Coating Methods
2.2. Microstructural Analysis
2.3. Electrochemical Corrosion Test
2.4. Tensile and Corrosion-Fatigue Testing
3. Results
3.1. Microstructure
3.2. Electrochemical Corrosion Behavior
3.2.1. Open Circuit Potential (OCP)
3.2.2. Potentiodynamic Polarization
3.2.3. Electrochemical Impedance Spectroscopy (EIS) Characteristic
3.3. Tensile Properties
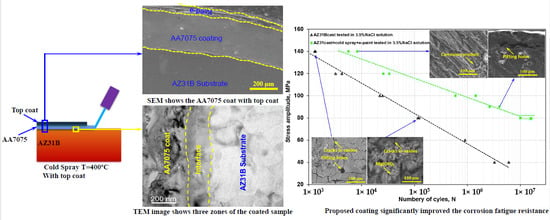
3.4. Corrosion-Fatigue Behavior
3.5. Fractographic Analysis
4. Discussion
4.1. Grain Refinement and Dislocation Density
4.2. Residual Stress
4.3. Effect of Intermetallics on Fatigue Performance
4.4. Other Factors Affecting the Fatigue Performance
5. Conclusions
- TEM microstructural analysis identified a continuous interfacial layer with a mixture of Al and Mg having a thickness of 200–300 nm. At the same time, ladder and columnar-like grain morphologies were observed in the AA7075 coating and AZ31B substrate at the interface.
- The electrochemical corrosion analysis shows that the corrosion resistance of the CS AA7075 alloy on AZ31B did not provide a significant improvement; this can be attributed to the fact that the compressive residual stress induced by the CS coating were suppressed by formation of corrosion pits and the presence of intermetallic. However, when the e-painting top coat was added to the CS coating it provided a corrosion resistance remarkably higher that of the AZ31B.
- A significant enhancement in fatigue life in the CS AA7075 followed by e-painted specimens was identified. This can be attributed due to the existence of passive oxide layer that increase the corrosion resistance. In addition, the high strength of the AA7075 alloy delayed the cracking in the coating of the e-painted and coated specimens. However, the CS coating without e-paint exhibited early cracking due to pit formation that acted as a pathway for NaCl to penetrate into the interface, creating localized pits. The localized pits led to stress concentration, which resulted in the nucleation of cracks caused a premature failure of the AZ31B substrate and hence lower fatigue life of the as-coated specimen.
- The fatigue life of the EP samples was lower than the CS + EP sample as there was no induced compressive residual stress and the brittle nature of the EP.
- The application of an e-paint on top of the CS coat proved to enhance significantly the corrosion-fatigue life of AZ31B cast.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karparvarfard, S.M.H.; Shaha, S.K.; Behravesh, S.B.; Jahed, H.; Williams, B.W. Microstructure, texture and mechanical behavior characterization of hot forged cast ZK60 magnesium alloy. J. Mater. Sci. Technol. 2017, 33, 907–918. [Google Scholar] [CrossRef]
- Joost, W.J.; Krajewski, P.E. Towards magnesium alloys for high-volume automotive applications. Scr. Mater. 2017, 128, 107–112. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Mutoh, Y.; Murai, T.; Iwakami, S. Corrosion fatigue behavior of extruded magnesium alloy AZ80-T5 in a 5% NaCl environment. Eng. Fract. Mech. 2010, 77, 1567–1576. [Google Scholar] [CrossRef]
- Liao, J.; Hotta, M.; Yamamoto, N. Corrosion behavior of fine-grained AZ31B magnesium alloy. Corros. Sci. 2012, 61, 208–214. [Google Scholar] [CrossRef]
- Kulekci, M.K. Magnesium and its alloys applications in automotive industry. Int. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.K.; Wang, B.J.; Han, E.H.; Dong, C. Effect of solution treatment on the fatigue behavior of an as-forged Mg-Zn-Y-Zr alloy. Sci. Rep. 2016, 6, 23955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahed, H.; Albinmousa, J. Multiaxial behaviour of wrought magnesium alloys—A review and suitability of energy-based fatigue life model. Theor. Appl. Fract. Mech. 2014, 73, 97–108. [Google Scholar] [CrossRef]
- Behravesh, S.B.; Jahed, H.; Lambert, S.B.; Chengji, M. Constitutive modeling for cyclic behavior of AZ31B magnesium alloy and its application. Adv. Mater. Res. 2014, 891, 809–814. [Google Scholar] [CrossRef]
- Roostaei, A.A.; Jahed, H. Role of loading direction on cyclic behaviour characteristics of AM30 extrusion and its fatigue damage modelling. Mater. Sci. Eng. 2016, 670, 26–40. [Google Scholar] [CrossRef]
- Pan, F.; Yang, M.; Chen, X. A review on casting magnesium alloys: Modification of commercial alloys and development of new alloys. J. Mater. Sci. Technol. 2016, 32, 1211–1221. [Google Scholar] [CrossRef]
- Toscano, D.; Shaha, S.K.; Behravesh, B.; Jahed, H.; Williams, B. Effect of forging on microstructure, texture, and uniaxial properties of cast AZ31B alloy. J. Mater. Eng. Perform. 2017, 26, 3090–3103. [Google Scholar] [CrossRef]
- Diab, M.; Pang, X.; Jahed, H. The effect of pure aluminum cold spray coating on corrosion and corrosion fatigue of magnesium (3% Al-1% Zn) extrusion. Surf. Coat. Technol. 2017, 309, 423–435. [Google Scholar] [CrossRef]
- Eliezer, A.; Medlinsky, O.; Haddad, J.; Ben-Hamu, G. Corrosion fatigue behavior of magnesium alloys under oil environments. Mater. Sci. Eng. A 2008, 477, 129–136. [Google Scholar] [CrossRef]
- Raman, R.S.; Jafari, S.; Harandi, S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: A review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]
- Unigovski, Y.; Eliezer, A.; Abramov, E.; Snir, Y.; Gutman, E.M. Corrosion fatigue of extruded magnesium alloys. Mater. Sci. Eng. A 2003, 360, 132–139. [Google Scholar] [CrossRef]
- Eliezer, A.; Gutman, E.M.; Abramov, E.; Unigovski, Y. Corrosion fatigue of die-cast and extruded magnesium alloys. J. Light Met. 2001, 1, 179–186. [Google Scholar] [CrossRef]
- Gutman, E.M.; Eliezer, A.; Unigovski, Y.; Abramov, E. Mechanoelectrochemical behavior and creep corrosion of magnesium alloys. Mater. Sci. Eng. A 2001, 302, 63–67. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Zhuang, W.Z. Nondestructive inspection of fatigue crack propagation beneath supersonic particle deposition coatings during fatigue testing. Int. J. Fatigue 2017, 102, 149–157. [Google Scholar] [CrossRef]
- Bu, H.; Yandouzi, M.; Lu, C.; MacDonald, D.; Jodoin, B. Cold spray blended Al + Mg17Al12 coating for corrosion protection of AZ91D magnesium alloy. Surf. Coat. Technol. 2012, 207, 155–162. [Google Scholar] [CrossRef]
- Ghelichi, R.; MacDonald, D.; Bagherifard, S.; Jahed, H.; Guagliano, M.; Jodoin, B. Microstructure and fatigue behavior of cold spray coated Al5052. Acta Mater. 2012, 60, 6555–6561. [Google Scholar] [CrossRef]
- Rokni, M.R.; Widener, C.A.; Crawford, G.A.; West, M.K. An investigation into microstructure and mechanical properties of cold sprayed 7075 Al deposition. Mater. Sci. Eng. A 2015, 625, 19–27. [Google Scholar] [CrossRef]
- Shayegan, G.; Mahmoudi, H.; Ghelichi, R.; Villafuerte, J.; Wang, J.; Guagliano, M.; Jahed, H. Residual stress induced by cold spray coating of magnesium AZ31B extrusion. Mater. Des. 2014, 60, 72–84. [Google Scholar] [CrossRef]
- Spencer, K.; Fabijanic, D.M.; Zhang, M.X. The use of Al-Al2O3 cold spray coatings to improve the surface properties of magnesium alloys. Surf. Coat. Technol. 2009, 204, 336–344. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, D.; Xiong, Y.; Birbilis, N.; Zhang, M.X. High resolution microstructure characterization of the interface between cold sprayed Al coating and Mg alloy substrate. Appl. Surf. Sci. 2014, 289, 366–369. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold spraying—A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Vezzu, S. Effect of cold spray deposition of similar material on fatigue behavior of Al 6082 alloy. Fract. Fatigue 2014, 7, 51–57. [Google Scholar]
- Luo, X.T.; Li, C.X.; Shang, F.L.; Yang, G.J.; Wang, Y.Y.; Li, C.J. High velocity impact induced microstructure evolution during deposition of cold spray coatings: A review. Surf. Coat. Technol. 2014, 254, 11–20. [Google Scholar] [CrossRef]
- McCune, R.C.; Donlon, W.T.; Popoola, O.O.; Cartwright, E.L. Cartwright characterization of copper layers produced by cold gas-dynamic spraying. J. Therm. Spray Technol. 2000, 9, 73–82. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, S.M.; Moridi, A.; Guagliano, M. Critical review of corrosion protection by cold spray coatings. Surf. Eng. 2015, 31, 803–815. [Google Scholar] [CrossRef]
- Yandouzi, M.; Richer, P.; Jodoin, B. SiC particulate reinforced Al-12Si alloy composite coatings produced by the pulsed gas dynamic spray process: Microstructure and properties. Surf. Coat. Technol. 2009, 203, 3260–3270. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, S.H.; Lee, S.Y.; You, Y.H.; Ko, K.H. Correlation between Al2O3 particles and interface of Al-Al2O3 coatings by cold spray. Appl. Surf. Sci. 2005, 252, 1891–1898. [Google Scholar] [CrossRef]
- Tao, Y.; Xiong, T.; Sun, C.; Kong, L.; Cui, X.; Li, T.; Song, G.L. Microstructure and corrosion performance of a cold sprayed aluminium coating on AZ91D magnesium alloy. Corros. Sci. 2010, 52, 3191–3197. [Google Scholar] [CrossRef]
- Tao, Y.; Xiong, T.; Sun, C.; Jin, H.; Du, H.; Li, T. Effect of α-Al2O3 on the properties of cold sprayed Al/α-Al2O3 composite coatings on AZ91D magnesium alloy. Appl. Surf. Sci. 2009, 256, 261–266. [Google Scholar] [CrossRef]
- Kalatehmollaei, E.; Mahmoudi-Asl, H.; Jahed, H. An asymmetric elastic–plastic analysis of the load-controlled rotating bending test and its application in the fatigue life estimation of wrought magnesium AZ31B. Int. J. Fatigue 2014, 64, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Dayani, S.B.; Shaha, S.K.; Ghelichi, R.; Wang, J.F.; Jahed, H. The impact of AA7075 cold spray coating on the fatigue life of AZ31B cast alloy. Surf. Coat. Technol. 2018, 337, 150–158. [Google Scholar] [CrossRef]
- Niu, L.Y.; Jiang, Z.H.; Li, G.Y.; Gu, C.D.; Lian, J.S. A study and application of zinc phosphate coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 200, 3021–3026. [Google Scholar] [CrossRef]
- Bastos, A.C.; Ferreira, M.G.S.; Simoes, A.M. Comparative electrochemical studies of zinc chromate and zinc phosphate as corrosion inhibitors for zinc. Prog. Org. Coat. 2005, 52, 339–350. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M.M. Evaluation of zinc phosphate and zinc chromate effectiveness via AC and DC methods. Prog. Org. Coat. 2005, 53, 191–194. [Google Scholar] [CrossRef]
- Beiro, M.; Collazo, A.; Izquierdo, M.; Nóvoa, X.R.; Pérez, C. Characterisation of barrier properties of organic paints: The zinc phosphate effectiveness. Prog. Org. Coat. 2003, 46, 97–106. [Google Scholar] [CrossRef]
- Davies, J.M. Lung cancer mortality among workers making lead chromate and zinc chromate pigments at three English factories. Occup. Environ. Med. 1984, 41, 158–169. [Google Scholar] [CrossRef]
- Hu, R.G.; Zhang, S.; Bu, J.F.; Lin, C.J.; Song, G.L. Progress in organic coatings recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2011, 73, 129–141. [Google Scholar] [CrossRef]
- Langård, S.; Norseth, T. A cohort study of bronchial carcinomas in workers producing chromate pigments. Occup. Environ. Med. 1975, 32, 62–65. [Google Scholar] [CrossRef]
- ASTM E8 / E8M-11: Standard Test Methods for Tension Testing of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2011.
- E-Coat Process Specifics. Available online: https://www.ppgcoatingsservices.com/services/electrocoating/e-coat-process-specifics (accessed on 20 July 2018).
- Ezhilselvi, V.; Nithin, J.; Balaraju, J.N.; Subramanian, S. The influence of current density on the morphology and corrosion properties of MAO coatings on AZ31B magnesium alloy. Surf. Coat. Technol. 2016, 288, 221–229. [Google Scholar] [CrossRef]
- Marzbanrad, B.; Jahed, H.; Toyserkani, E. On the evolution of substrate’s residual stress during cold spray process: A parametric study. Mater. Des. 2018, 138, 90–102. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.; Maeva, E.; Leshchinsky, E.; Maev, R.G. Corrosion protection of light alloys using low pressure cold spray. J. Therm. Spray Technol. 2012, 21, 304–313. [Google Scholar] [CrossRef]
- Zhu, L.; Song, G. Improved corrosion resistance of AZ91D magnesium alloy by an aluminium-alloyed coating. Surf. Coat. Technol. 2006, 200, 2834–2840. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Wagner, C. On the interpretation of corrosion processes through the superposition of electrochemical partial processes and on the potential of mixed electrodes. Ztschr. Elecktrochem 1938, 44, 391. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Shao, Y.; Zhang, T.; Wang, Y.; Wang, F.; Cheng, X.; Dong, C.; Li, X. Effect of microstructures on corrosion behavior of nickel coatings: (II) competitive effect of grain size and twins density on corrosion behavior. J. Mater. Sci. Technol. 2016, 32, 465–469. [Google Scholar] [CrossRef]
- Quach, N.C.; Uggowitzer, P.J.; Schmutz, P. Corrosion behaviour of an Mg-Y-RE alloy used in biomedical applications studied by electrochemical techniques. C.R. Chim. 2008, 11, 1043–1054. [Google Scholar] [CrossRef]
- Niu, Y.; Cui, R.; He, Y.; Yu, Z. Wear and corrosion behavior of Mg-Gd-Y-Zr alloy treated by mixed molten-salt bath. J. Alloy. Compd. 2014, 610, 294–300. [Google Scholar] [CrossRef]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Rammelt, U.; Reinhard, G. On the applicability of a constant phase element (CPE) to the estimation of roughness of solid metal electrodes. Electrochim. Acta 1990, 35, 1045–1049. [Google Scholar] [CrossRef]
- Nady, H.; El-Rabiei, M.M.; Samy, M. Corrosion behavior and electrochemical properties of carbon steel, commercial pure titanium, copper and copper–aluminum–nickel alloy in 3.5% sodium chloride containing sulfide ions. Egypt. J. Pet. 2017, 26, 79–94. [Google Scholar] [CrossRef]
- Wei, Y.K.; Li, Y.J.; Zhang, Y.; Luo, X.T.; Li, C.J. Corrosion resistant nickel coating with strong adhesion on AZ31B magnesium alloy prepared by an in-situ shot-peening-assisted cold spray. Corros. Sci. 2018, 138, 105–115. [Google Scholar] [CrossRef]
- Mahdavian, M.M.; Attar, M.M. Another approach in analysis of paint coatings with EIS measurement: Phase angle at high frequencies. Corros. Sci. 2006, 48, 4152–4157. [Google Scholar] [CrossRef]
- Jahed, H.; Ghelichi, R. Residual stresses and fatigue life enhancement of cold spray. Mod. Cold Spray 2015, 225–252. [Google Scholar]
- Nan, Z.Y.; Ishihara, S.; Goshima, T. Corrosion fatigue behavior of extruded magnesium alloy AZ31 in sodium chloride solution. Int. J. Fatigue 2008, 30, 1181–1188. [Google Scholar] [CrossRef]
- Xie, Z.H.; Chen, F.; Xiang, S.R.; Zhou, J.L.; Song, Z.W.; Yu, G. Studies of several pickling and activation processes for electroless Ni-P plating on AZ31 magnesium alloy. J. Electrochem. Soc. 2015, 162, D115–D123. [Google Scholar] [CrossRef]
- Wang, S.G.; Huang, Y.J.; Han, H.B.; Sun, M.; Long, K.; Zhang, Z.D. The electrochemical corrosion characterization of bulk nanocrystalline aluminium by X-ray photoelectron spectroscopy and ultra-violet photoelectron spectroscopy. J. Electroanal. Chem. 2014, 724, 95–102. [Google Scholar] [CrossRef]
- Moy, C.K.; Cairney, J.; Ranzi, G.; Jahedi, M.; Ringer, S.P. Investigating the microstructure and composition of cold gas-dynamic spray (CGDS) Ti powder deposited on Al 6063 substrate. Surf. Coat. Technol. 2010, 204, 3739–3749. [Google Scholar] [CrossRef]
- Zou, Y.; Qin, W.; Irissou, E.; Legoux, J.G.; Yue, S.; Szpunar, J.A. Dynamic recrystallization in the particle/particle interfacial region of cold-sprayed nickel coating: Electron backscatter diffraction characterization. Scr. Mater. 2009, 61, 899–902. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Honkanen, M.; Vuoristo, P. Cold-sprayed copper and tantalum coatings—detailed FESEM and TEM analysis. Surf. Coat. Technol. 2010, 204, 2353–2361. [Google Scholar] [CrossRef]
- Li, W.; Li, D.Y. Variations of work function and corrosion behaviors of deformed copper surfaces. Appl. Surf. Sci. 2005, 240, 388–395. [Google Scholar] [CrossRef]
- Laleh, M.; Kargar, F. Effect of surface nanocrystallization on the microstructural and corrosion characteristics of AZ91D magnesium alloy. J. Alloy. Compd. 2011, 509, 9150–9156. [Google Scholar] [CrossRef]
- Balani, K.; Laha, T.; Agarwal, A.; Karthikeyan, J.; Munroe, N. Effect of carrier gases on microstructural and electrochemical behavior of cold-sprayed 1100 aluminum coating. Surf. Coat. Technol. 2005, 195, 272–279. [Google Scholar] [CrossRef]
- Meydanoglu, O.; Jodoin, B.; Kayali, E.S. Microstructure, mechanical properties and corrosion performance of 7075 Al matrix ceramic particle reinforced composite coatings produced by the cold gas dynamic spraying process. Surf. Coat. Technol. 2013, 235, 108–116. [Google Scholar] [CrossRef]
- Ghelichi, R.; Bagherifard, S.; Mac Donald, D.; Brochu, M.; Jahed, H.; Jodoin, B.; Guagliano, M. Fatigue strength of Al alloy cold sprayed with nanocrystalline powders. Int. J. Fatigue 2014, 65, 51–57. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Han, Q.; Li, J.; Xu, R.; Zhao, K. A comparison of AA2024 and AA7150 subjected to ultrasonic shot peening: Microstructure, surface segregation and corrosion. Surf. Coat. Technol. 2018, 337, 552–560. [Google Scholar] [CrossRef]
- Sun, Q.; Han, Q.; Xu, R.; Zhao, K.; Li, J. Localized corrosion behaviour of AA7150 after ultrasonic shot peening: Corrosion depth vs. impact energy. Corros. Sci. 2018, 130, 218–230. [Google Scholar] [CrossRef]
- Reboul, M.C.; Warner, T.J.; Mayer, H.; Barouk, B. A Ten Step Mechanism for the Pitting Corrosion of Aluminium Alloys. Corros. Rev. 1997, 15, 471–496. [Google Scholar] [CrossRef]












| Alloy | Mg | Al | Zn | Mn | Cu | Fe | Cr | Others |
|---|---|---|---|---|---|---|---|---|
| AZ31 | balance | 3.29 | 1.33 | 0.37 | 0 | 0 | 0 | >0.01 |
| AA7075 | 2.35 | balance | 5.2 | 0 | 1.55 | 0.35 | 0.25 | 0.3 |
| Specimens | Ecorr (V) | icorr (μA·cm−2) | Corrosion Rate (mm/year) |
|---|---|---|---|
| Uncoated | −1.52 | 117.6 | 2.7 |
| CS | −1.37 | 80.7 | 1.8 |
| CS + EP | −1.24 | 7 × 10−2 | 1.6 × 10−3 |
| Specimen | Rct (Ω∙cm2) | Qdl (µF·cm−2∙sn−1) | ndl | Rads (Ω∙cm2) | Lads (mH·cm−2) |
|---|---|---|---|---|---|
| uncoated | 1.64 × 102 | 23.59 | 0.95 | 1.45 × 102 | 0.786 |
| Specimen | Qcoat (µF·cm−2∙sn−1) | ncoat | Rcoat (Ω∙cm2) | Rct (Ω∙cm2) | Qdl (µF·cm−2∙sn−1) | ndl |
|---|---|---|---|---|---|---|
| CS | 0.13720 | 0.87 | 1.833 × 102 | 1.42 × 103 | 0.92 | 0.58 |
| CS + EP | 0.05301 | 0.81 | 8.756 × 106 | 4.13 × 107 | 0.21 | 0.41 |
| Sample ID | YS (MPa) | UTS (MPa) | Fracture Strain (%) |
|---|---|---|---|
| Uncoated | 88.75 | 195.88 | 11.32 |
| 85.50 | 196.25 | 13.26 | |
| CS | 112.65 | 194.56 | 12.15 |
| 107.50 | 199.21 | 11.88 | |
| CS + EP | 108.95 | 196.43 | 10.97 |
| 113.25 | 195.78 | 12.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaha, S.K.; Dayani, S.B.; Xue, Y.; Pang, X.; Jahed, H. Improving Corrosion and Corrosion-Fatigue Resistance of AZ31B Cast Mg Alloy Using Combined Cold Spray and Top Coatings. Coatings 2018, 8, 443. https://doi.org/10.3390/coatings8120443
Shaha SK, Dayani SB, Xue Y, Pang X, Jahed H. Improving Corrosion and Corrosion-Fatigue Resistance of AZ31B Cast Mg Alloy Using Combined Cold Spray and Top Coatings. Coatings. 2018; 8(12):443. https://doi.org/10.3390/coatings8120443
Chicago/Turabian StyleShaha, Sugrib Kumar, Siavash Borhan Dayani, Yuna Xue, Xin Pang, and Hamid Jahed. 2018. "Improving Corrosion and Corrosion-Fatigue Resistance of AZ31B Cast Mg Alloy Using Combined Cold Spray and Top Coatings" Coatings 8, no. 12: 443. https://doi.org/10.3390/coatings8120443