Mechanical and Barrier Properties of Potato Protein Isolate-Based Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparation
2.3. Cast Films
2.4. Residual Moisture Determination
2.5. Protein Solubility
2.6. Thickness Measurement
2.7. Determination of the Degree of Swelling, Degree of Cross-Linking and the Cross-Linking Density
2.8. Determination of Mechanical Properties
2.9. Determination of Barrier Properties
2.10. Statistical Evaluation
3. Results and Discussion
3.1. Plasticizer Effect and Compatibility
3.1.1. Effects of Glycerol, Sorbitol, and PEG 400
3.1.2. Effects of EG and PG
3.2. Intermolecular Characteristics
3.2.1. Cross-Linking Density and Degree of Cross-Linking
3.2.2. Degree of Swelling
3.3. Mechanical Properties
3.4. Barrier Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tolinski, M. Plastics and Sustainability: Towards a Peaceful Coexistence between Bio-Based and Fossil Fuel-Based Plastics; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Coltelli, M.B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Muller, K.; Rodriguez, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Denavi, G.; Tapia-Blácido, D.R.; Añón, M.C.; Sobral, P.J.A.; Mauri, A.N.; Menegalli, F.C. Effects of drying conditions on some physical properties of soy protein films. J. Food Eng. 2009, 90, 341–349. [Google Scholar] [CrossRef]
- Zubeldía, F.; Ansorena, M.R.; Marcovich, N.E. Wheat gluten films obtained by compression molding. Polym. Test. 2015, 43, 68–77. [Google Scholar] [CrossRef]
- Tihminlioglu, F.; Atik, İ.D.; Özen, B. Water vapor and oxygen-barrier performance of corn–zein coated polypropylene films. J. Food Eng. 2010, 96, 342–347. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Wojnowska, I.; Poznanski, S.; Bednarski, W. Processing of potato protein concentrates and their properties. J. Food Sci. 1982, 47, 167–172. [Google Scholar] [CrossRef]
- Newson, W.R.; Rasheed, F.; Kuktaite, R.; Hedenqvist, M.S.; Gallstedt, M.; Plivelic, T.S.; Johansson, E. Commercial potato protein concentrate as a novel source for thermoformed bio-based plastic films with unusual polymerisation and tensile properties. RSC Adv. 2015, 5, 32217–32226. [Google Scholar] [CrossRef]
- Van Koningsveld, G.A.; Gruppen, H.; de Jongh, H.H.J.; Wijngaards, G.; van Boekel, M.A.J.S.; Walstra, P.; Voragen, A.G.J. The solubility of potato proteins from industrial potato fruit juice as influenced by pH and various additives. J. Sci. Food Agric. 2002, 82, 134–142. [Google Scholar] [CrossRef]
- Strætkvern, K.O.; Schwarz, J.G. Recovery of native potato protein comparing expanded bed adsorption and ultrafiltration. Food Bioprocess Technol. 2012, 5, 1939–1949. [Google Scholar] [CrossRef] [Green Version]
- Ralet, M.-C.; Guéguen, J. Fractionation of potato proteins: Solubility, thermal coagulation and emulsifying properties. LWT Food Sci. Technol. 2000, 33, 380–387. [Google Scholar] [CrossRef]
- Løkra, S.; Helland, M.H.; Claussen, I.C.; Strætkvern, K.O.; Egelandsdal, B. Chemical characterization and functional properties of a potato protein concentrate prepared by large-scale expanded bed adsorption chromatography. LWT Food Sci. Technol. 2008, 41, 1089–1099. [Google Scholar] [CrossRef]
- Pots, A.M. Physico-Chemical Properties and Thermal Aggregation of Patatin, the Major Potato Tuber Protein; Landbouwuniversiteit Wageningen: Wageningen, The Netherlands, 1999. [Google Scholar]
- Van Koningsveld, G.A.; Walstra, P.; Gruppen, H.; Wijngaards, G.; van Boekel, M.A.J.S.; Voragen, A.G.J. Formation and stability of foam made with various potato protein preparations. J. Agric. Food Chem. 2002, 50, 7651–7659. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xiong, Y.L.; Chen, J. Antioxidant and emulsifying properties of potato protein hydrolysate in soybean oil-in-water emulsions. Food Chem. 2010, 120, 101–108. [Google Scholar] [CrossRef]
- Pouvreau, L.; Gruppen, H.; Piersma, S.R.; van den Broek, L.A.M.; van Koningsveld, G.A.; Voragen, A.G.J. Relative abundance and inhibitory distribution of protease inhibitors in potato juice from cv. Elkana. J. Agric. Food Chem. 2001, 49, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Van Koningsveld, G.A.; Gruppen, H.; de Jongh, H.H.; Wijngaards, G.; van Boekel, M.A.; Walstra, P.; Voragen, A.G. Effects of pH and heat treatments on the structure and solubility of potato proteins in different preparations. J. Agric. Food Chem. 2001, 49, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Hammann, F.; Schmid, M. Determination and quantification of molecular interactions in protein films: A review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.B.; Monahan, F.J.; O’Riordan, E.D.; O’Sullivan, M. Physical properties of wpi films plasticized with glycerol, xylitol, or sorbitol. J. Food Sci. 2002, 67, 164–167. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey-protein-coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 2012, 562381. [Google Scholar] [CrossRef]
- Schmid, M. Properties of cast films made from different ratios of whey protein isolate, hydrolysed whey protein isolate and glycerol. Materials 2013, 6, 3254–3269. [Google Scholar] [CrossRef] [PubMed]
- McHugh, T.H.; Aujard, J.F.; Krochta, J.M. Plasticized whey-protein edible films—Water vapor permeability properties. J. Food Sci. 1994, 59, 416–419. [Google Scholar] [CrossRef]
- AOCS BA 11-65 Nitrogen Solubility Index (NSI); American Oil Chemists’ Society: Urbana, IL, USA, 1993.
- AACC Method 46-23 Nitrogen Solubility Index. Approved methods of the American Association of Cereal Chemists (AACC); American Association of Cereal Chemists: St. Paul, MN, USA, 1983.
- Schmid, M.; Pröls, S.; Kainz, D.M.; Hammann, F.; Stäbler, A. Impact of hydrolyzed whey protein on the molecular interactions and cross-linking density in whey protein isolate-based films. Int. J. Polym. Sci. 2016, 2016, 3723758. [Google Scholar] [CrossRef]
- Schmid, M.; Pröls, S.; Kainz, D.M.; Hammann, F.; Grupa, U. Effect of thermally induced denaturation on molecular interaction-response relationships of whey protein isolate based films and coatings. Prog. Org. Coat. 2017, 104, 161–172. [Google Scholar] [CrossRef]
- DIN EN ISO 175:2010-10 Kunststoffe-Prüfverfahren zur Bestimmung des Verhaltens gegen flüssige Chemikalien; Deutsches Institut für Normung e.V.: Berlin, Germany, 2010. (In German)
- Pęksa, A.; Miedzianka, J. Amino acid composition of enzymatically hydrolysed potato protein preparations. Czech J. Food Sci. 2014, 32, 265–272. [Google Scholar]
- Ahmed, K.; Nizami, S.S.; Raza, N.Z.; Mahmood, K. Mechanical, swelling, and thermal aging properties of marble sludge-natural rubber composites. Int. J. Ind. Chem. 2012, 3, 21. [Google Scholar] [CrossRef]
- DIN EN ISO 527-1 Kunststoffe-Bestimmung der Zugeigenschaften; Deutsches Institut für Normung e.V.: Berlin, Germany, 2012. (In German)
- DIN 53122-1 Bestimmung der Wasserdampfdurchlässigkeit. Teil 1: Gravimetrisches Verfahren; Deutsches Institut für Normung e.V.: Berlin, Germany, 2001. (In German)
- DIN 53380-3 Bestimmung der Gasdurchlässigkeit. Teil 3: Sauerstoffspezifisches Trägergas-Verfahren zur Messung an Kunststoff-Folien und Kunststoff-Formteilen; Deutsches Institut für Normung e.V.: Berlin, Germany, 1998. (In German)
- Ronniger, C.U. Taschenbuch der Statistischen Qualitäts-und Zuverlässigkeitsmethoden: Die Wichtigsten Methoden und Verfahren für Die Praxis; CRGRAPH: Starnberg, Germany, 2013. (In German) [Google Scholar]
- Sothornvit, R.; Krochta, J. Plasticizer effect on oxygen permeability of β-lactoglobulin films. J. Agric. Food Chem. 2000, 48, 6298–6302. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, C.J.R.; van den Berg, L.E. Extrusion processing and properties of protein-based thermoplastics. Macromol. Mater. Eng. 2010, 295, 10–21. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizer effect on mechanical properties of β-lactoglobulin films. J. Food Eng. 2001, 50, 149–155. [Google Scholar] [CrossRef]
- Griffin, W.; Lynch, M. Polyhydric alcohols. In Handbook of Food Additives, 2nd ed.; Furia, T., Ed.; CRC Press: Cleveland, OH, USA, 1980; Volume 2, pp. 431–455. [Google Scholar]
- Cuq, B.; Gontard, N.; Cuq, J.-L.; Guilbert, S. Selected functional properties of fish myofibrillar protein-based films as affected by hydrophilic plasticizers. J. Agric. Food Chem. 1997, 45, 622–626. [Google Scholar] [CrossRef]
- Jangchud, A.; Chinnan, M.S. Properties of peanut protein film: Sorption isotherm and plasticizer effect. LWT Food Sci. Technol. 1999, 32, 89–94. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Water and glycerol as plasticizers affect mechanical and water vapor barrier properties of an edible wheat gluten film. J. Food Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Pocas, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef] [Green Version]
- CHERIC. Pure Component Properties. Available online: https://www.cheric.org/research/kdb/hcprop/cmpsrch.php (accessed on 10 December 2017).
- Orliac, O.; Rouilly, A.; Silvestre, F.; Rigal, L. Effects of various plasticizers on the mechanical properties, water resistance and aging of thermo-moulded films made from sunflower proteins. Ind. Crop. Prod. 2003, 18, 91–100. [Google Scholar] [CrossRef]
- Viroben, G.; Barbot, J.; Mouloungui, Z.; Guéguen, J. Preparation and characterization of films from pea protein. J. Agric. Food Chem. 2000, 48, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Vanin, F.M.; Sobral, P.J.A.; Menegalli, F.C.; Carvalho, R.A.; Habitante, A.M.Q.B. Effects of plasticizers and their concentrations on thermal and functional properties of gelatin-based films. Food Hydrocoll. 2005, 19, 899–907. [Google Scholar] [CrossRef]
- Donhowe, I.G.; Fennema, O. The effects of plasticizers on crystallinity, permeability, and mechanical properties of methylcellulose films. J. Food Process. Preserv. 1993, 17, 247–257. [Google Scholar] [CrossRef]
- Nemet, N.T.; Šošo, V.M.; Lazić, V.L. Effect of glycerol content and pH value of film-forming solution on the functional properties of protein-based edible films. Acta Period. Technol. 2010, 57–67. [Google Scholar] [CrossRef] [Green Version]
- De Wit, J. Lehrbuch der Molke und Molkenerzeugnisse; European Whey Products Association: Brüssel, Belgium, 2001. [Google Scholar]
- Creusot, N.; Wierenga, P.A.; Laus, M.C.; Giuseppin, M.L.F.; Gruppen, H. Rheological properties of patatin gels compared with β-lactoglobulin, ovalbumin, and glycinin. J. Sci. Food Agric. 2011, 91, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Vikelouda, M.; Kiosseoglou, V. The use of carboxymethylcellulose to recover potato proteins and control their functional properties. Food Hydrocoll. 2004, 18, 21–27. [Google Scholar] [CrossRef]
- Montalvo-Paquini, C.; Rangel-Marron, M.; Palou, E.; Lopez-Malo, A. Effect of pH on physical properties of edible films from faba bean protein. Recent Adv. Chem. Eng. Biochem. Comput. Chem. 2013, 29–34. [Google Scholar]
- Venugopal, V. Marine Products for Healthcare: Functional and Bioactive Nutraceutical Compounds from the Ocean; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Peppas, N.A.; Ottenbrite, R.M.; Park, K.; Okano, T. Biomedical Applications of Hydrogels Handbook; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Schmidt, M.; Rodler, N.; Miesbauer, O.; Rojahn, M.; Vogel, T.; Dörfler, R.; Kucukpinar, E.; Langowski, H.-C. Adhesion and barrier performance of novel barrier adhesives used in multilayered high-barrier laminates. J. Adhes. Sci. Technol. 2012, 26, 2405–2436. [Google Scholar] [CrossRef]
- Schmid, M.; Prinz, T.K.; Müller, K.; Haas, A. UV radiation induced cross-linking of whey protein isolate-based films. Int. J. Polym. Sci. 2017, 2017, 1846031. [Google Scholar] [CrossRef]
- Schmid, M.; Prinz, T.K.; Stäbler, A.; Sängerlaub, S. Effect of sodium sulfite, sodium dodecyl sulfate, and urea on the molecular interactions and properties of whey protein isolate-based films. Front. Chem. 2016, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Sängerlaub, S.; Wege, L.; Stäbler, A. Properties of transglutaminase crosslinked whey protein isolate coatings and cast films. Packag. Technol. Sci. 2014, 27, 799–817. [Google Scholar] [CrossRef]
- Schmid, M.; Reichert, K.; Hammann, F.; Stäbler, A. Storage time-dependent alteration of molecular interaction–property relationships of whey protein isolate-based films and coatings. J. Mater. Sci. 2015, 50, 4396–4404. [Google Scholar] [CrossRef]
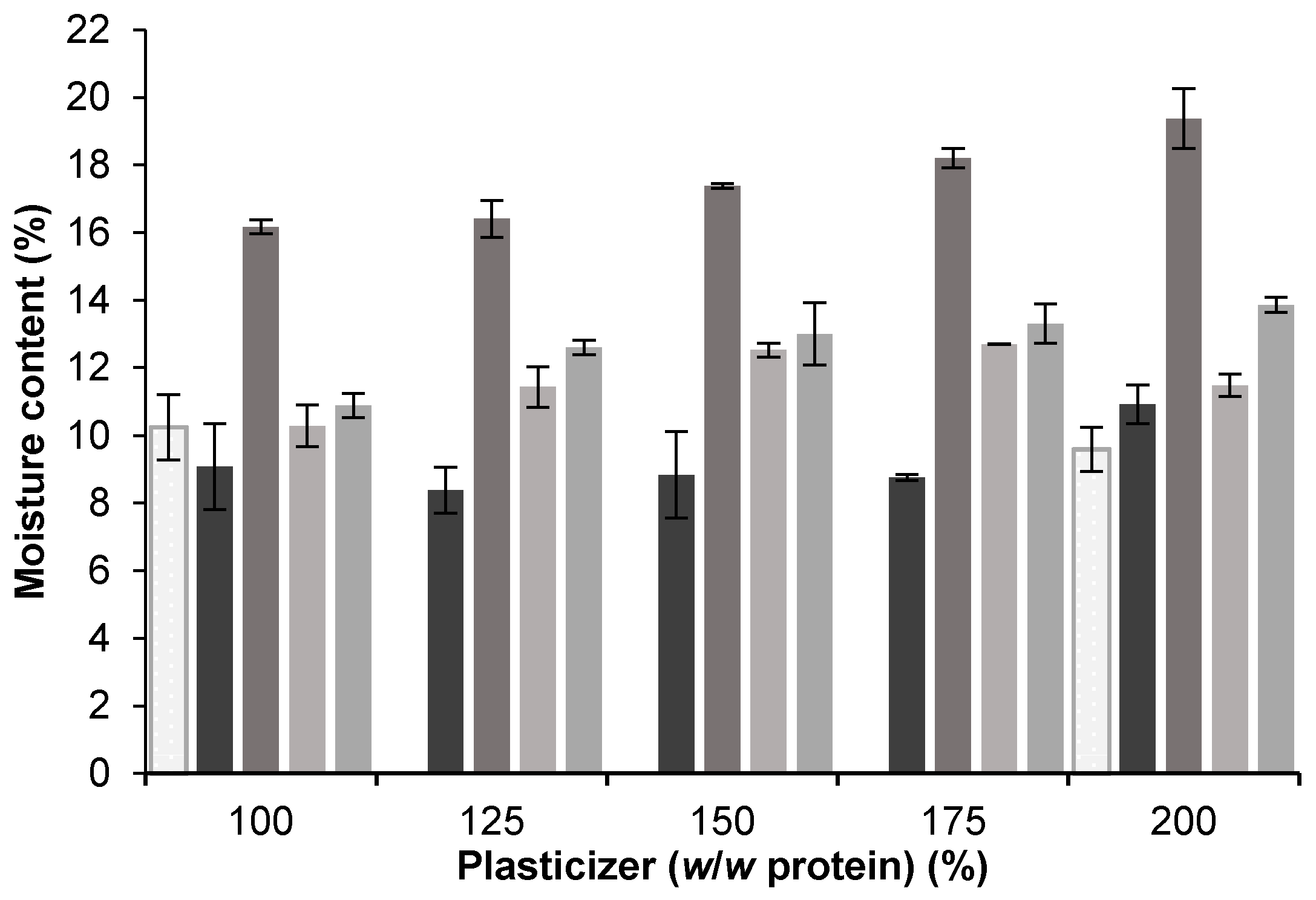
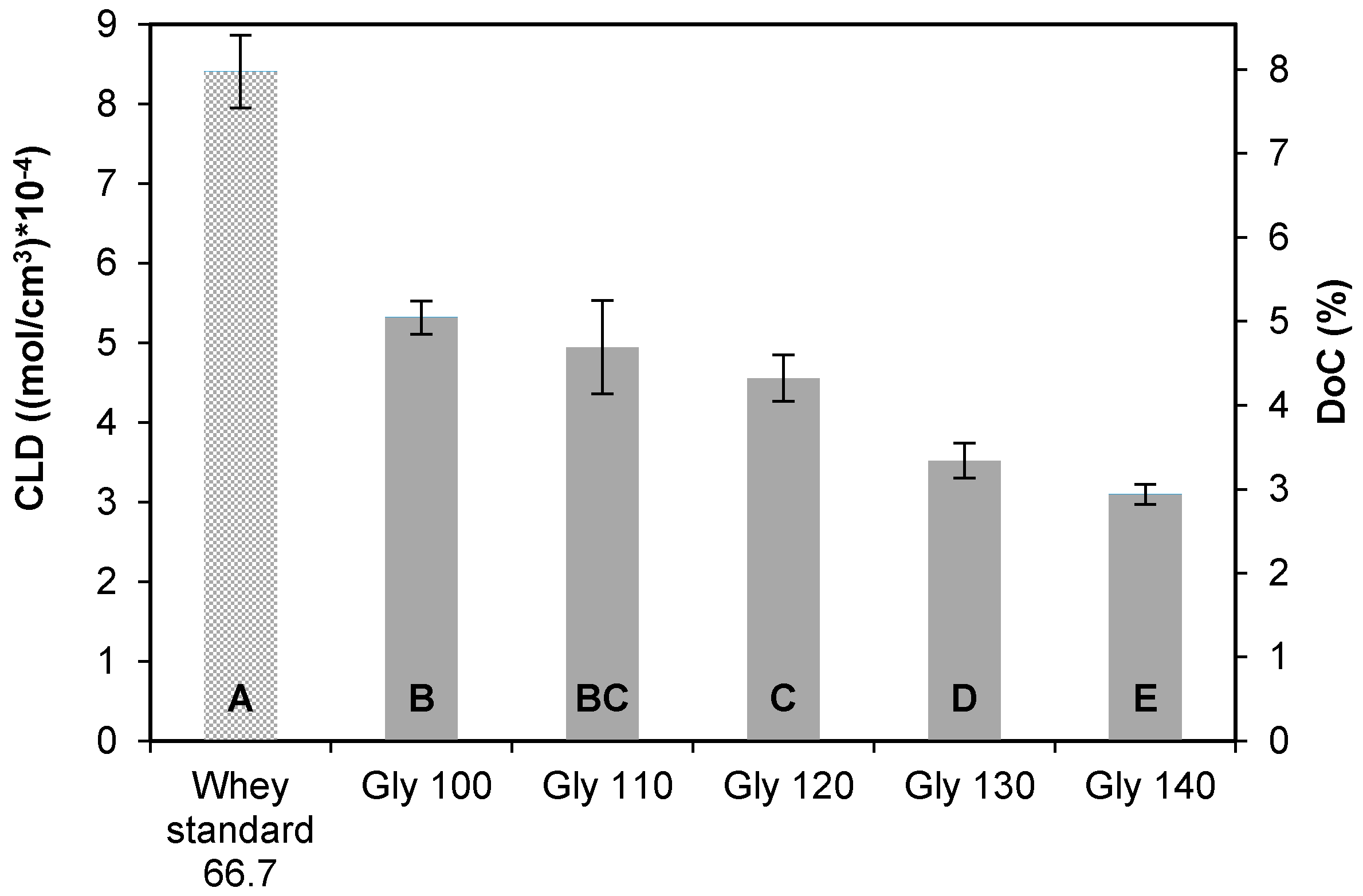
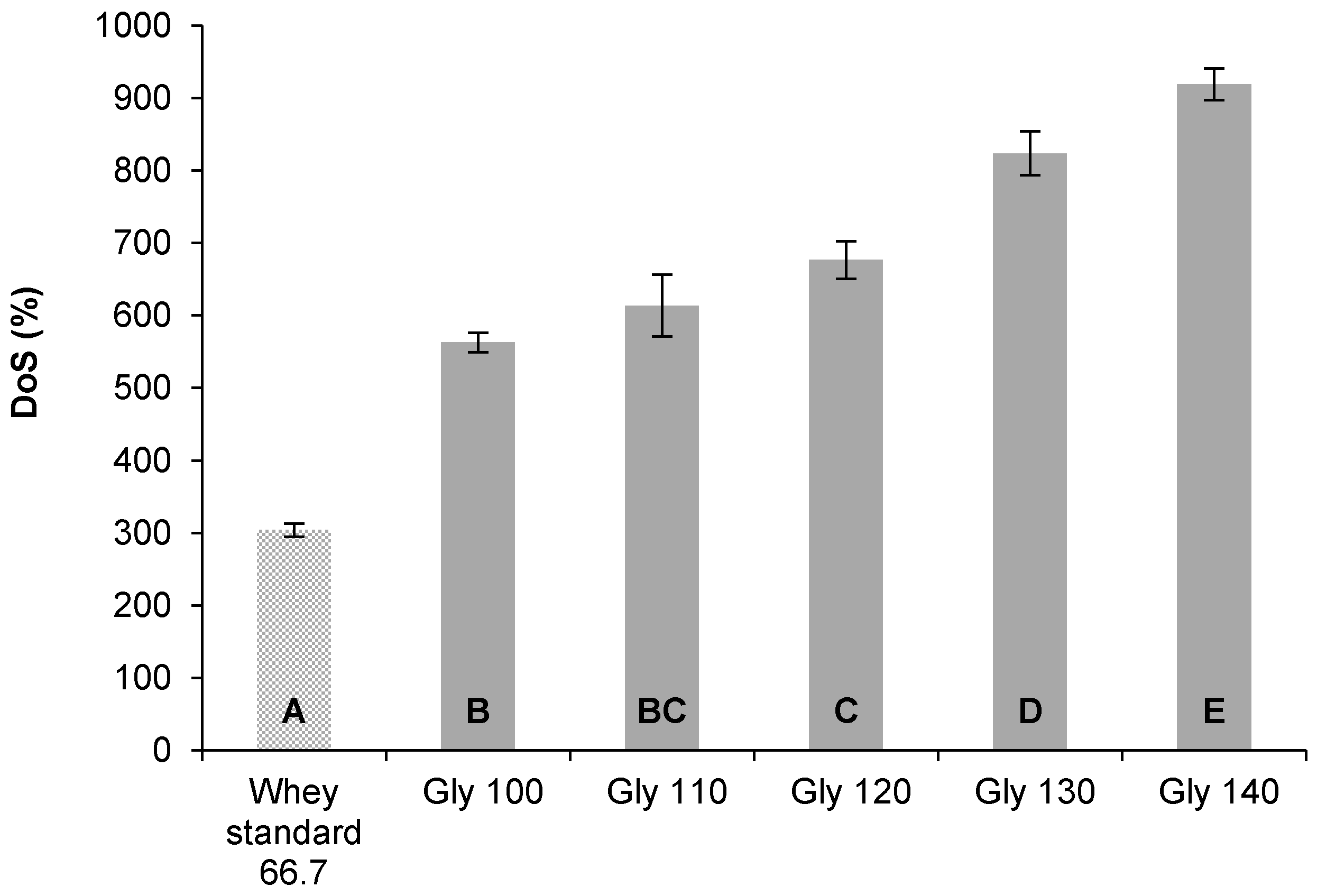
| Plasticizer Type | MW (g/mol) | Formula | Hydrophilic Groups (%) |
|---|---|---|---|
| Ethylene glycol (EG) | 62 | C2H6O2 | 54.8 |
| Propylene glycol (PG) | 76 | C3H8O2 | 44.7 |
| Glycerol (Gly) | 92 | C3H8O3 | 55.4 |
| Sorbitol (Sor) | 182 | C6H14O6 | 56.0 |
| Polyethylene glycol (PEG) | 400 | H(OCH2–CH2)8OH | 36.5 |
| Plasticizer | Plasticizer (w/w Protein) | ||||
|---|---|---|---|---|---|
| 100% | 125% | 150% | 175% | 200% | |
| Ethylene glycol |  |  | |||
| Propylene glycol |  |  |  |  |  |
| Glycerol |  |  |  |  |  |
| Sorbitol |  |  |  |  |  |
| Polyethylene glycol 400 |  |  |  |  |  |
| Sample | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break *(%) |
|---|---|---|---|
| Whey standard 66.7 | 90.8 ± 7.2 a | 4.01 ± 0.34 a | 90.0 ± 16.2 a |
| Gly 100 | 48.2 ± 2.3 b | 1.88 ± 0.09 b | 11.7 ± 1.3 b |
| Gly 110 | 39.7 ± 0.2 c | 1.67 ± 0.05 c | 11.4 ± 1.5 b |
| Gly 120 | 35.6 ± 1.8 d | 1.47 ± 0.08 d | 10.5 ± 2.9 b |
| Gly 130 | 20.9 ± 2.1 e | 0.93 ± 0.09 e | 9.0 ± 4.5 b,c |
| Gly 140 | 13.7 ± 2.6 f | 0.64 ± 0.07 f | 6.1 ± 1.4 c |
| Sample | Oxygen Permeability Q100 * (cm3/m2·d·bar) | Water vapor Transmission Rate Q100 (g/(m2·d)) |
|---|---|---|
| Whey standard 66.7 | 104.8 ± 4.3 a | 392.9 ± 40.5 a |
| Gly 100 | 230.8 ± 7.5 b | 792.7 ± 65.3 b |
| Gly 110 | 256.0 ± 11.2 b | 883.0 ± 82.5 b,c |
| Gly 120 | 282.5 ± 1.5 b | 1055.9 ± 172.5 c,d |
| Gly 130 | 339.9 ± 35.1 b,c | 1237.0 ± 48.7 d,e |
| Gly 140 | 396.7 ± 20.7 c | 1341.3 ± 130.6 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, D.; Reinelt, M.; Stäbler, A.; Schmid, M. Mechanical and Barrier Properties of Potato Protein Isolate-Based Films. Coatings 2018, 8, 58. https://doi.org/10.3390/coatings8020058
Schäfer D, Reinelt M, Stäbler A, Schmid M. Mechanical and Barrier Properties of Potato Protein Isolate-Based Films. Coatings. 2018; 8(2):58. https://doi.org/10.3390/coatings8020058
Chicago/Turabian StyleSchäfer, David, Matthias Reinelt, Andreas Stäbler, and Markus Schmid. 2018. "Mechanical and Barrier Properties of Potato Protein Isolate-Based Films" Coatings 8, no. 2: 58. https://doi.org/10.3390/coatings8020058





