1. Introduction
At thermal power plants in Russia, repair costs account for 12% of the total cost of the electricity generated [
1]. This is caused by corrosion and wear of parts operating at high temperature and sustaining wear. The protection of parts and surfaces by means of thermally-sprayed (TS) coatings is one of the rapidly developing fields in surface engineering. Arc spraying (AS) is one of the most cost-effective methods among other TS alternatives.
Corrosion, fouling, and slagging of superheaters are serious problems in boilers utilizing fuels with high alkali and chlorine content. It has been reported that the combustion of biomass [
2,
3], waste [
4,
5], and high-chlorine coals [
6,
7] cause severe materials wastage in superheaters.
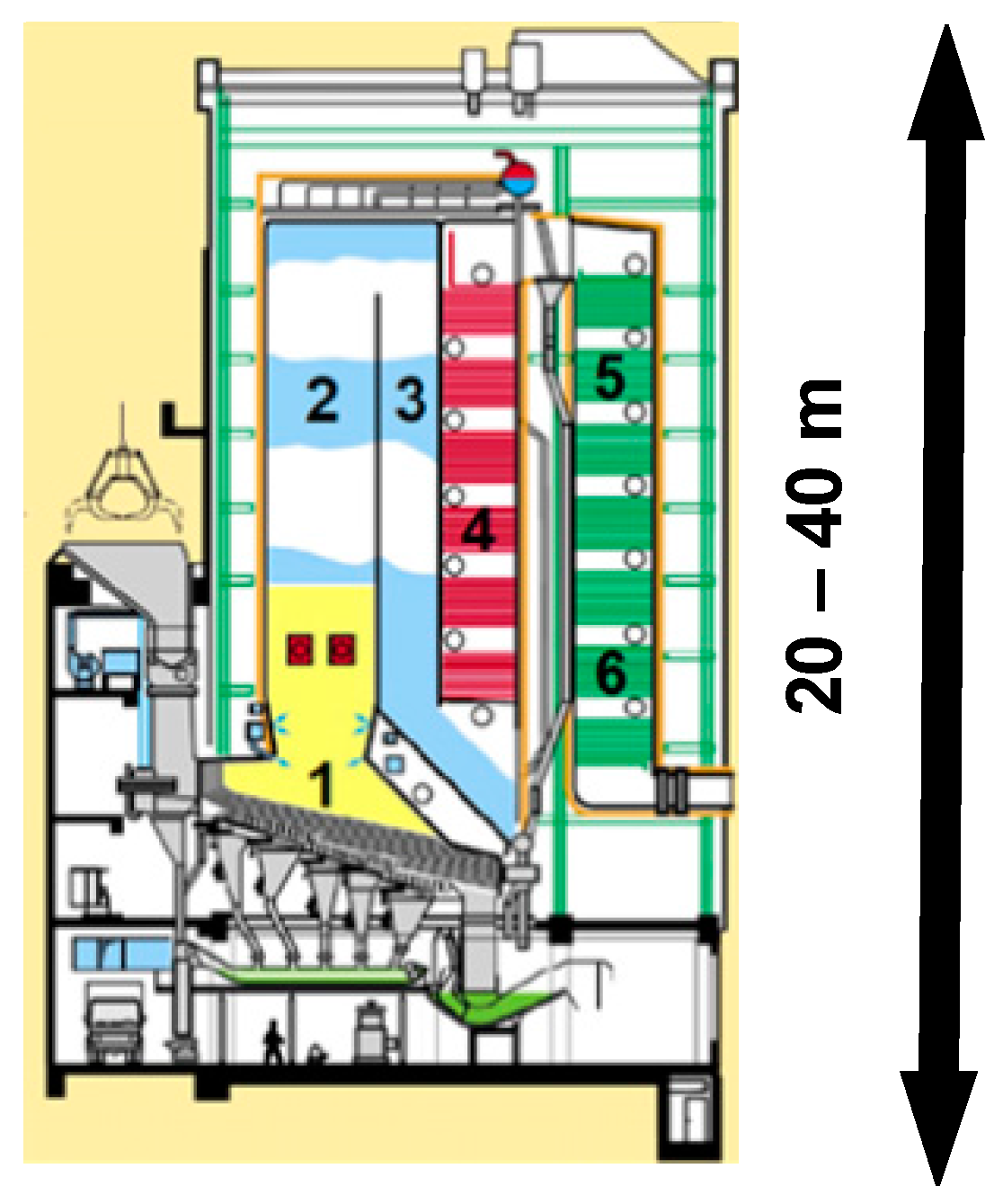
Applied thermal and aggressive loads in the coal-fired power plant are divided into groups due to the operating temperature, up to 800 °C, and the particulate content. In a gas corrosion medium, a small amount of particulate is present. In another typical group, the temperature drops, but the share of particulates increases. Various correlations between temperatures and particulate content cause changes in the wear type (gas corrosion, cavitation, abrasive, etc.). A typical incineration plant, shown in
Figure 1, is subjected to similar loads, which are specified in
Table 1.
Arc spraying is supremely suitable for protecting a large coating area due to the low operating costs and the simplicity of handling [
9,
10,
11]. Active arc spraying (AAS) was applied for coating deposition in the present study. The process differs from a typical AS process by the use of propane-air combustion products as a carrier gas instead of compressed air. This increases the particle velocity and temperature and forms a reducing atmosphere in the arc burning zone. These features can decrease the burnout of spraying metal in flight, increase the adhesion strength of the coating, and lower the porosity of the coating [
12,
13].
Various Fe-based solid and cored wires are used as feedstock materials for the formation of coatings for wear- and heat-resistant applications.
Wear resistant coatings with a metastable austenite structure are of particular interest due to low alloying costs and high resistance in various conditions of mechanical wear (abrasion, impact, cavitation, etc.). Here, the energy of the external load which is applied to the working surface is dissipated due to the transformation of austenite to disperse martensite. This improves the reliability by synergetic combination of surface hardening and reducing internal stresses [
14,
15,
16,
17]. Pukasiewicz et al. showed that in Fe-Mn-Cr-Si arc-sprayed coatings cavitation resistance increased due to strain-induced martensite transformation [
18].
Fe-Cr-Al alloys are traditionally used as bulk materials and as coatings for protecting against gas corrosion [
19,
20]. The heat resistance of the Fe-Cr-Al alloy is provided by a surface alumina layer, which forms at high operating temperatures. This oxide layer is characterized by a high melting temperature, chemical and thermal stability, and a low rate of growth [
21]. Alloying the Fe-Cr-Al by B increases the wear resistance of the alloy by forming reinforcing carboboride phases [
22]. Arc sprayed Fe-Cr-Al-B coatings save their protective properties when the erosion is combined with corrosion at elevated temperatures. The coatings demonstrate a 10–30-fold improvement in heat resistance at elevated temperatures of 600 and 700 °C compared with the steel 12Cr1MoV [
23]. The addition of yttrium is favorable for protecting against gas corrosion and wear for the following reasons: First, Y-containing Fe-Cr-Al alloys showed good oxide adherence on the arc-sprayed coatings during operations undertaken at high temperatures under thermal cycling [
24,
25]. Second, during the AS process, alloying element burnout can take place. Yttrium’s affinity to oxygen is higher than that of other alloying elements [
26]. Therefore, yttrium protects them from oxidation during the transition from the feedstock to the coating and preserves the ability of the alloying elements to resist against gas corrosion and wear.
A number of Fe-based cored wires for wear- and heat-resistant applications were developed [
20,
27,
28] and patented with the participation of authors. The aim of the study was an analysis of wear- and heat-resistant properties of the coatings from the developed Fe-based cored wires.
2. Experimental Details
ASME 1020 steel were used as a substrate. An ADM-10 (UWI, Ekaterinburg, Russia) AAS gun was used for spraying the tested coatings. Current and voltage differed according to the type of test (see
Table 2). The other employed parameters were kept constant: spraying distance 100 mm, input pressure of gases: air—0.34 MPa, propane—0.32 MPa, traverse velocity 200 mm/s.
A 1.5Cr8Ti2Al cored wire 1.6 mm in diameter was used as a feedstock in tests up to 200 °C. This alloy belongs to the metastable austenite type of alloy, which is used for increasing wear resistance via arc spraying [
27]. The coatings were examined in an as-sprayed state and after additional rolling down of the surface, which was carried out via a “ball-on-plate” reciprocating technique. The test parameters were as follows: ball diameter: 10 mm; ball steel: E52100 ASTM (HRC 65); traverse speed: 0.16 m/s; stroke: 125 mm; friction path: 5 m; and axial load: 100 N, which corresponds to pressure of 2000 MPa [
29]. This allows to reproduce loads sufficient for martensitic transformation in steels with metastable austenite [
17].
Two Cr13Al5B5 cored wires 2.0 mm in diameter were used for high temperature tests. The composition of the second wire differs because of the alloying of yttrium. The Y content in the wire was designated according to the neural network model, which has been developed with respect to arc spraying [
30]. The chemical composition of the cored wires is given in
Table 3.
All coatings were subjected to a two-body abrasive test via the pin-on-plate reciprocating technique. Corundum abrasive paper with a grain size up to 150 μm, was fixed on the plate and the as-sprayed sample, 50 mm × 10 mm × 10 mm, was fixed in a holder. The test conditions were as follows: sliding distance: 60 m; pin velocity: 0.16 m/s; and specific load: 1 MPa. The pin is made of 1020 steel. To reduce the lapping period during the wear tests, the sprayed work surface area, 10 mm × 10 mm, on the pin was polished to
Ra 0.8. A combination of corundum microhardness 22.900 MPa [
31] and a specific load of 1 MPa provide the scratching abrasive wear. This type of test was chosen for the following reasons:
For wear-resistant coatings, the following studies were performed. Microhardness was measured with a PMT-3 device (LOMO, St. Petersburg, Russia) under a 100 g load. The load was assigned ensuring the ratio (indentation depth)/(coating thickness) < 0.1 [
34]. To verify, microhardness measurements were made with a successive decrease in the load, 0.981, 0.49, and 0.196 N. The deviations of the average values of the corresponding series from five measurements of each did not exceed 4%. The coating structure was studied with a MET 2 microscope (Altami, St. Petersburg, Russia), a DRON-3.0 X-ray diffractometer (Bourevestnik Inc., St. Petersburg, Russia) in Fe- and Co- Kα radiation, and a Philips SEM 525 scanning electron microscope (FEI, Hillsboro, OR, USA).
The following studies were performed to evaluate the heat-resistant coatings. The amount of oxygen in the coating was measured by melting the coating in an inert gas flow with an ON-900 analyzer (Eltra, Haan, Germany). The porosity of the coatings was determined via the metallographic method on the transverse sections using commercial SIAMS 700 hardware and software (SIAMS, Ekaterinburg, Russia) to estimate the distribution of pores by size, area, and volume. The results of the porosity determination were averaged for the five fields of view in accordance with ASTM E2109-01(14).
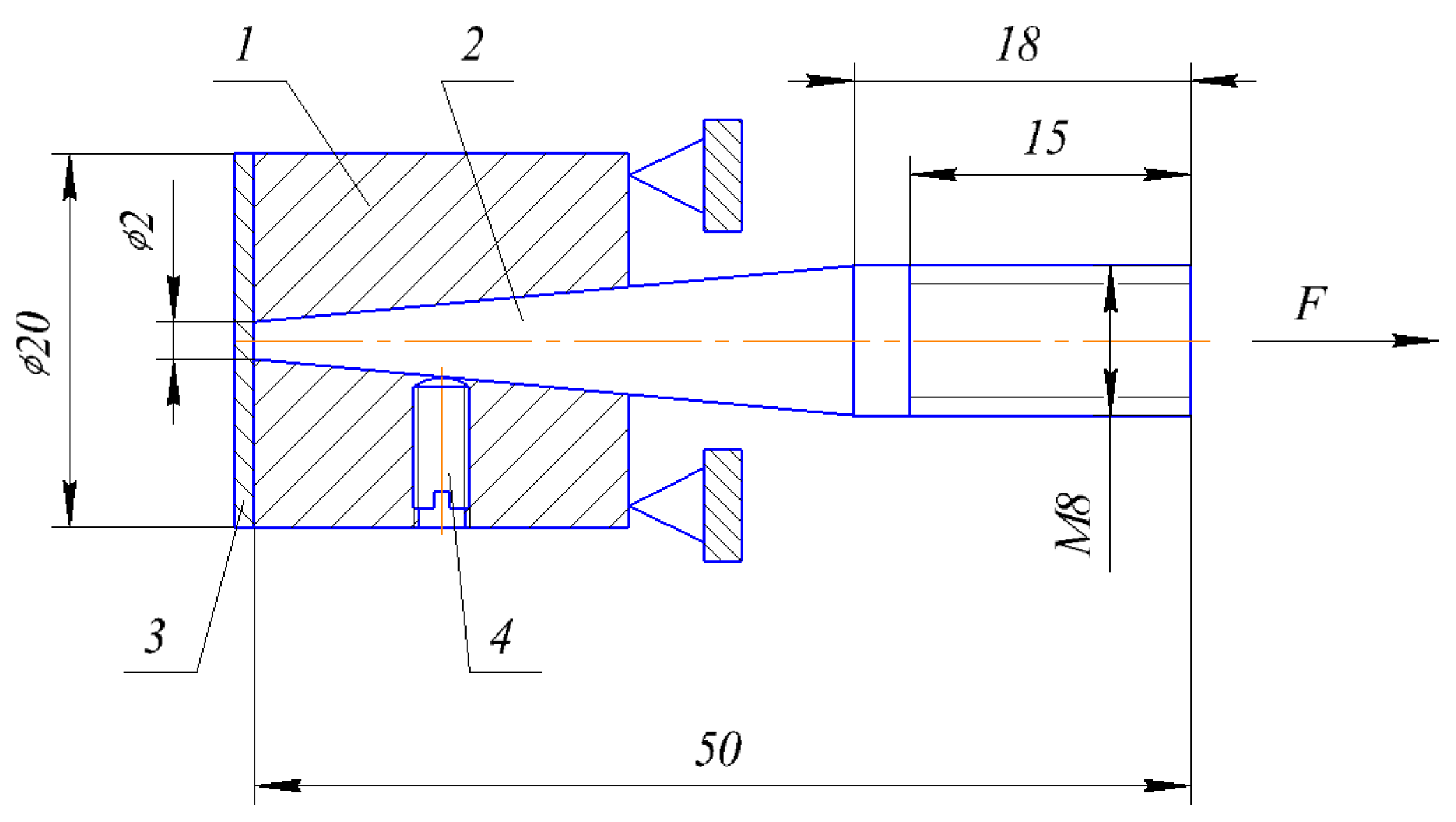
The adhesion strength of the coatings was determined by the separation of a conical pin. A schematic view of the test is presented in
Figure 2. Here, washer 1 serves as the base; pin 2 is inserted into the hole so that its end face is flush with the external plane of the washer. The pin is fixed by screw 4. The total surface of the pin and the washer after preparation is coated with coating 3. The test consists of pulling the pin by applying force (
F). The quantitative characteristic of the adhesive strength is the ratio of the maximum applying force to the pin face area. The results were averaged from three samples. The method is characterized by the versatility and high-speed performance. In case of a pin end diameter of 2 mm and a coating thickness of more than 300 μm, reliable results are possible [
34].
The heat resistance of the coatings from these wires was evaluated in accordance with GOST 9.312 [
35]. The gravimetric method was used according to the weight-loss measurements of samples exposed for 100 h in air at 700 °C. To decrease measuring errors, the 30 mm × 20 mm × 3 mm samples were deposited with a 10 μm galvanic nickel layer in order to protect the sample from non-controlled oxidation at elevated temperatures. A special evaluation of the heat resistance of the galvanized Ni were not carry out. However, oxidation of Ni and Fe differ as follows: A passivizing oxide film is formed on a Ni surface at 635 °C, and it is preserved up to 1200 °C [
36]. On the Fe surface, at a temperature above 572 °C, the film is mainly composed of wuestite, which has weak protective properties due to the predominant diffusion of Fe [
37]. After the exposition there were no signs of disturbance of the Ni oxide film. Before spraying, the layer from one side of the specimen surface (30 mm × 20 mm) was removed by grinding. This side was subjected to grit blasting with corundum sand; after that, the arc-spray coatings with a thickness of 700 μm were deposited. The galvanized surfaces were not specially prepared, so the coating that formed on them was easily removed. The sprayed surface was ground to a thickness of 400 μm.
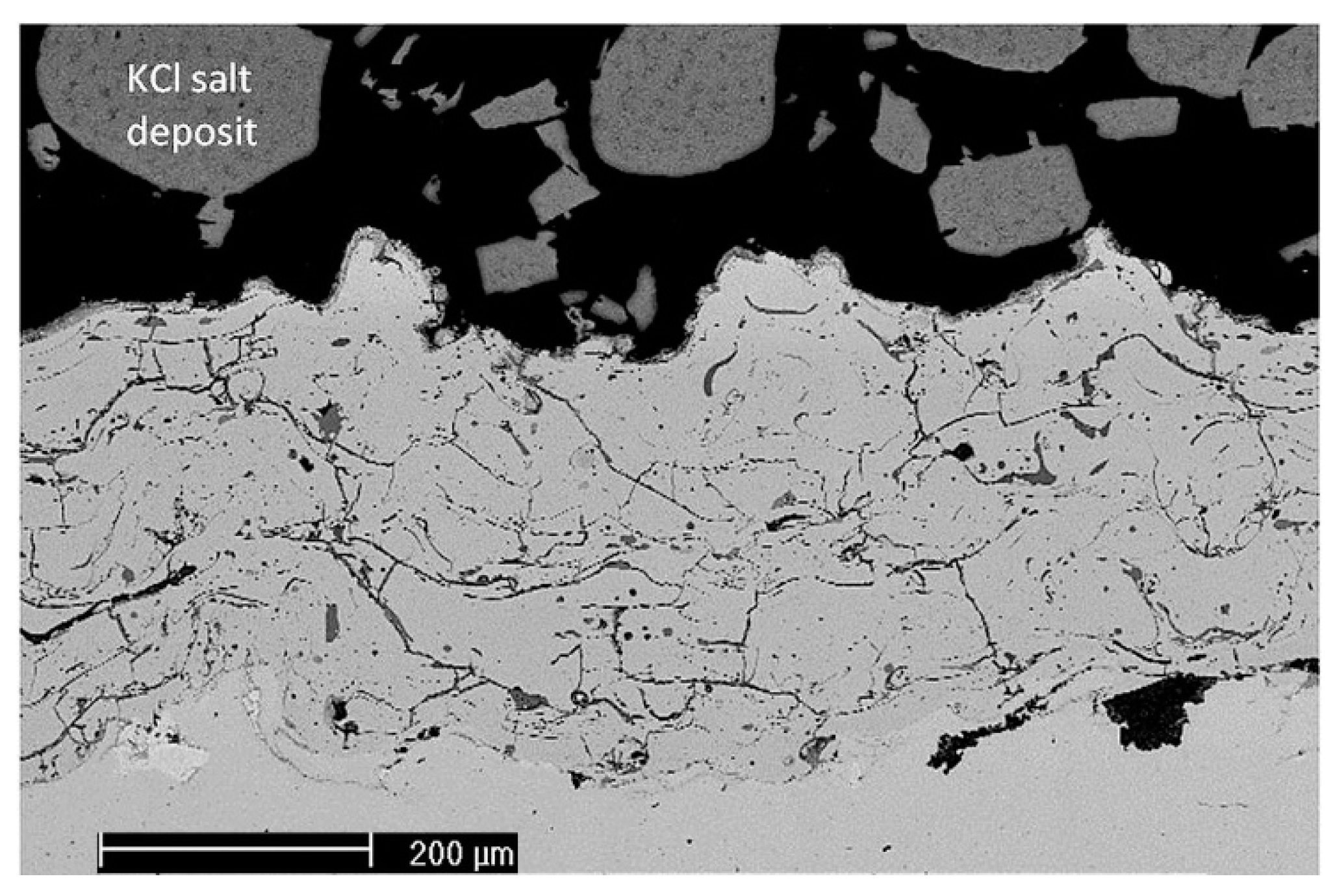
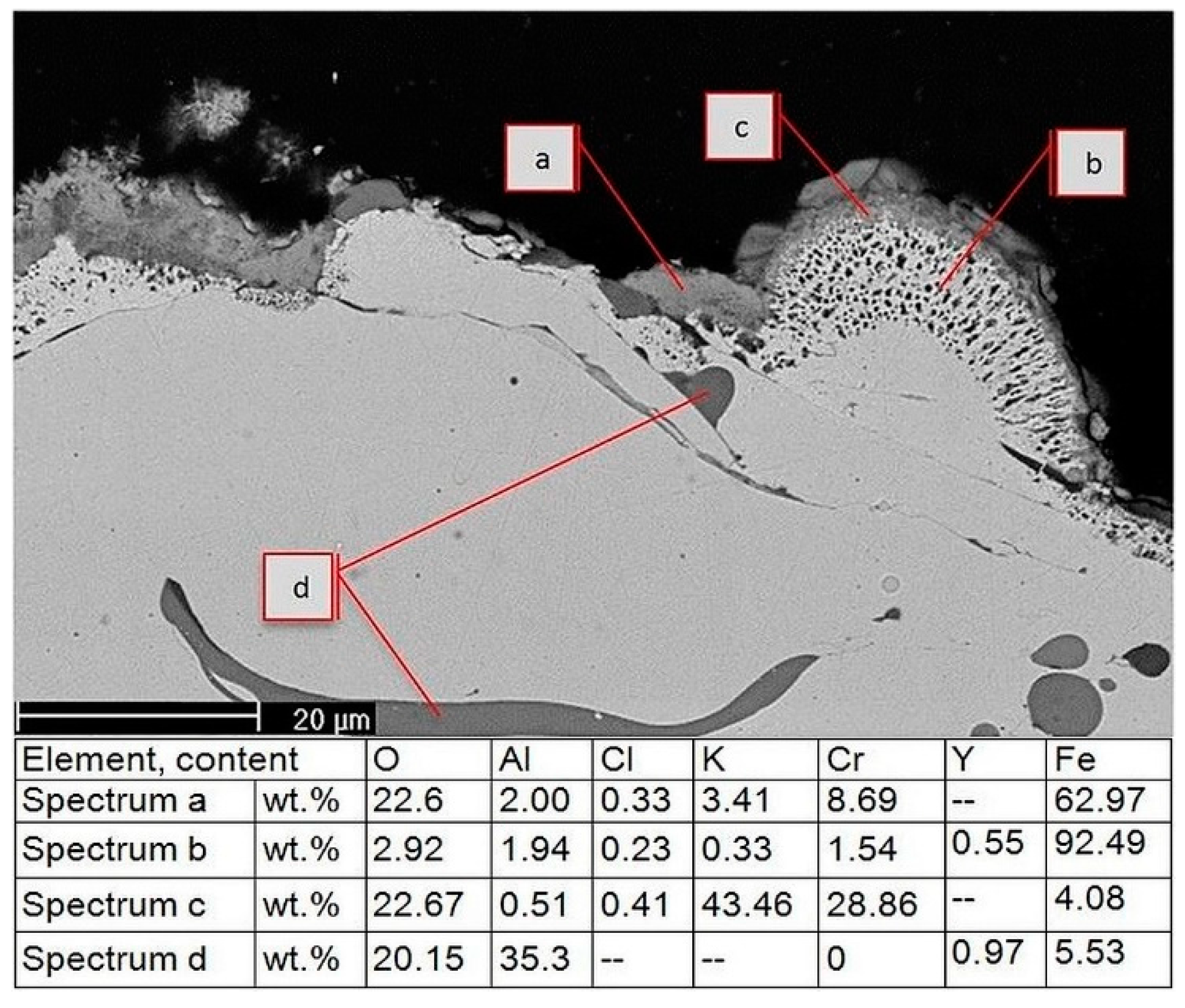
The high-temperature corrosion test was performed in an alumina tube furnace at 550 °C for a duration of 168 h. The coating samples on ASME 1020 steel were cut to a size of 20 mm × 20 mm, while an area of about 255 mm2 (an 18 mm-diameter circle) was covered with approximately 1 g of KCl salt. The salt was finely ground in a ceramic mortar and mixed with ethanol to create a paste and facilitate the deposition. The furnace environment was purged with 1.5 L/min of air with the addition of water vapor to reach 12% of the specific humidity.
Microhardness measurements were performed with a Leica VMHT device (Walter Uhl, Aßlar, Germany) under 50 and 300 g loads. When measuring the average hardness value before wear testing, a load of 300 g is taken. After the wear tests, it is important to evaluate the microhardness of the thinner surface layer subjected to friction hardening. This requires a smaller load on the indenter [
34] and a procedure of verifying the test results was carried similar to wear-resistant coatings.
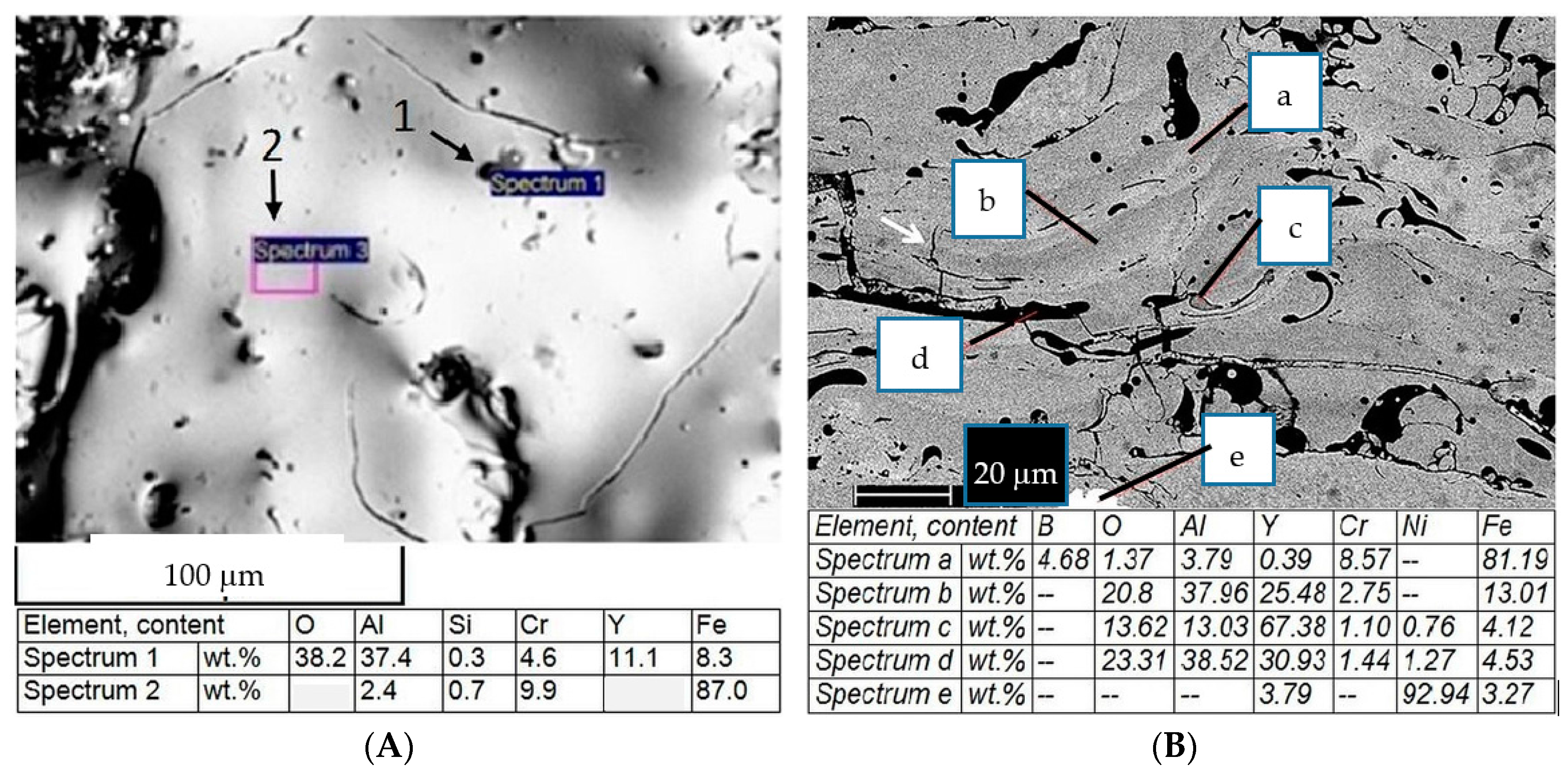
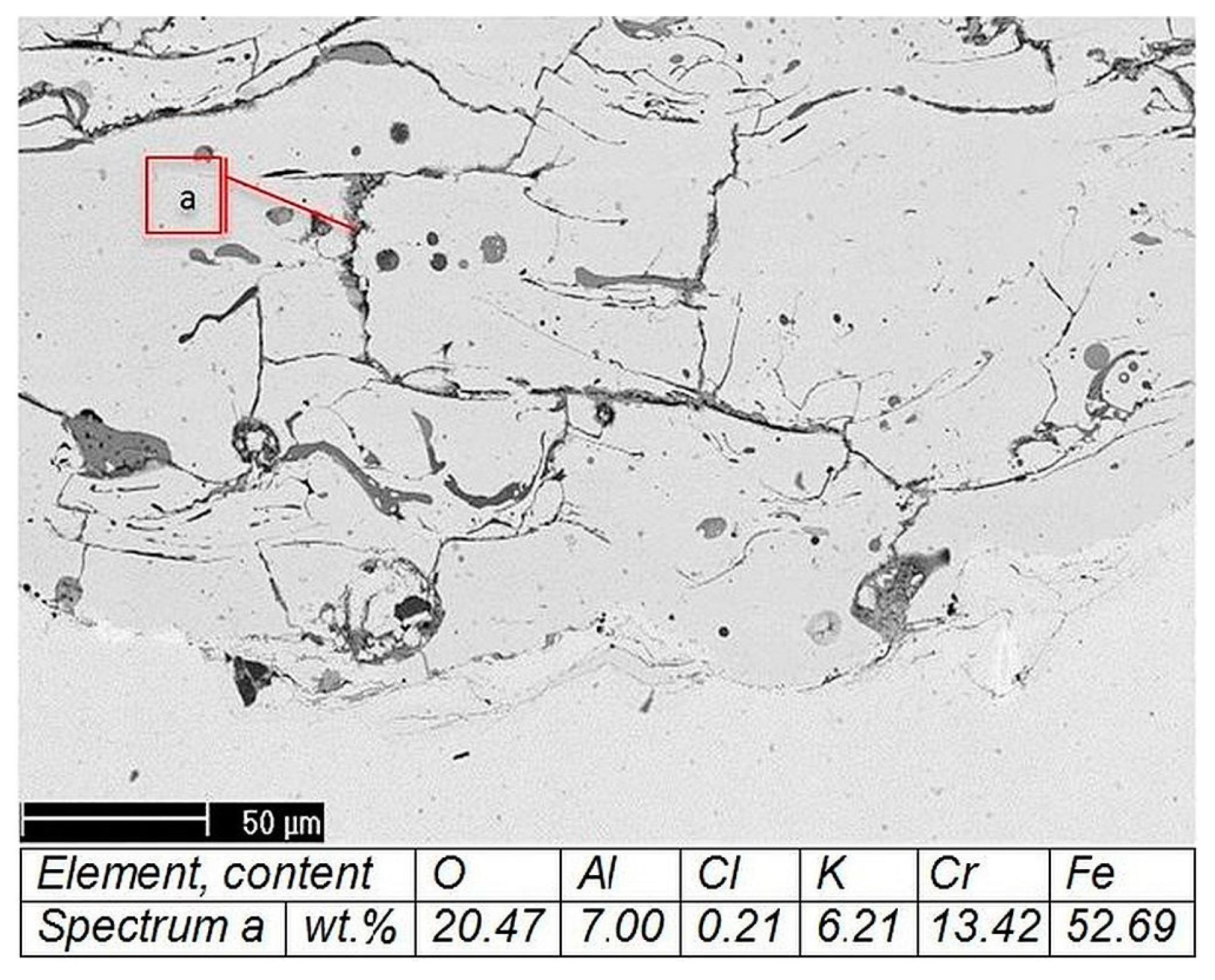
A microstructural study of the as-sprayed coating from a Y-containing wire was performed as follows: Phase composition on the coating surface was determined by a XRD-7000 X-ray diffractometer (SHIMADZU, Kyoto, Japan, Cr-Kα radiation). The microchemical structure and composition of the coatings was studied with a VEGA II XMU scanning electron microscope (Tescan, Brno, Czech Republic). It was equipped with an INCA Wave 700 wavelength dispersive X-ray spectrometer and INCA Energy 450XT microanalyzers (Centre “Plastometria”, the Institute of Engineering Science, Ural Branch of RAS, Ekaterinburg, Russia).
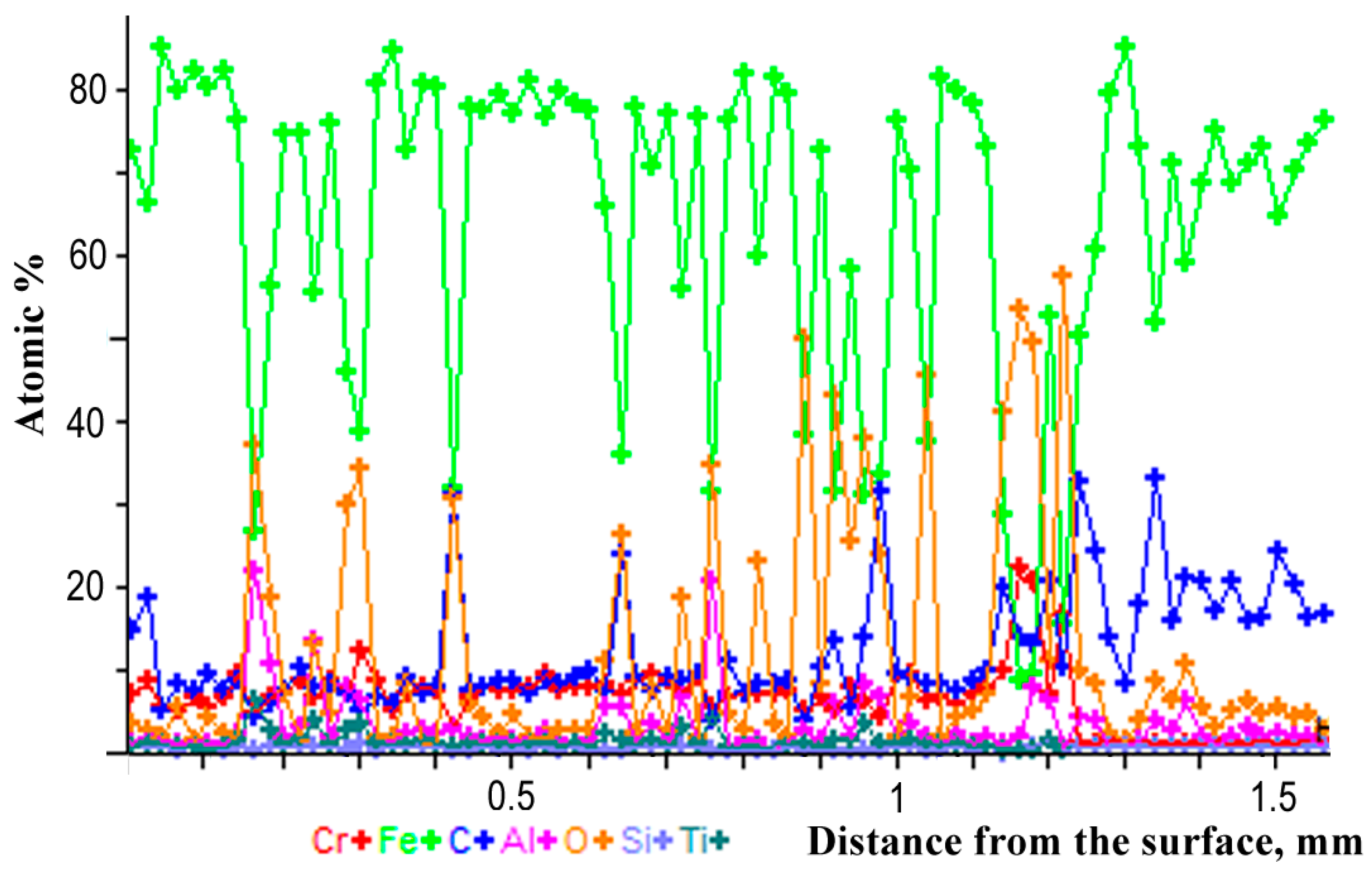
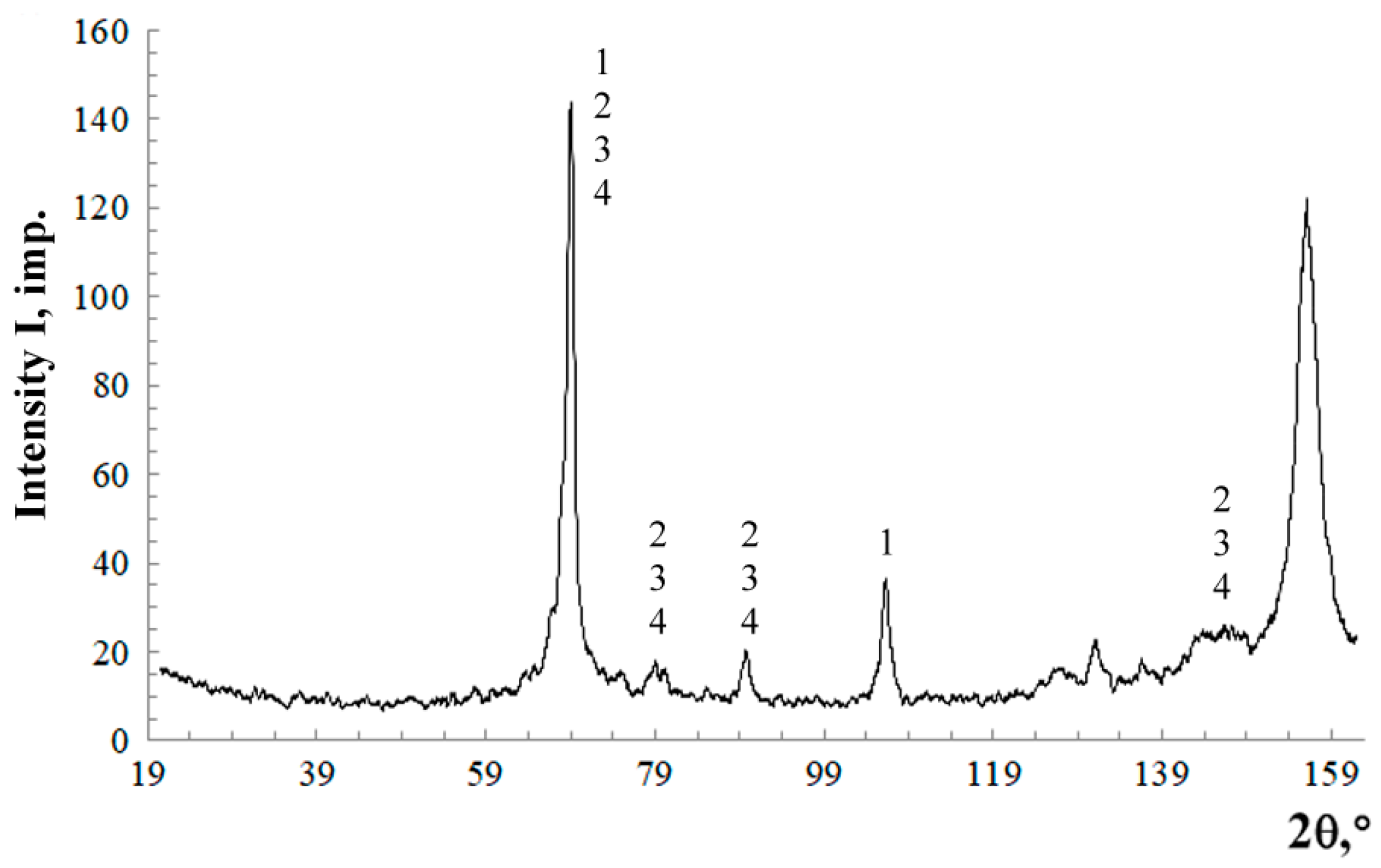
The microstructure of the coating after a corrosion test from an AAS-Y wire was investigated via a scanning electron microscope (SEM) and a microanalysis of the cross-section was studied with a Philips XL30 equipped with an energy dispersive X-ray (EDX). Phase composition was assessed by X-ray diffractometry (XRD, Empyrean, PANAnalytical, Co-Kα radiation, the wavelength is 1.79021 Å) of the surface of the coating. The experimental conditions include the scanning range from 20° to 120° in 2θ, at a scanning step rate of 0.02°, a beam mask of 20 mm, a programmable divergent slit fixed at ½ degree, a Fe-filter, and a PANAnalytical PIXcel 3D detector (Malvern Panalytical B.V., Almelo, The Netherlands). Phase identification was performed using the PANAlytical X’Pert High Score Plus software (version 4.0) using the ICDD JCPDF-2 database (International Centre of Diffraction Data, Newtown Square, PA, USA).
5. Conclusions
Cored wires of the base alloying system Fe-Cr-C were used as a feedstock for arc spraying. They included 1.5Cr8Ti2Al cored wire and two Cr13Al5B5 cored wires. One of the last was additionally alloyed by yttrium. The coatings were investigated in the range of 20–700 °C. The studies comprise the following: wear and heat resistance tests, microhardness measurements, high-temperature corrosion testing, porosity and adhesion strength evaluation, and SEM and XRD analyzes.
A metastable austenite structure forms in the coating from 1.5Cr8Ti2Al cored wire. As a result, after loading at room temperature, a share of the martensitic phase rises. The strain-induced martensite nucleation on each surface layer is facilitated by the impact of droplets during the formation of the coating.
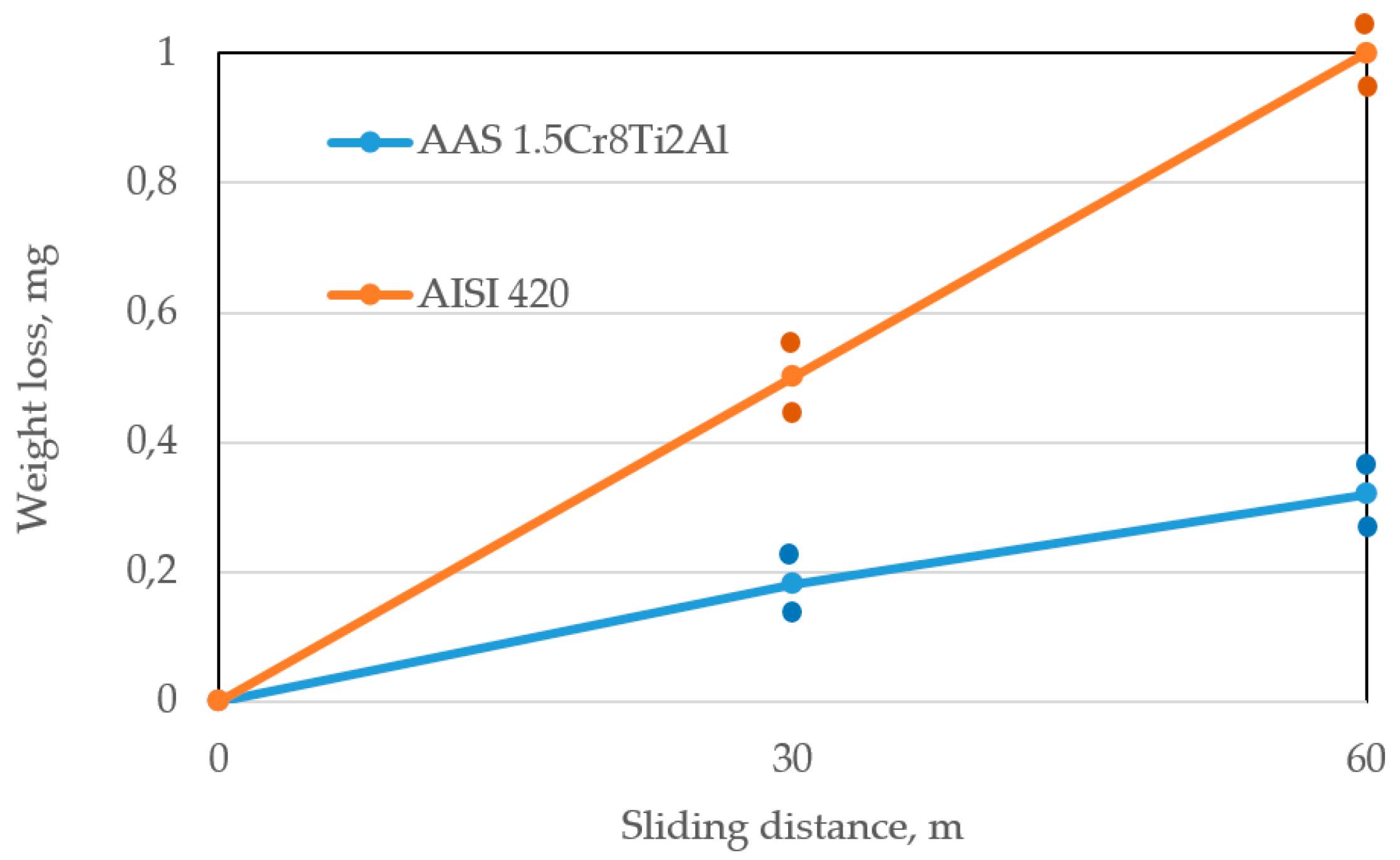
This leads to an increase of abrasive wear resistance, which is three times larger compared to the arc-sprayed coating from the AISI 420 (X20Cr13) solid wire. Thus, the wear resistance of the coating improves via the following synergetic effects:
Internal stresses in the coating drop due to the change in the crystal lattice volume;
The energy of the contact load is dissipated by the strain-induced nucleation of martensite;
Surface hardening takes place.
Addition of yttrium to the Cr13Al5B5 coating results in the following, as compared to the Y-free coating:
Oxygen content is 1.8 times less, which indicates a lowering of the burnout of other alloying elements during spraying, Thus their proper content is saved for further operations;
The formation of complex oxides like (Fe, Al, Y)2O3 leads to an increase in the coating’s oxidation resistance by 20%;
Adhesion strength is 1.2 times higher and the porosity drops by 25% due to less oxidation of sprayed particles in flight; and
Wear resistance increases by 20% due to an influence of amplified aluminothermic reactions on the uniformity of boron-containing components melting and the coating density.
Borides of the (Fe, Cr)2(B, C) type, which form in the case of the Cr13Al5B5 coating, are more effective as strengthening phases at abrasive wear than martensite and chromium carbides in the case of the 1.5Cr8Ti2Al coating. Consequently, the wear resistance in the former case is twice as high as in the latter one.
Chlorine-induced corrosion takes place mainly on the surface of the coating from the yttrium-added Cr13Al5B5 wire. Corrosion product thickness on the surface of the coating is quite low and of the same magnitude as the comparable Ni-based arc-sprayed coatings tested under similar conditions.
Values of wear- and heat resistance of the coating from the yttrium-added Cr13Al5B5 cored wire are an order of magnitude higher than those of pearlite and martensite-ferritic steels that are typical for boiler applications.
Economically alloyed Fe-Cr-C-based arc sprayed coatings can be applied in wear- and heat-resistant conditions in the range of 20–700 °C. Possible applications in power engineering include protection of the parts of fossil energy systems and waste incinerators.