The Synthesis and Morphology of a Perfluoroalkyl Oligosiloxane@SiO2 Resin and Its Performance in Anti-Fingerprint Coating
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Reagents
2.2. Synthesis of Hydro- and Oleo-Phobic Intermediate
2.3. Synthesis of Nano SiO2 Hybrid Fluorinated Alkyl Oligosiloxane Resin
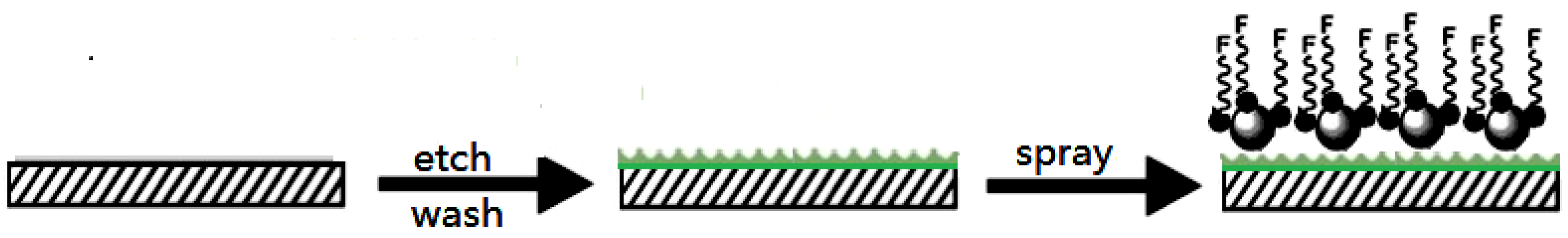
2.4. Preparation of the FSi@SiO2 Anti-Fingerprint Coating
2.5. Characterization
3. Results and Discussion
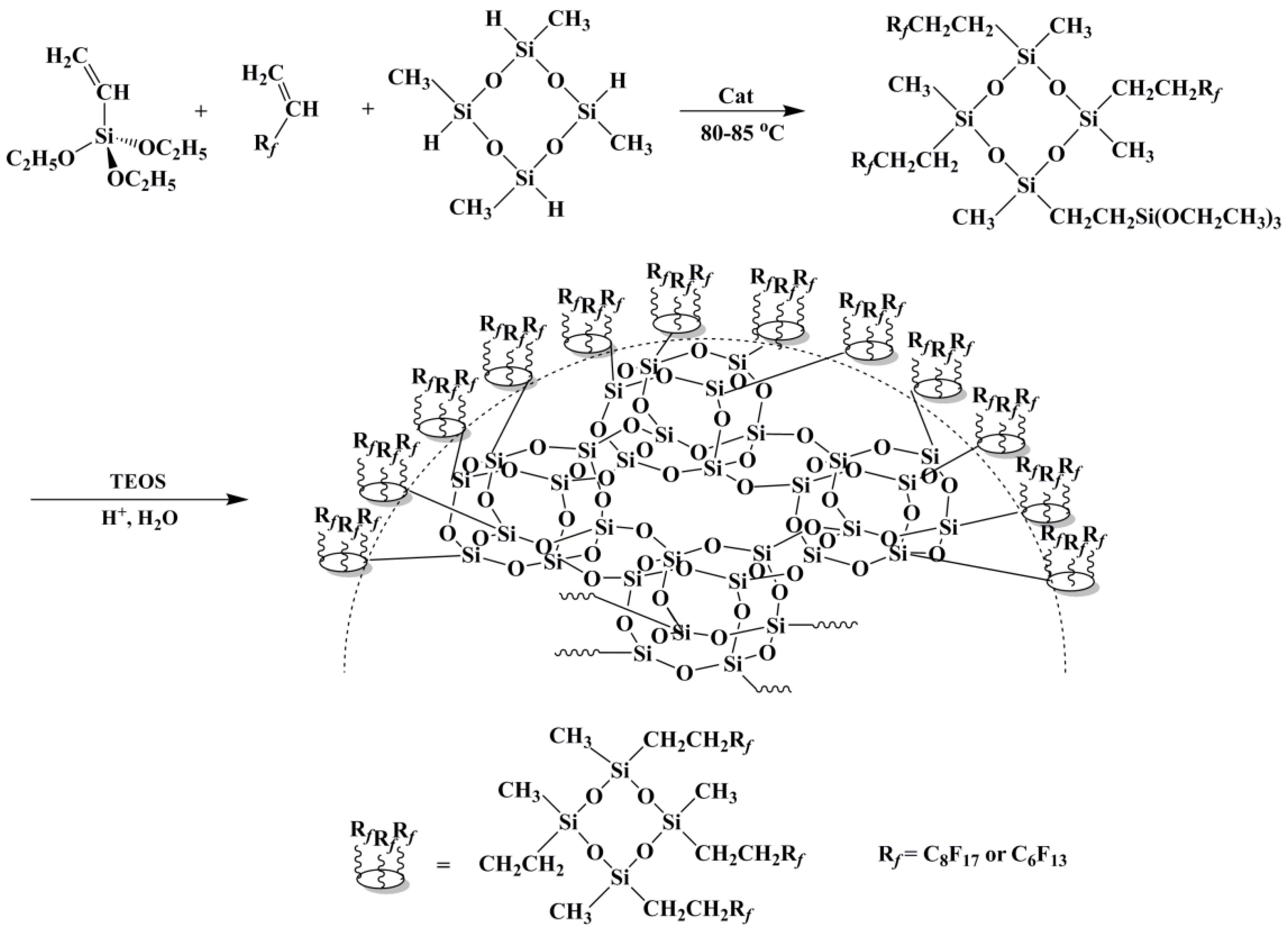
3.1. About Design and Synthesis of FSi@SiO2
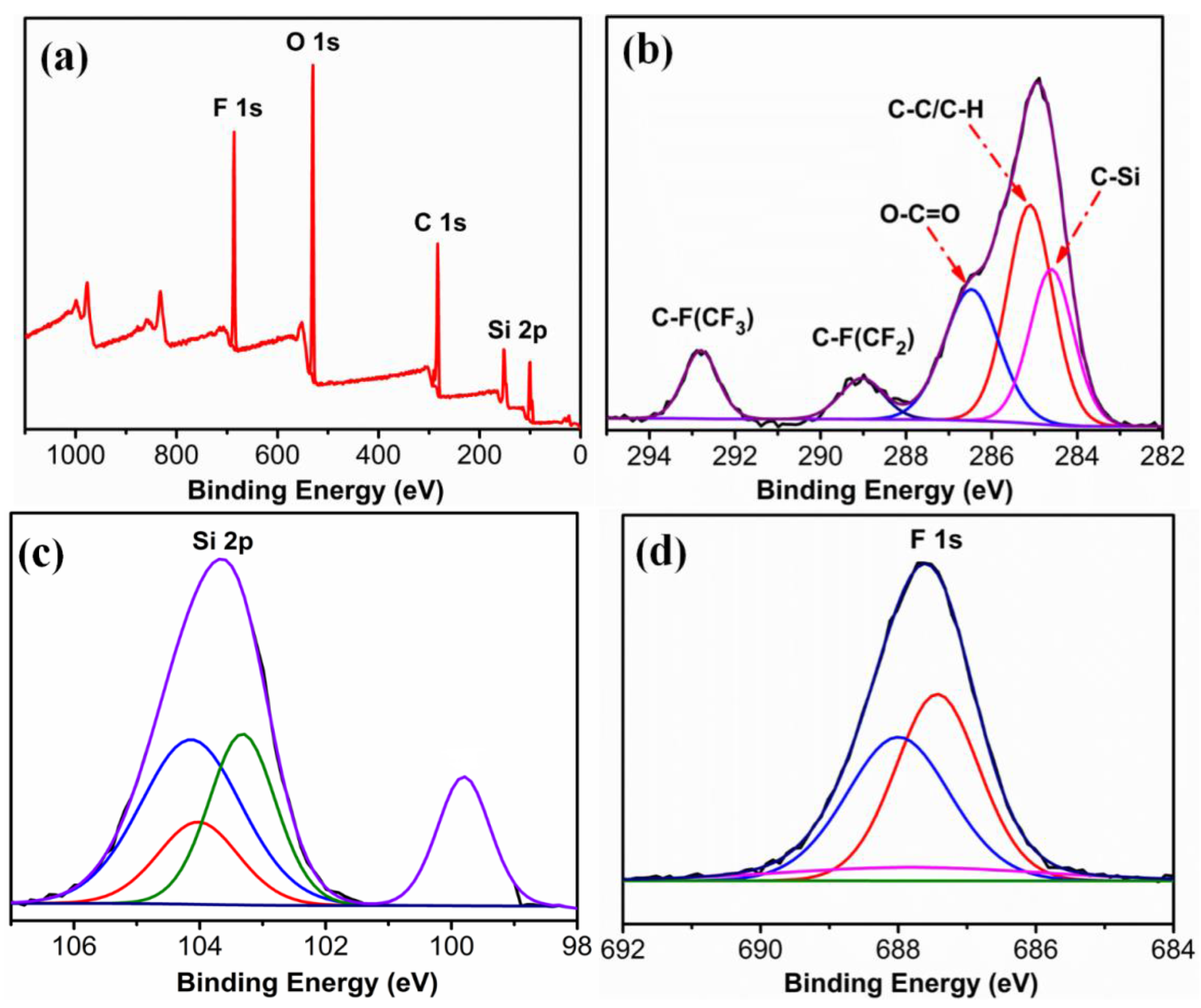
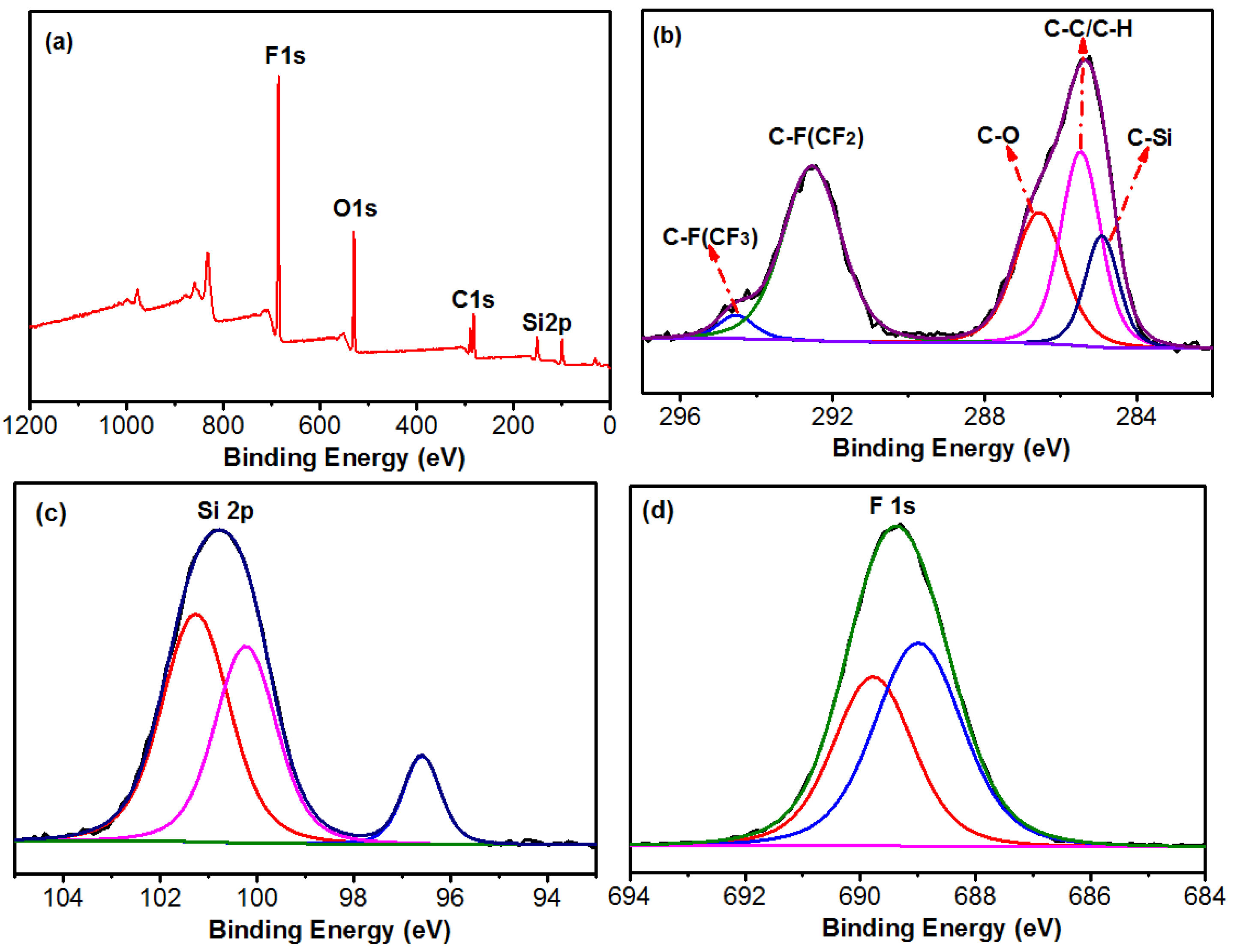
3.2. Characterization of FVPS and FSi@SiO2
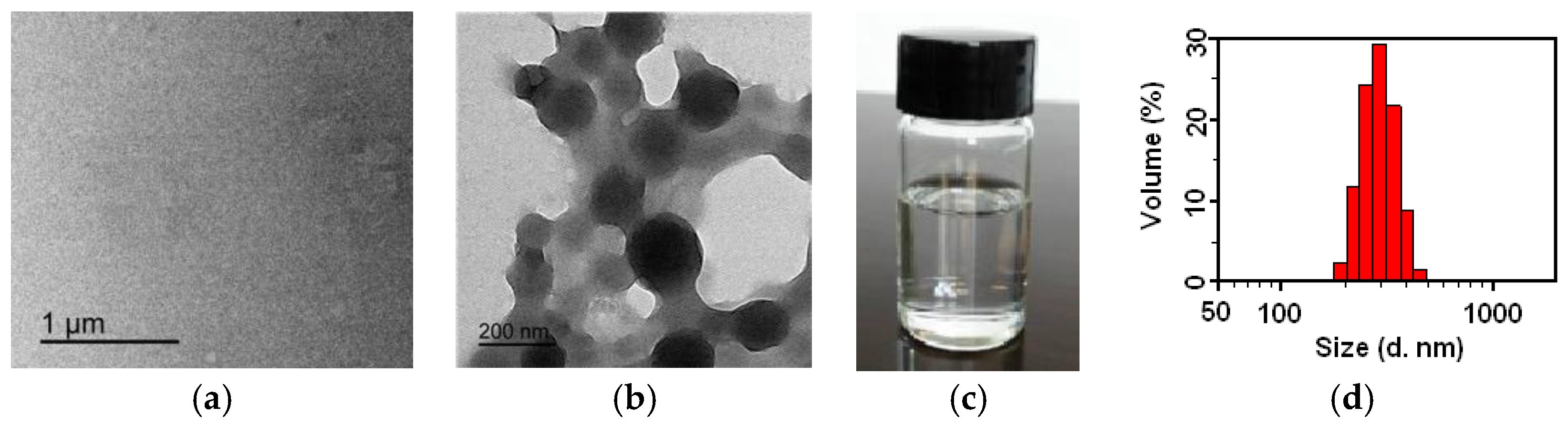
3.3. State and Particle Size Distribution of the FSi@SiO2 Resin Solution
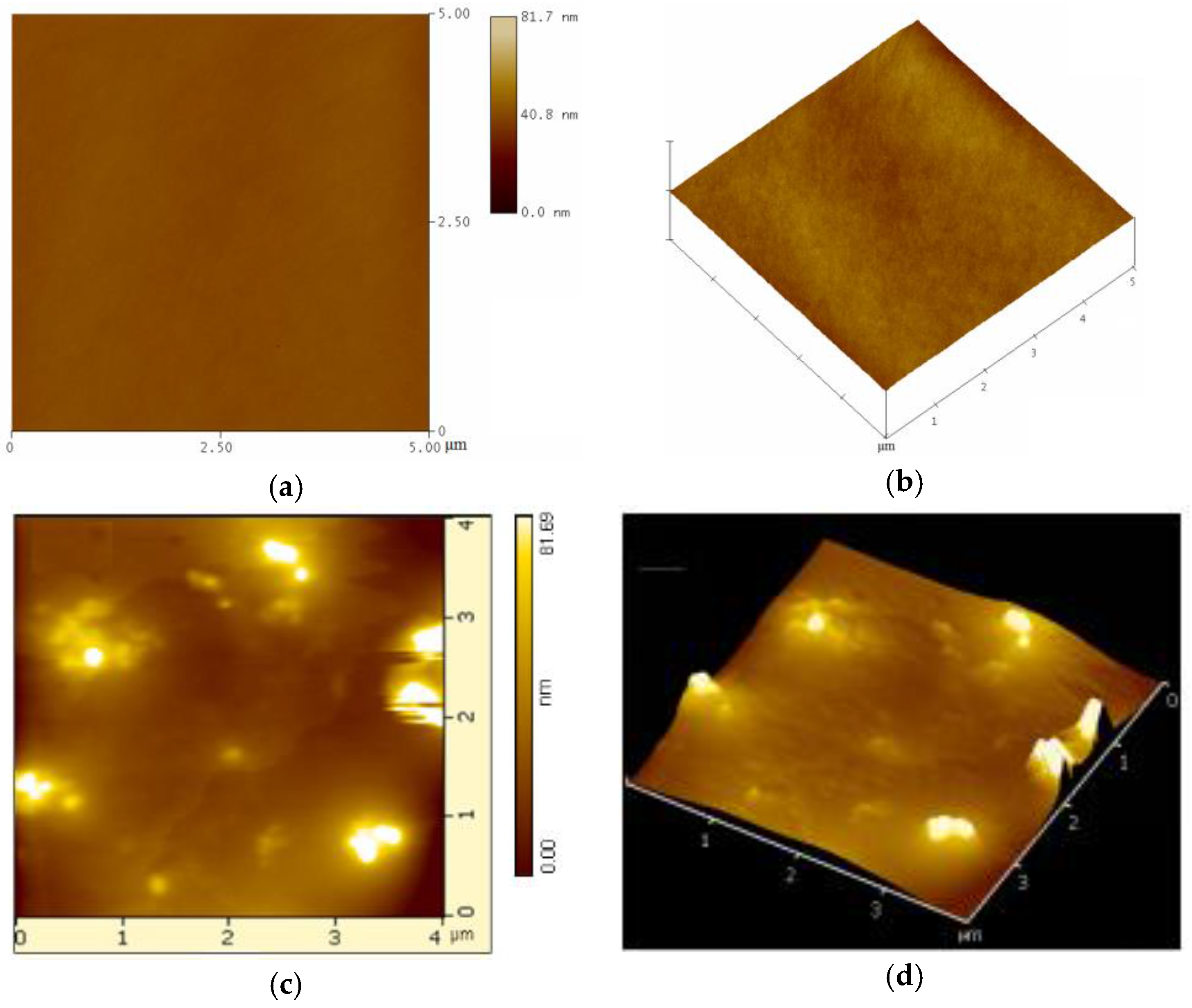
3.4. Morphology and Hydrophobic Properties of the FSi@SiO2 Anti-Fingerprint Coating on Substrates
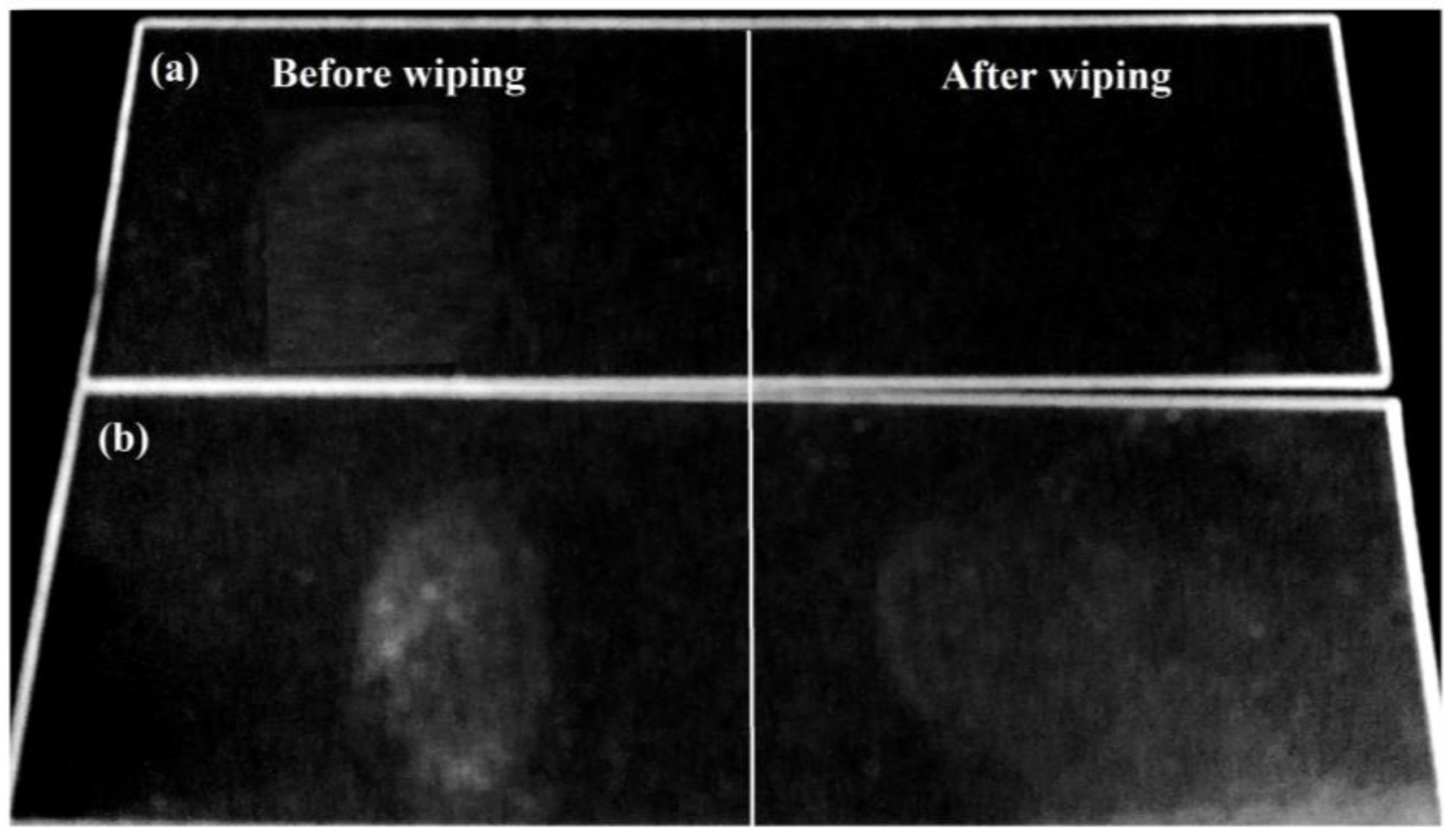
3.5. Performance of the FVPS and FSi@SiO2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, G.L.; Reynoldson, T.E. Some observation on fingerprint deposits. J. Phys D Appl. Phys. 1975, 8, 724–729. [Google Scholar] [CrossRef]
- Uhlmann, P.; Frenzel, R.; Voit, B.; Mock, U.; Szyszka, B.; Schmidt, B.; Ondratschek, D.; Gochermann, J.; Roths, K. Research agenda surface technology: Future demands for research in the field of coatings materials. Process Org. Coat. 2007, 58, 122–126. [Google Scholar] [CrossRef]
- Colaiacovo, F.; Tortora, S.; Totoreto, L.; Mauro, S.; Salamone, G. Method for Restoring or Executing an Anti Fingerprint Coating onto Sheets of Stainless Steel. U.S. Patent No. 20,090,087,649, 2 April 2009. [Google Scholar]
- Siriviryanun, A.; Imae, T. Anti-fingerprint properties of non-fluorinated organosiloxane self-assembled monolayer-coated glass surfaces. Chem. Eng. J. 2014, 246, 254–259. [Google Scholar] [CrossRef]
- Scruton, B.; Robins, B.W.; Blott, B.H. The deposition of fingerprint films. Appl. Phys. 1975, 8, 714–723. [Google Scholar] [CrossRef]
- Choi, H.; Park, K.C.; Lee, H.; Crouzier, T.; Rubner, M.F.; Cohen, R.E.; Barbastathis, G.; Mckinley, G.H. Superoleophilic titania nanoparticle coatings with fast fingerprint decomposition and high transparency. ACS Appl. Mater. Interfaces 2017, 9, 8354–8360. [Google Scholar] [CrossRef] [PubMed]
- Parale, V.G.; Mahadik, D.B.; Mahadik, S.A. OTES modified transparent dip coated silica coatings. Ceram. Int. 2013, 39, 835–840. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, J.; Sanderson, S. Superhydrophobic and transparent coatings based on removable polymeric spheres. J. Mater. Chem. 2009, 19, 655–660. [Google Scholar] [CrossRef]
- Bae, G.Y.; Min, B.G.; Jeong, Y.G.; Lee, S.C.; Jang, J.H.; Koo, G.H. Superhydrophobicity of cotton fabrics treated with silica nanoparticles and water-repellent agent. J. Colloid Interface Sci. 2009, 337, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuning the wetting properties of siloxane-nanoparticle coatings to induce superhydrophobicity and superoleophobicity for stone protection. Mater. Des. 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Li, J.; Weng, R.; Di, X.; Yao, Z. Gradient and weather resistant hybrid super-hydrophobic coating based on fluorinated epoxy resin. Appl. Polym. Sci. 2014, 10, 40955. [Google Scholar] [CrossRef]
- Basu, B.J.; Dinesh, V.K.; Anandan, C. Surface studies on superhydrophobic and oleophobic polydimethylsiloxane–silica nanocomposite coating system. Appl. Surf. Sci. 2012, 261, 807–814. [Google Scholar] [CrossRef]
- Navarrini, W.; Tortelli, V.; Colaianna, P.; Abusleme, J.A. Perfluorodioxoles, the Preparation Process Thereof, and Homopolymers and Copolymers Therefrom. U.S. Patent No. 5,646,223, 8 July 1997. [Google Scholar]
- Yang, H.; Cheng, Y.; Xiao, F. Thermal stable superhydrophobic polyphenylsilsesquioxane/nanosilica composite coatings. Appl. Surf. Sci. 2011, 258, 1572–1580. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, B.; Men, X.; Li, Y. Mechanically durable, superhydrophobic coatings prepared by dual-layer method for anti-corrosion and self-cleaning. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 182–188. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Yang, M.; Peng, L.; Zong, S.; Yu, G. Multifunctional superhydrophobic surfaces templated from innately microstructured hydrogel matrix. Nano Lett. 2014, 14, 4803–4809. [Google Scholar] [CrossRef] [PubMed]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic coatings on cellulose-based materials: Fabrication, properties, and applications. Adv. Mater. Interfaces 2013, 1, 1300026. [Google Scholar] [CrossRef]
- Vecellio, M. Opportunities and developments in fluoropolymeric coatings. Prog. Org. Coat. 2000, 40, 225–242. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Cheng, T.; Ding, J.; Qu, L.; Dai, L.; Wang, X.; Lin, T. One-step coating of fluoro-containing silica nanoparticles for universal generation of surface superhydrophobicity. Chem. Commun. 2008, 7, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhai, J.; Wang, Y.; Jiang, L. Fabrication of superhydrophobic surfaces by self-assembly and their water-adhesion properties. Phys. Chem. 2005, 109, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, R.G.; Lu, C.; Zhang, Z.; Yang, S. Highly transparent superhydrophobic surfaces from the coassembly of nanoparticles (≤100 nm). Langmuir 2011, 27, 4594–4602. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Zhai, L.; Wu, Z.; Cohen, R.E.; Rubner, M.F. Transparent superhydrophobic films based on silica nanoparticles. Langmuir 2007, 23, 7293–7298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Xu, X. Facile fabrication of a superamphiphobic surface on the copper substrate. J. Colloid Interface Sci. 2012, 367, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Park, I.J.; Lee, S.B. Hydrophobicity and sliding behavior of liquid droplets on the fluorinated latex films. Macromolecules 2005, 38, 736–744. [Google Scholar] [CrossRef]
- Xiong, S.; Guo, X.; Li, L.; Wu, S.; Chu, P.; Xu, Z. Preparation and characterization of fluorinated acrylate copolymer latexes by miniemulsion polymerization under microwave irradiation. J. Fluor. Chem. 2010, 131, 417–425. [Google Scholar] [CrossRef]
- Yang, T.; Yao, L.; Peng, H.; Cheng, S.; Park, I. Characterization of a low-wettable surface based on perfluoroalkyl acrylate copolymers. J. Fluor. Chem. 2006, 127, 1105–1110. [Google Scholar] [CrossRef]
- Cui, X.; Zhong, S.; Yan, J.; Wang, C.; Zhang, H.; Wang, H. Synthesis and characterization of core–shell SiO2-fluorinated polyacrylate nanocomposite latex particles containing fluorine in the shell. Colloid Surf. A 2010, 360, 41–46. [Google Scholar] [CrossRef]
- Xue, C.; Guo, X.; Ma, J.; Jia, S. Fabrication of robust and antifouling superhydrophobic surfaces via surface-initiated atom transfer radical polymerization. ACS. Appl. Mater. Interface 2015, 7, 8251–8259. [Google Scholar] [CrossRef] [PubMed]
- Block, S.; Hupfield, P.; Itami, Y.; Kitaura, E.; Kleyer, D.; Masutani, T.; Nakai, Y. New Anti-Fingerprint Coatings. Paint Coat. Industry 2008, 24, 88–92. [Google Scholar]








| Treating Solutions | wt % | WCA (°) | OCA (°) | T % | Appearance | Abrasion Resistant/Cycles | AF property |
|---|---|---|---|---|---|---|---|
| FSi@SiO2 | 0.8 | 120.3 | 87.5 | 95 | transparent | 5000 | ◎ |
| FVPS | 0.8 | 110.2 | 65.8 | 92 | transparent | 5000 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Q.; Lyu, Z.; Shangguan, W.; Qiao, B.; Qin, P. The Synthesis and Morphology of a Perfluoroalkyl Oligosiloxane@SiO2 Resin and Its Performance in Anti-Fingerprint Coating. Coatings 2018, 8, 100. https://doi.org/10.3390/coatings8030100
An Q, Lyu Z, Shangguan W, Qiao B, Qin P. The Synthesis and Morphology of a Perfluoroalkyl Oligosiloxane@SiO2 Resin and Its Performance in Anti-Fingerprint Coating. Coatings. 2018; 8(3):100. https://doi.org/10.3390/coatings8030100
Chicago/Turabian StyleAn, Qiufeng, Zhujun Lyu, Wenchao Shangguan, Bianli Qiao, and Pengwei Qin. 2018. "The Synthesis and Morphology of a Perfluoroalkyl Oligosiloxane@SiO2 Resin and Its Performance in Anti-Fingerprint Coating" Coatings 8, no. 3: 100. https://doi.org/10.3390/coatings8030100




