Superhydrophobic, Superoleophobic and Antimicrobial Coatings for the Protection of Silk Textiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Surface Characterization of Coated Silk Samples
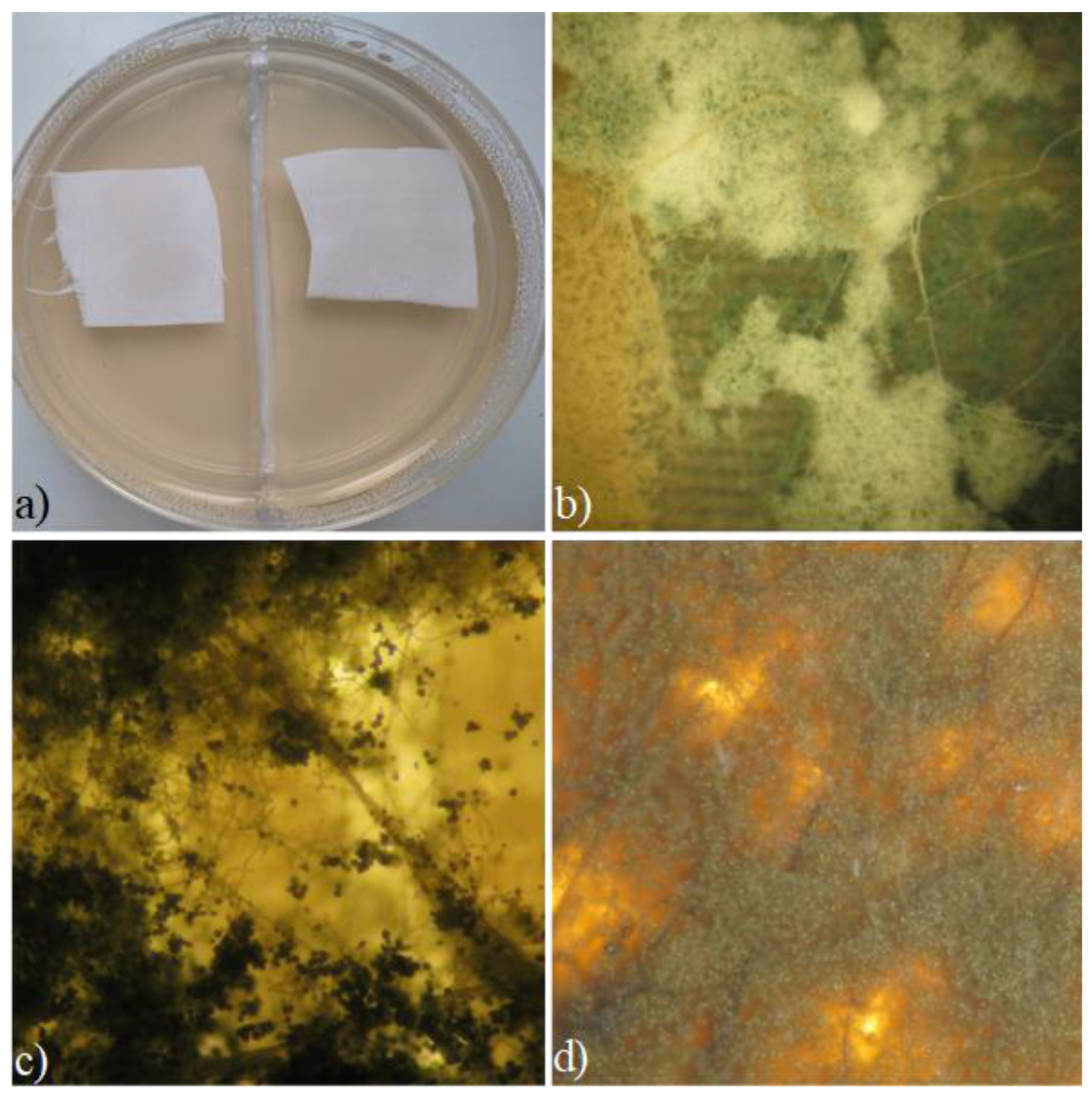
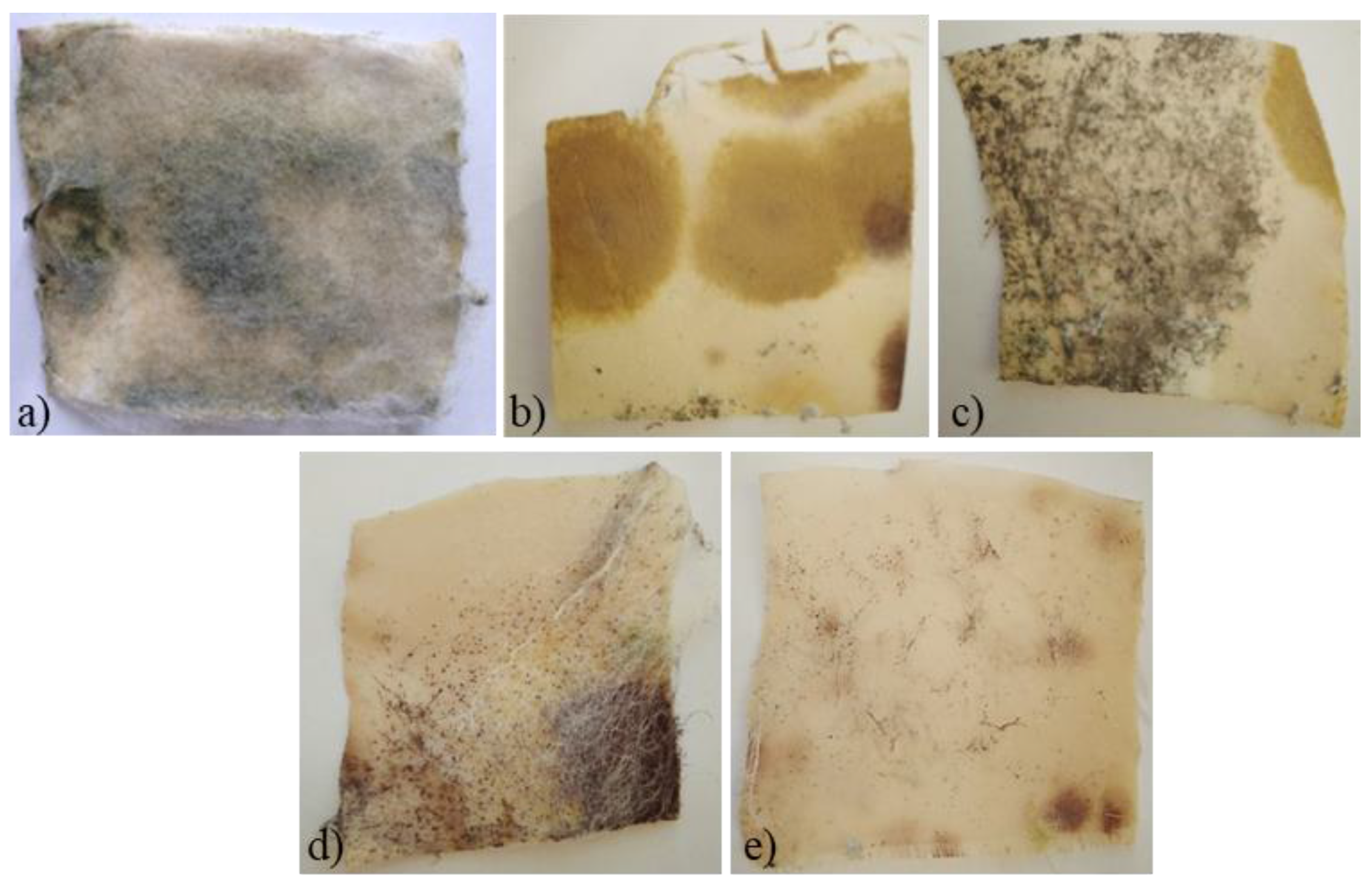
2.2. Evaluation of the Antimicrobial Properties of Treated Silk
2.3. Other Evaluation Tests
3. Results and Discussion
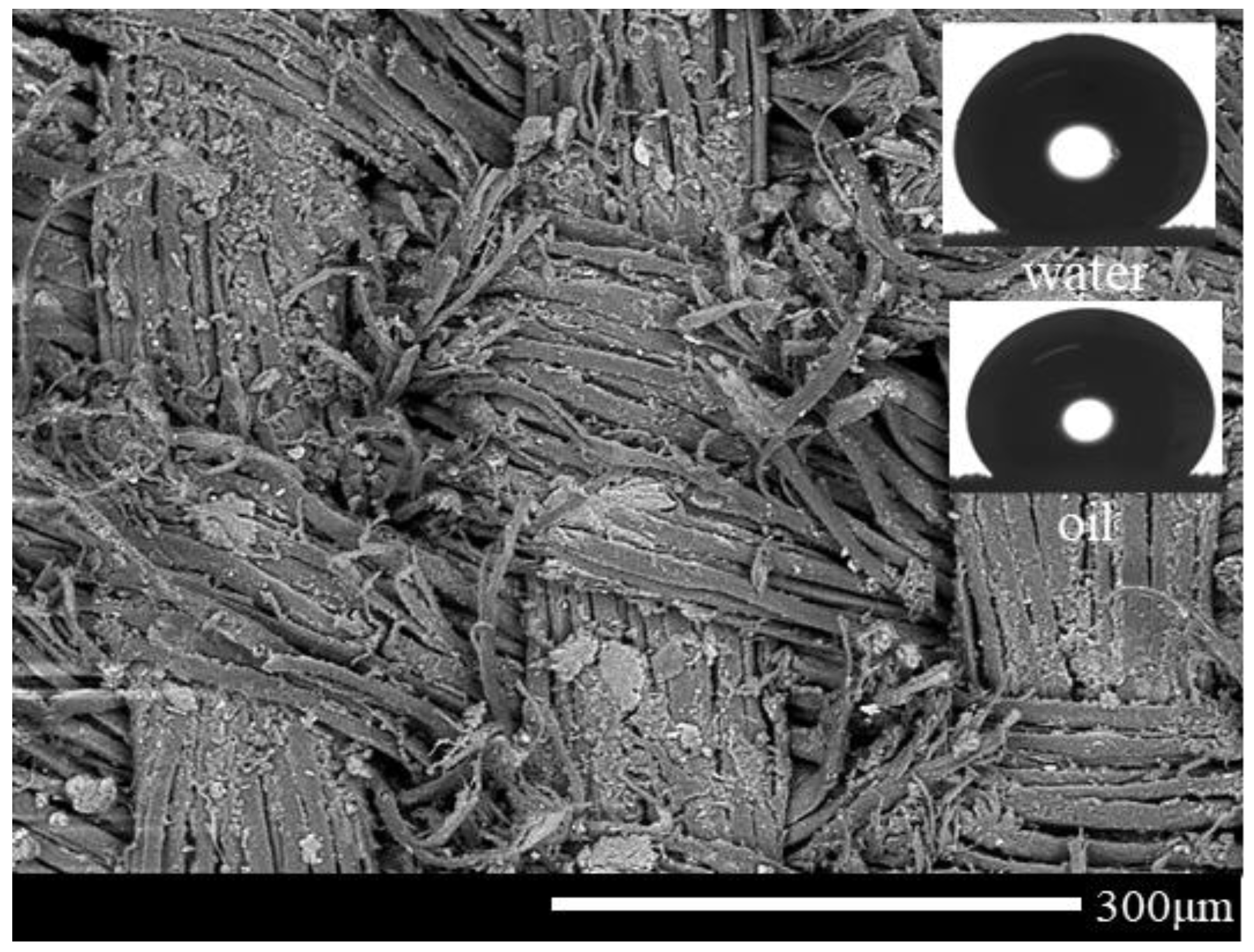
3.1. SEM-EDX Characterization
3.2. Wetting Properties and Surface Structures
3.3. Antimicrobial Activity
3.4. Other Evaluation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Rana, M.; Hao, B.; Mu, L.; Chen, L.; Ma, P.C. Development of multi-functional cotton fabrics with Ag/AgBr-TiO2. Compos. Sci. Technol. 2016, 122, 104–112. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, D.; Zhang, X.; Meng, Y.; Cheng, C.; Bao, C.; Ding, X.; Cao, H.; Tian, X. Construction of intumescent flame retardant and antimicrobial coating on cotton fabric via layer-by-layer assembly technology. Surf. Coat. Technol. 2015, 276, 726–734. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.K.; Chen, Z.; Lin, C.L. Recent Progress on the superhydrophobic surfaces with special adhesion: From natural to biomimetic to functional. J. Nanoeng. Nanomanuf. 2011, 1, 18–34. [Google Scholar] [CrossRef]
- Eadie, L.; Ghosh, T.K. Biomimicry in textiles: Past, present and potential. An overview. J. R. Soc. Interface 2011, 8, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Gurav, A.B.; Maruti, C.S.; Vhatkar, R.S. Recent progress in preparation of superhydrophobic surfaces: A review. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 76–94. [Google Scholar]
- Karapanagiotis, I.; Manoudis, P.N. Superhydrophobic surfaces. JMBM 2012, 21, 21–32. [Google Scholar]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Superhydrophobic surfaces: From the lotus leaf to the submarine. C. R. Mecanique 2012, 340, 18–34. [Google Scholar] [CrossRef]
- Zhang, P.; Lv, F.Y. A review of the recent advances in superhydrophobic surfaces and the emerging energy-related applications. Energy 2015, 82, 1068–1087. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, S.; Wang, L.; Zheng, Y. Lotus effect in wetting and self-cleaning. Biotribology 2016, 5, 31–43. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I. Modification of the wettability of polymer surfaces using nanoparticles. Prog. Org. Coat. 2014, 77, 331–338. [Google Scholar] [CrossRef]
- Bhushan, B.; Koch, K.; Jung, Y.C. Fabrication and characterization of the hierarchical structure for superhydrophobicity and self-cleaning. Ultramicroscopy 2009, 109, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Bernagozzi, I.; Torrengo, S.; Minati, L.; Ferrari, M.; Chiappini, A.; Armellini, C.; Toniutti, L.; Lunelli, L.; Speranza, G. Synthesis and characterization of PMMA based superhydrophobic surfaces. Colloid Polym. Sci. 2012, 290, 315–322. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bharathidasan, T.; Basu, B.J. Superhydrophobic sol–gel nanocomposite coatings with enhanced hardness. Appl. Surf. Sci. 2011, 257, 10421–10426. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Pavlou, A.; Manoudis, P.N.; Aifantis, K.E. Water repellent ORMOSIL films for the protection of stone and other materials. Mater. Lett. 2014, 131, 276–279. [Google Scholar] [CrossRef]
- Wang, T.; Isimjan, T.T.; Chen, J.; Rohani, S. Transparent nanostructured coatings with UV-shielding and superhydrophobicity properties. Nanotechnology 2011, 22, 265708. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Zhu, X.; Men, X.; Ge, B.; Zhou, X. Fabrication of a superhydrophobic coating with high adhesive effect to substrates and tunable wettability. Appl. Surf. Sci. 2015, 328, 475–481. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Gupta, N. Superhydrophobic polytetrafluoroethylene surfaces with leaf-like micro-protrusions through Ar + O2 plasma etching process. Vacuum 2014, 99, 42–48. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Grosu, D.; Aslanidou, D.; Aifantis, K.E. Facile method to prepare superhydrophobic and water repellent cellulosic paper. J. Nanomater. 2015, 16, 11. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Manoudis, P.N.; Karapanagiotis, I. Fabrication of water repellent coatings using waterborne resins for the protection of the cultural heritage. Macromol. Symp. 2013, 331, 158–165. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N.; Savva, A.; Panayiotou, C. Superhydrophobic polymer-particle composite films produced using various particle sizes. Surf. Interface Anal. 2012, 44, 870–875. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A Mater. 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Ovaskainena, L.; Rodriguez-Meizoso, I.; Birkin, N.A.; Howdle, S.M.; Gedde, U.; Wågberg, L.; Turner, C. Towards superhydrophobic coatings made by non-fluorinated polymers sprayed from a supercritical solution. J. Supercrit. Fluids 2013, 77, 134–141. [Google Scholar] [CrossRef]
- Quana, C.; Werner, O.; Wågberg, L.; Turner, C. Generation of superhydrophobic paper surfaces by a rapidly expanding alkyl ketene dimer—Supercritical carbon dioxide solution. J. Supercrit. Fluids 2009, 49, 117–124. [Google Scholar] [CrossRef]
- Cao, L.; Price, T.P.; Weiss, M.; Gao, D. Super water- and oil-repellent surfaces on intrinsically hydrophilic and oleophilic porous silicon films. Langmuir 2008, 24, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.; Choi, W.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Robust omniphobic surfaces. Proc. Natl. Acad. Sci. USA 2008, 105, 18200–18205. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Tuteja, A.; Chhatre, S.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. Fabrics with tunable oleophobicity. Adv. Mater. 2009, 21, 2190–2195. [Google Scholar] [CrossRef]
- Kota, A.K.; Li, Y.; Mabry, J.M.; Tuteja, A. Hierarchically structured superoleophobic surfaces with ultralow contact angle hysteresis. Adv. Mater. 2012, 24, 5838–5843. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Lafuma, A.; Quéré, D. Slippery pre-suffused surfaces. Europhys. Lett. 2011, 96, 56001. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Quéré, D. Bouncing water drops. Europhys. Lett. 2000, 50, 769–775. [Google Scholar] [CrossRef]
- Wu, Y.; Su, B.; Jiang, L.; Heeger, A.J. “Liquid-liquid-solid”-type superoleophobic surfaces to pattern polymeric semiconductors towards high-quality organic field-effect transistors. Adv. Mater. 2013, 25, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Grynyov, R.; Chaniel, G.; Taitelbaum, H.; Bormashenko, Y. Robust technique allowing manufacturing superoleophobic surfaces. Appl. Surf. Sci. 2013, 270, 98–103. [Google Scholar] [CrossRef]
- Pechook, S.; Kornblum, N.; Pokroy, B. Bio-inspired superoleophobic fluorinated wax crystalline surfaces. Adv. Funct. Mater. 2013, 23, 4572–4576. [Google Scholar] [CrossRef]
- Fujii, T.; Aoki, Y.; Habazaki, H. Fabrication of super-oil-repellent dual pillar surfaces with optimized oillar intervals. Langmuir 2011, 27, 11752–11756. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, T.; Guittard, F.; Amigoni, S.; de Givenchy, E.T.; Noblin, X.; Kofman, R.; Celestini, F. Superoleophobic behavior of fluorinated conductive polymer films combining electropolymerization and lithography. Soft Matter 2011, 7, 1053–1057. [Google Scholar] [CrossRef]
- Steele, A.; Bayer, I.; Loth, E. Inherently superoleophobic nanocomposite coatings by spray atomization. Nano Lett. 2009, 9, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.C.; Chang, W.H.; Chen, W.C.; Chang, Y.H.; Huang, Y.C.; Chang, F.C. Non-fluorinated superamphiphobic surfaces through sol–gel processing of methyltriethoxysilane and tetraethoxysilane. Mater. Chem. Phys. 2009, 114, 63–68. [Google Scholar] [CrossRef]
- Hoefnagels, H.F.; Wu, D.; de With, G.; Ming, W. Biomimetic superhydrophobic and highly oleophobic cotton textiles. Langmuir 2007, 23, 13158–13163. [Google Scholar] [CrossRef] [PubMed]
- Artusa, G.R.J.; Zimmermann, J.; Reifler, F.A.; Brewer, S.A.; Seeger, S.A. Superoleophobic textile repellent towards impacting drops of alkanes. Appl. Surf. Sci. 2012, 258, 3835–3840. [Google Scholar] [CrossRef]
- Leng, B.X.; Shao, Z.Z.; de With, G.; Ming, W.H. Superoleophobic cotton textiles. Langmuir 2009, 25, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, M.; Zang, D.; Gao, Z.; Wang, C. Fabrication of superhydrophobic/superoleophilic cotton for application in the field of water/oil separation. Carbohydr. Polym. 2014, 103, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Wang, C.; Li, J. A facile method to fabricate superhydrophobic cotton fabrics. Appl. Surf. Sci. 2012, 261, 561–566. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Lin, T. One-step vapour-phase formation of patternable, electrically conductive, superamphiphobic coatings on fibrous materials. Soft Matter 2011, 7, 8158–8161. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Xu, X.; Men, X.; Zhu, X. Fabrication of super-repellent cotton textiles with rapid reversible wettability switching of diverse liquid. Appl. Surf. Sci. 2013, 276, 571–577. [Google Scholar] [CrossRef]
- Saraf, R.; Lee, H.J.; Michielsen, S.; Owens, J.; Willis, C.; Stone, C.; Wilusz, E. Comparison of three methods for generating superhydrophobic, superoleophobic nylon nonwoven surface. J. Mater. Sci. 2011, 46, 5751–5760. [Google Scholar] [CrossRef]
- Hayn, R.A.; Owens, J.R.; Boyer, S.A.; McDonald, R.S.; Lee, H.J. Preparation of highly hydrophobic and oleophobic textile surfaces using microwave-promoted silane coupling. J. Mater. Sci. 2011, 46, 2503–2509. [Google Scholar] [CrossRef]
- Shirgholami, M.A.; Khalil-Abad, M.S.; Khajavi, R.; Yazdanshenas, M.E. Fabrication of superhydrophobic polymethylsilsesquioxane nanostructures on cotton textiles by a solution immersion process. J. Colloid Interface Sci. 2011, 359, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Sataev, M.S.; Koshkarbaeva, S.T.; Tleuova, A.B.; Perni, S.; Aidarova, S.B.; Prokopovich, P. Novel process for coating textile materials with silver to prepare antimicrobial fabrics. Colloids Surfaces A 2014, 442, 146–151. [Google Scholar] [CrossRef]
- Hassan, M.M. Antimicrobial coatings for textiles. In Handbook of Antimicrobial Coatings; Atul, T., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 321–355. [Google Scholar]
- Coradia, M.; Zanettia, M.; Valério, A.; de Oliveira, D.; da Silva, O.; de Arruda, S.M.; de Souza, G.U.; de Souza, A.A.U. Production of antimicrobial textiles by cotton fabric functionalization and pectinolytic enzyme immobilization. Mater. Chem. Phys. 2018, 208, 28–34. [Google Scholar] [CrossRef]
- Terzioglu, F.; Grethe, T.; Both, C.; Joßen, A.; Mahltig, B.; Rabe, M. Coating technologies for antimicrobial textile surfaces: State of the art and future prospects for textile finishing. In Handbook of Antimicrobial Coatings; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 123–135. [Google Scholar]
- Ulaeto, S.B.; Rajan, R.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Developments in smart anticorrosive coatings with multifunctional characteristics. Prog. Org. Coat. 2017, 111, 294–314. [Google Scholar] [CrossRef]
- Pospiech, D.; Jehnichen, D.; Stark, S.; Müller, F.; Bünker, T.; Wollenberg, A.; Häußler, L.; Simone, F.; Grundke, K.; Oertel, U.; et al. Multifunctional methacrylate-based coatings for glass and metal. Appl. Surf. Sci. 2017, 399, 205–214. [Google Scholar] [CrossRef]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Constr. Build. Mater. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- Zarzuela, R.; Carbú, M.; Gil, M.L.A.; Cantoral, J.M.; Mosquera, M.J. CuO/SiO2 nanocomposites: A multifunctional coating for application on building stone. Mater. Des. 2017, 114, 364–372. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Terlizzi, V.; Munafò, P. Multifunctional TiO2-based hybrid coatings on limestone: Initial performances and durability over time. J. Build. Eng. 2017, 14, 134–149. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifuntional TiO2 coatings for cultural heritage. Prog. Org. Coat. 2012, 74, 186–191. [Google Scholar] [CrossRef]
- Attia, N.F.; Moussa, M.; Sheta, A.M.F.; Taha, R.; Gamal, H. Synthesis of effective multifunctional textile based on silica nanoparticlesOriginal. Prog. Org. Coat. 2017, 106, 41–49. [Google Scholar] [CrossRef]
- Aranzabe, E.; Arriortua, M.I.; Larrañaga, A.; Aranzabe, A.; Villasante, P.M.; March, R. Designing multifunctional pigments for an improved energy efficiency in buildings. Energy Build. 2017, 147, 9–13. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Beake, B.D.; Yamamoto, K.; Aguirre, M.H.; Veldhuis, S.C.; Dosbaeva, G.; Elfizy, A.; Biksa, A.; Shuster, L.S. Structure, properties and wear performance of nano-multilayered. TiAlCrSiYN/TiAlCrN coatings during machining of Ni-based aerospace superalloys. Surf. Coat. Technol. 2010, 204, 3698–3706. [Google Scholar] [CrossRef]
- Cannavale, A.; Fiorito, F.; Manca, M.; Tortorici, G.; Cingolani, R.; Gigli, G. Multifunctional bioinspired sol-gel coatings for architectural glasses. Build. Environ. 2010, 45, 1233–1243. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Superhydrophobic, superoleophobic coatings for the protection of silk textiles. Prog. Org. Coat. 2016, 97, 44–52. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuneable textile cleaning and disinfection process based on supercritical CO2 and Pickering emulsions. J. Supercrit. Fluids 2016, 118, 128–139. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Rozman, U.; Zavec Pavlinić, D.; Pal, E.; Gönc, V.; Šostar Turk, Z. Efficiency of Medical Workers’ Uniforms with Antimicrobial Textiles for Advanced Applications; Kumar, B., Thakur, S., Eds.; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Neoh, K.G.; Shi, Z.; Kang, E.T. Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. J. Membr. Sci. 2008, 320, 259–267. [Google Scholar] [CrossRef]
- Wessels, S.; Ingmer, H. Modes of action of three disinfectant active substances: A review. Regul. Toxicol. Pharmacol. 2013, 67, 456–467. [Google Scholar] [CrossRef] [PubMed]


| Drop | CA (°) on Coated Silk | |||
|---|---|---|---|---|
| Siloxane | Siloxane+AM | Siloxane+SiO2 | Siloxane+AM+SiO2 | |
| Water | 148.7 ± 2.8 | 143.0 ± 2.5 | 164.3 ± 3.3 | 155.3 ± 2.7 |
| Oil | 138.4 ± 4.8 | 141.4 ± 2.1 | 147.6 ± 5.9 | 157.0 ± 3.3 |
| Coating on Silk | Treatment Time | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| ΔW (g/cm2) | |||||
| Uncoated silk (reference) | 0.312 | 0.304 | 0.297 | 0.281 | 0.285 |
| Siloxane+AM | 0.198 | 0.198 | 0.202 | 0.201 | 0.201 |
| Siloxane+SiO2 | 0.230 | 0.229 | 0.232 | 0.190 | 0.212 |
| Siloxane+AM+SiO2 | 0.216 | 0.193 | 0.199 | 0.205 | 0.206 |
| %RVP | |||||
| Siloxane+AM | 36.5 | 34.9 | 32.0 | 28.5 | 29.5 |
| Siloxane+SiO2 | 26.3 | 24.7 | 21.9 | 32.4 | 25.6 |
| Siloxane+AM+SiO2 | 30.8 | 36.5 | 33.0 | 27.0 | 27.7 |
| Drop | CA (°) on Coated Silk | |||
|---|---|---|---|---|
| Siloxane | Siloxane+AM | Siloxane+SiO2 | Siloxane+AM+SiO2 | |
| Water | 152.0 ± 3.3 | 144.3 ± 2.3 | 163.8 ± 3.1 | 153.1 ± 3.3 |
| Oil | 135.3 ± 4.2 | 144.6 ± 4.4 | 144.0 ± 5.6 | 145.1 ± 7.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslanidou, D.; Karapanagiotis, I. Superhydrophobic, Superoleophobic and Antimicrobial Coatings for the Protection of Silk Textiles. Coatings 2018, 8, 101. https://doi.org/10.3390/coatings8030101
Aslanidou D, Karapanagiotis I. Superhydrophobic, Superoleophobic and Antimicrobial Coatings for the Protection of Silk Textiles. Coatings. 2018; 8(3):101. https://doi.org/10.3390/coatings8030101
Chicago/Turabian StyleAslanidou, Dimitra, and Ioannis Karapanagiotis. 2018. "Superhydrophobic, Superoleophobic and Antimicrobial Coatings for the Protection of Silk Textiles" Coatings 8, no. 3: 101. https://doi.org/10.3390/coatings8030101
APA StyleAslanidou, D., & Karapanagiotis, I. (2018). Superhydrophobic, Superoleophobic and Antimicrobial Coatings for the Protection of Silk Textiles. Coatings, 8(3), 101. https://doi.org/10.3390/coatings8030101






