Detection of AFB1 via TiO2 Nanotubes/Au Nanoparticles/Enzyme Photoelectrochemical Biosensor
Abstract
:1. Introduction
2. Materials and Methods
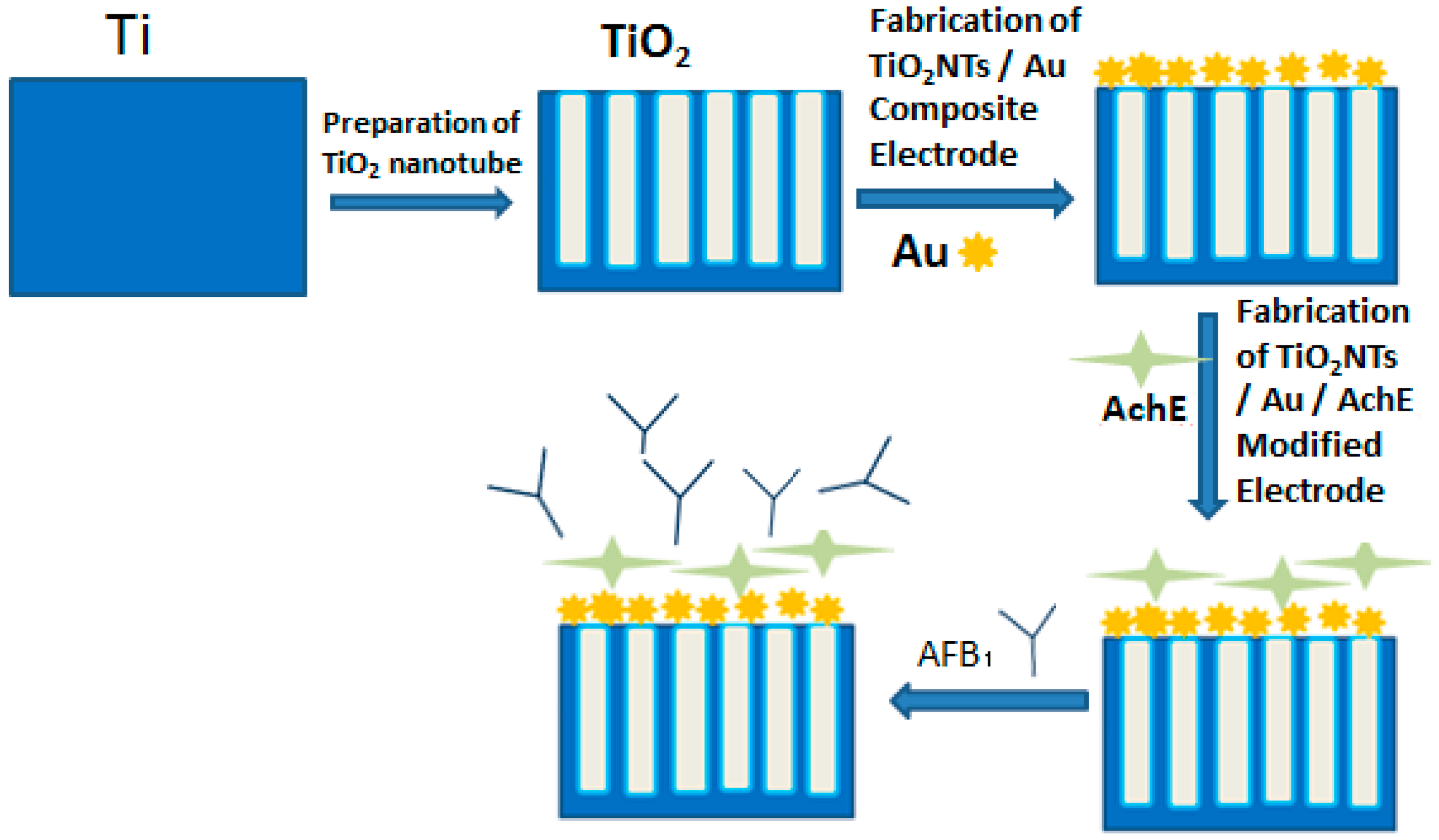
2.1. Biosensors Preparation
2.1.1. Fabrication of TiO2NTs Electrode
2.1.2. Fabrication of Au/TiO2NTs Composite Electrode
2.1.3. Fabrication of Au/AchE/TiO2NTs Modified Electrode
2.2. Procedure for Electrochemical Biosensor
3. Results and Discussion
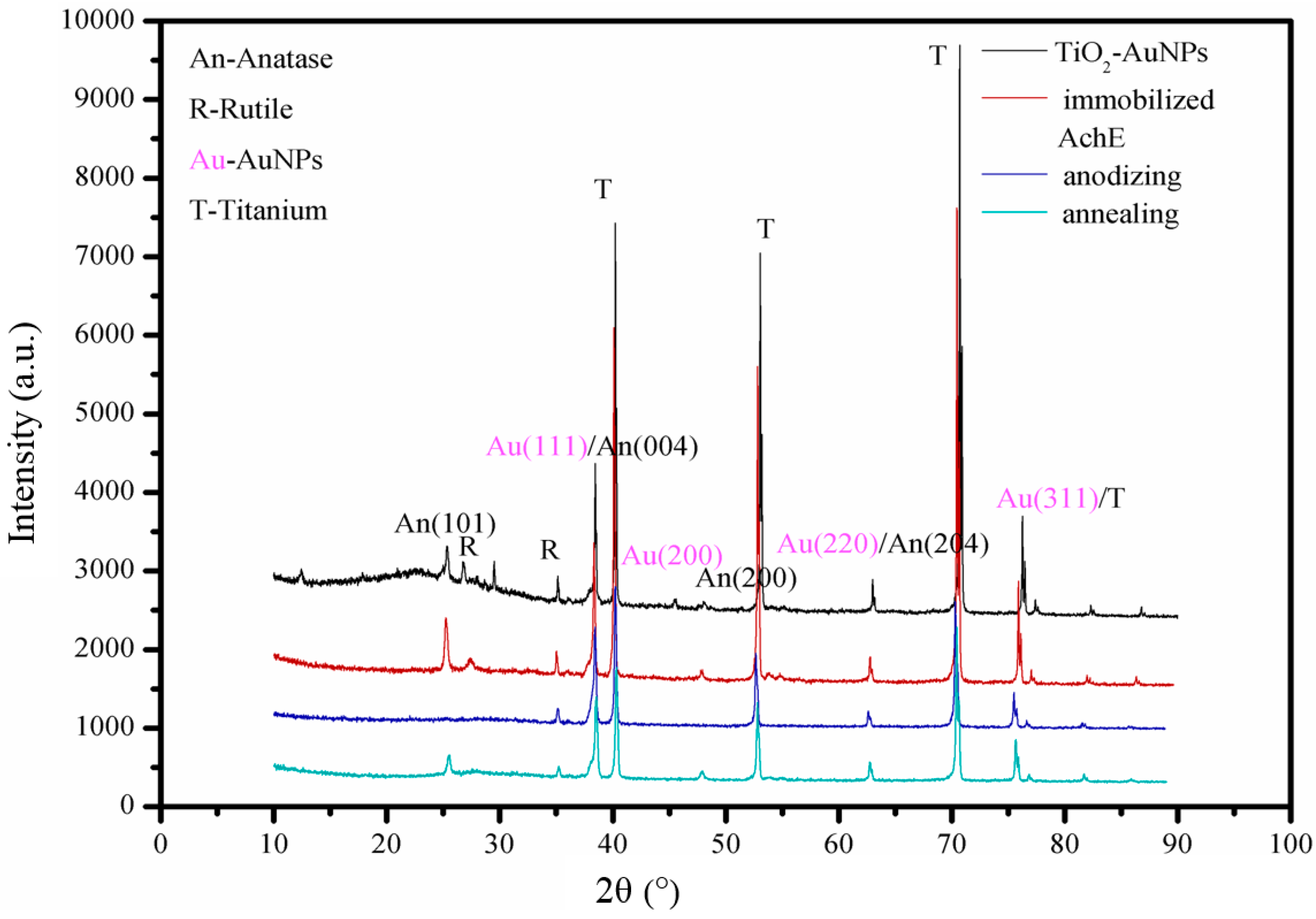
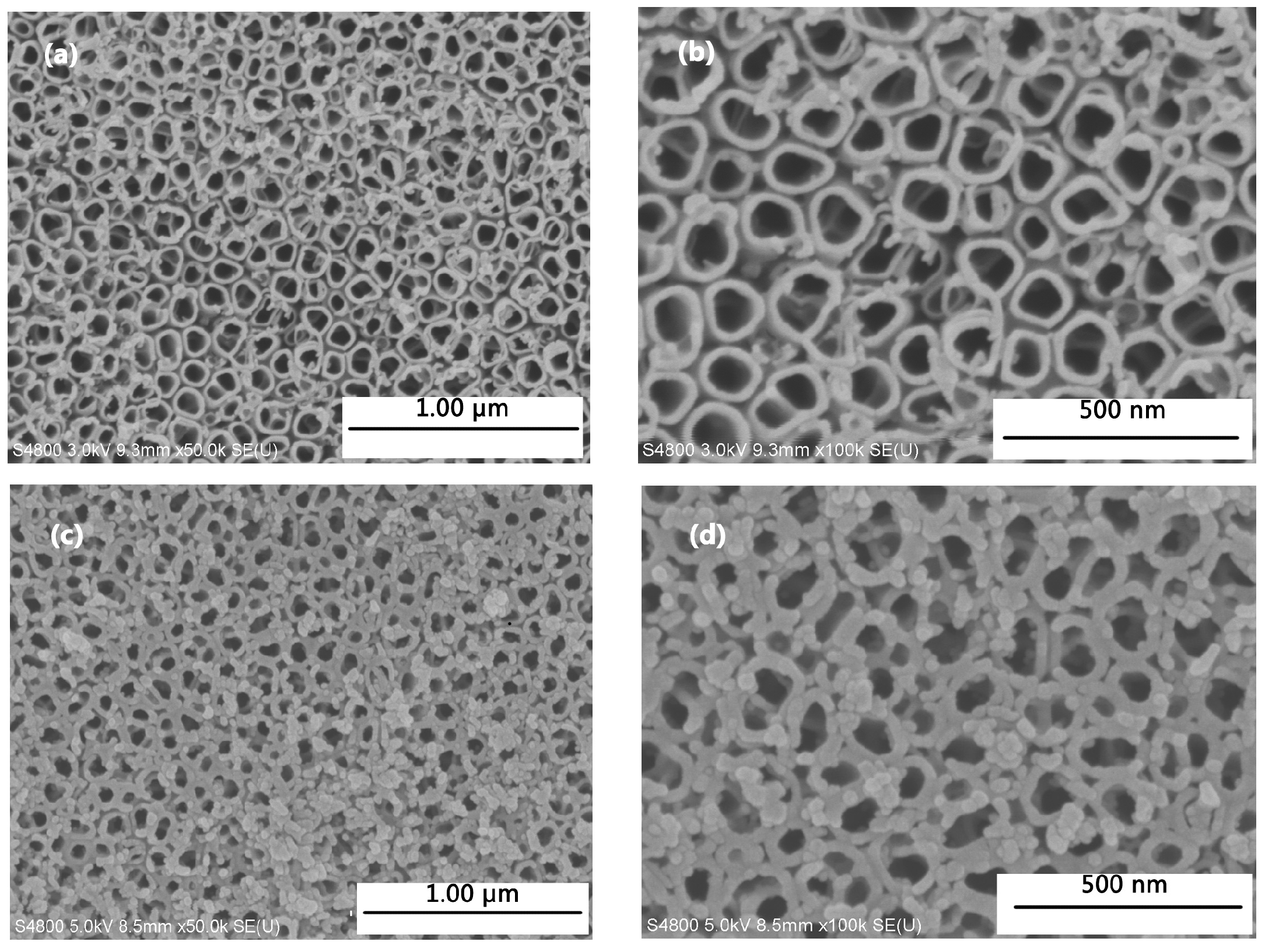
3.1. Characterizationn
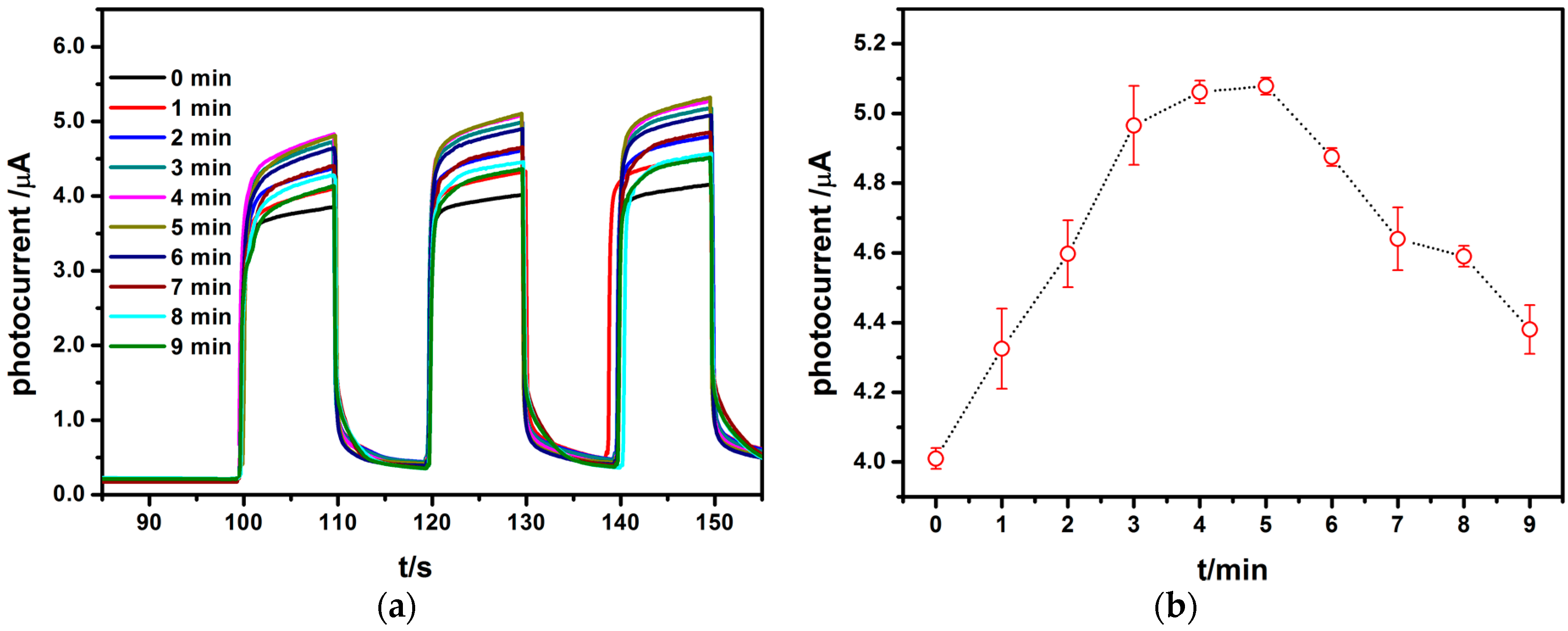
3.2. Effect of AuNPs on Photocatalytic Effect of TiO2NTs
3.3. Effect of Acetylcholine Concentration on Photocurrent Response of Enzyme Biosensor
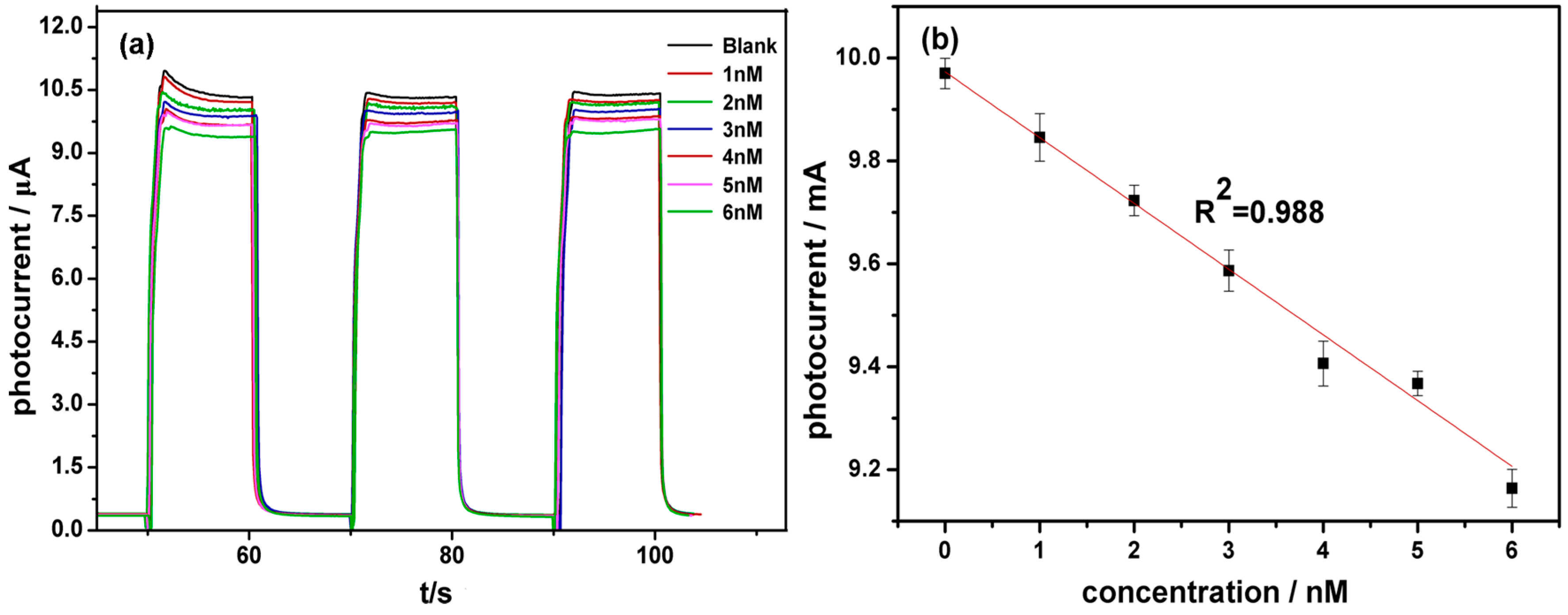
3.4. Performance of the Biosensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shyu, R.H.; Shyu, H.F.; Liu, H.W.; Tang, S.S. Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon 2002, 40, 255–258. [Google Scholar] [CrossRef]
- Egner, P.A.; Wang, J.B.; Zhu, Y.R.; Zhang, B.C.; Wu, Y.; Zhang, Q.N.; Qian, G.S.; Kuang, S.Y.; Gange, S.J.; Jacobson, L.P. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14601–14606. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, Y.; Shao, S.; Cai, Z.; Feng, L.; Pan, H.; Wang, Z. Simultaneous determination of multi-component mycotoxin contaminants in foods and feeds by ultra-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1143, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Amirkhizi, B.; Arefhosseini, S.R.; Ansarin, M.; Nemati, M. Aflatoxin B1 in eggs and chicken livers by dispersive liquid-liquid microextraction and HPLC. Food Addit. Contam. Part B 2015, 8, 245–249. [Google Scholar]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- Wang, P.; Dai, W.; Ge, L.; Yan, M.; Ge, S.; Yu, J. Visible light photoelectrochemical sensor based on Au nanoparticles and molecularly imprinted poly(o-phenylenediamine)-modified TiO2 nanotubes for specific and sensitive detection chlorpyrifos. Analyst 2013, 138, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, Z.; Liu, X.; Zhao, S.; Liu, S. Nanostructured photoelectrochemical biosensor for highly sensitive detection of organophosphorous pesticides. Biosens. Bioelectron. 2015, 64, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Xu, Q.; Yang, Z.; Hu, X. A derivative photoelectrochemical sensing platform for 4-nitrophenolate contained organophosphates pesticide based on carboxylated perylene sensitized nano-TiO2. Anal. Chim. Acta 2013, 766, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Da, P.; Wu, H.; Zhao, D.; Zheng, G. Controlled Sn-doping in TiO2 nanowire photoanodes with enhanced photoelectrochemical conversion. Nano Lett. 2012, 12, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dasgupta, N.P.; Yang, P. Cheminform abstract: Semiconductor nanowires for artificial photosynthesis. Cheminform 2014, 45, 415–422. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.M.; Liu, C.; Andrews, S.C.; Hahn, C.; Yang, P. Large-scale synthesis of transition-metal-doped TiO2 nanowires with controllable overpotential. J. Amer. Chem. Soc. 2013, 135, 9995–9998. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, L.M.; Lang, X.F.; Wang, H.Y.; Lou, X.W.; Aydil, E.S. Doping high-surface-area mesoporous TiO2 microspheres with carbonate for visible light hydrogen production. Energy Environ. Sci. 2014, 7, 2592–2597. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Amer. Chem. Soc. 2014, 136, 458–565. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Miao, J.; Liu, B. Self-assembly of aligned rutile@anatase TiO2 nanorod@CdS quantum dots ternary core-shell heterostructure: Cascade electron transfer by interfacial design. Mater. Horiz. 2014, 1, 259–263. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Kaur, R.; Pal, B. Size and shape dependent attachments of Au nanostructures to TiO2 for optimum reactivity of Au–TiO2 photocatalysis. J. Mol. Catal. A 2012, 355, 39–43. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, H.; Xu, L.; Lu, D.; Tang, L.; Jin, L.; Xu, Z.; Zhang, W. Visible-light-activated photoelectrochemical biosensor for the study of acetylcholinesterase inhibition induced by endogenous neurotoxins. Biosens. Bioelectron. 2013, 45, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Spectrophotomeric assay of aflatoxin B1 using acetylcholinesterase immobilized on standard microplates. Anal. Lett. 2013, 46, 1306–1315. [Google Scholar] [CrossRef]
- Pohanka, M.; Kuca, K.; Jun, D. Aflatoxin assay using an amperometric sensor strip and acetylcholinesterase as recognition element. Sens. Lett. 2008, 6, 450–453. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Arduini, F.; Arvinte, A.; Amine, A.; Gargouri, M.; Micheli, L.; Bala, C.; Moscone, D.; Palleschi, G. Development of a bio-electrochemical assay for aflatoxin B1 detection in olive oil. Biosens. Bioelectron. 2009, 24, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Soldatkin, O.O.; Burdak, O.S.; Sergeyeva, T.A.; Arkhypova, V.M.; Dzyadevych, S.V.; Soldatkin, A.P. Acetylcholinesterase-based conductometric biosensor for determination of aflatoxin B1. Sens. Actuators B 2013, 188, 999–1003. [Google Scholar] [CrossRef]
- Baker, D.R.; Kamat, P.V. Photosensitization of TiO2 nanostructures with CdS quantum dots: Particulate versus tubular support architectures. Adv. Funct. Mater. 2009, 19, 805–811. [Google Scholar] [CrossRef]
- Li, G.; Wu, L.; Li, F.; Xu, P.; Zhang, D.; Li, H. Photoelectrocatalytic degradation of organic pollutants via a CdS quantum dots enhanced TiO2 nanotube array electrode under visible light irradiation. Nanoscale 2013, 5, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- He, L.L.; Oybek, T.; Abidov, A.; Song, J.J.; Jeong, S.W.; Kim, S.Y.; Kim, T.Y. Study of formation and investigation of properties of metal doped titanium dioxide nanotube arrays synthesized by anodizing method. Afore 2012, 11, 466. [Google Scholar]
- Shibu, E.S.; Kimura, K.; Pradeep, T. Gold nanoparticle superlattices: Novel surface enhanced Raman scattering active substrates. Chem. Mater. 2009, 21, 3773–3781. [Google Scholar] [CrossRef]
- Mori, T.; Sharma, A.; Hegmann, T. Significant enhancement of the chiral correlation length in nematic liquid crystals by gold nanoparticle surfaces featuring axially chiral binaphthyl ligands. ACS Nano 2016, 10, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Zhou, Y.; Xu, B.Q.; Chen, Y.F.; Liu, H.Z. Effect of gold doping on the photocatalytic activity of the anatase TiO2. Acta Phys. Chim. Sin. 2008, 24, 459–464. [Google Scholar]
- Silman, H.I.; Karlin, A. Effect of local pH changes caused by substrate hydrolysis on the activity of membrane-bound acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1967, 58, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Grafius, M.A.; Millar, D.B. Reversible aggregation of acetylcholinesterase. Ii. Interdependencee of pH and ionic strength. Biochemistry 1967, 6, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Cometa, M.F.; Lorenzini, P.S.; Volpe, M.T.; Meneguz, A.; Palmery, M. In vitro inhibitory effect of aflatoxin B1 on acetylcholinesterase activity in mouse brain. Toxicology 2005, 206, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Chen, J.H.; Cao, H.; Yao, D.S.; Liu, D.L. Amperometric biosensor for aflatoxin B1 based on aflatoxin-oxidase immobilized on multiwalled carbon nanotubes. Food Control 2011, 22, 43–49. [Google Scholar] [CrossRef]
- Ordóñez, L.C.; Escobar, B.; Barbosa, R.; Verde-Gómez, Y. Enhanced performance of direct ethanol fuel cell using pt/mwcnts as anodic electrocatalyst. J. Appl. Electrochem. 2015, 45, 1205–1210. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, D.; You, J.M.; Han, H.S.; Jeon, S. Non-enzymatic superoxide anion radical sensor based on pt nanoparticles covalently bonded to thiolated MWCNTs. Electrochim. Acta 2012, 81, 31–36. [Google Scholar] [CrossRef]
- Hansmann, T.; Sanson, B.; Stojan, J.; Weik, M.; Marty, J.L.; Fournier, D. Kinetic insight into the mechanism of cholinesterasterase inhibition by aflatoxin B1 to develop biosensors. Biosens. Bioelectron. 2009, 24, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, Y.D.T.; Ferreira, L.F. Amperometric biosensing of carbamate and organophosphate pesticides utilizing screen-printed tyrosinase-modified electrodes. Anal. Chim. Acta 2007, 596, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Darain, F.; Park, S.U.; Shim, Y.B. Disposable amperometric immunosensor system for rabbit igg using a conducting polymer modified screen-printed electrode. Biosens. Bioelectron. 2003, 18, 773–780. [Google Scholar] [CrossRef]
- Stepurska, K.V.; Soldatkin, C.O.; Kucherenko, I.S.; Arkhypova, V.M.; Dzyadevych, S.V.; Soldatkin, A.P. Feasibility of application of conductometric biosensor based on acetylcholinesterase for the inhibitory analysis of toxic compounds of different nature. Anal. Chim. Acta 2015, 854, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kirdeciler, S.K.; Soy, E.; Oztürk, S.; Kucherenko, I.; Soldatkin, O.; Dzyadevych, S.; Akata, B. A novel urea conductometric biosensor based on zeolite immobilized urease. Talanta 2011, 85, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Luo, X.; Huang, J.; Cui, X.T.; Yun, M. Detection of cardiac biomarkers using single polyaniline nanowire-based conductometric biosensors. Biosensors 2012, 2, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Guedri, H.; Durrieu, C. A self-assembled monolayers based conductometric algal whole cell biosensor for water monitoring. Microchim. Acta 2008, 163, 179–184. [Google Scholar] [CrossRef]
- Moffatt, P.; Smith, C.E.; St-Arnaud, R.; Nanci, A. Evaluation of aflatoxin B1-acetylcholinesterase dissociation kinetic using the amperometric biosensor technology: Prospect for toxicity mechanism. Protein Pept. Lett. 2010, 17, 340–342. [Google Scholar]
- Xu, X.; Ying, Y.; Li, Y. A simple, competitive biosensor for rapid detection of aflatoxin B1 based on aggregation of gold nanorods. In Proceedings of the 2012 IEEE Sensors, Taipei, Taiwan, 28–31 October 2012; pp. 1–4. [Google Scholar]
- Xu, X.; Liu, X.; Li, Y.; Ying, Y. A simple and rapid optical biosensor for detection of aflatoxin B1 based on competitive dispersion of gold nanorods. Biosens. Bioelectron. 2013, 47, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Taitt, C.R.; Fertig, S.; Moore, M.H.; Lassman, M.E.; Maragos, C.M.; Shriverlake, L.C. Indirect competitive immunoassay for detection of aflatoxin B1 in corn and nut products using the array biosensor. Biosens. Bioelectron. 2006, 21, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Okwu, G.I.; Achar, P.N.; Sharma, S.K. Quantification of aflatoxin B1 in ready-to-use food thickeners in south-east geo-political zone in nigeria. Afr. J. Microbiol. Res. 2010, 4, 1788–1793. [Google Scholar]


| Methods | Detection Limit or IC50 | Ref. |
|---|---|---|
| Au/AchE/TiO2NTs | 0.33 nM | This experiment |
| AFO/MWCNTS/Pt | 1.6 nM | [32,33,34] |
| Amperometric, screen printed electrode modified with CoPc | 302 μM | [35,36,37] |
| AchE-based Conductometric Biosensor | 0.05 μg/mL | [38,39,40,41] |
| Amperometric, screen printed electrode | IC50 = 100 ppb | [42] |
| Aflatoxin B1 based on aggregation of gold nanorods | 0.04 ppb | [43,44] |
| Detection of aflatoxin B1 in corn and nut products using the array biosensor | 0.3 ng/mL | [45,46] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Q.; He, C.; Mo, R.; He, L.; Zhou, C.; Hong, P.; Sun, S.; Li, C. Detection of AFB1 via TiO2 Nanotubes/Au Nanoparticles/Enzyme Photoelectrochemical Biosensor. Coatings 2018, 8, 90. https://doi.org/10.3390/coatings8030090
Yuan Q, He C, Mo R, He L, Zhou C, Hong P, Sun S, Li C. Detection of AFB1 via TiO2 Nanotubes/Au Nanoparticles/Enzyme Photoelectrochemical Biosensor. Coatings. 2018; 8(3):90. https://doi.org/10.3390/coatings8030090
Chicago/Turabian StyleYuan, Qiong, Chuxian He, Rijian Mo, Lei He, Chunxia Zhou, Pengzhi Hong, Shengli Sun, and Chengyong Li. 2018. "Detection of AFB1 via TiO2 Nanotubes/Au Nanoparticles/Enzyme Photoelectrochemical Biosensor" Coatings 8, no. 3: 90. https://doi.org/10.3390/coatings8030090





