Investigation of Element Effect on High-Temperature Oxidation of HVOF NiCoCrAlX Coatings
Abstract
1. Introduction
2. Materials and Experiment
3. Simulation
3.1. Short-Range Diffusion
3.2. Long-Range Diffusion
4. Results and Discussion
4.1. Experimental Results Analysis
4.1.1. Heat-Treated Coating
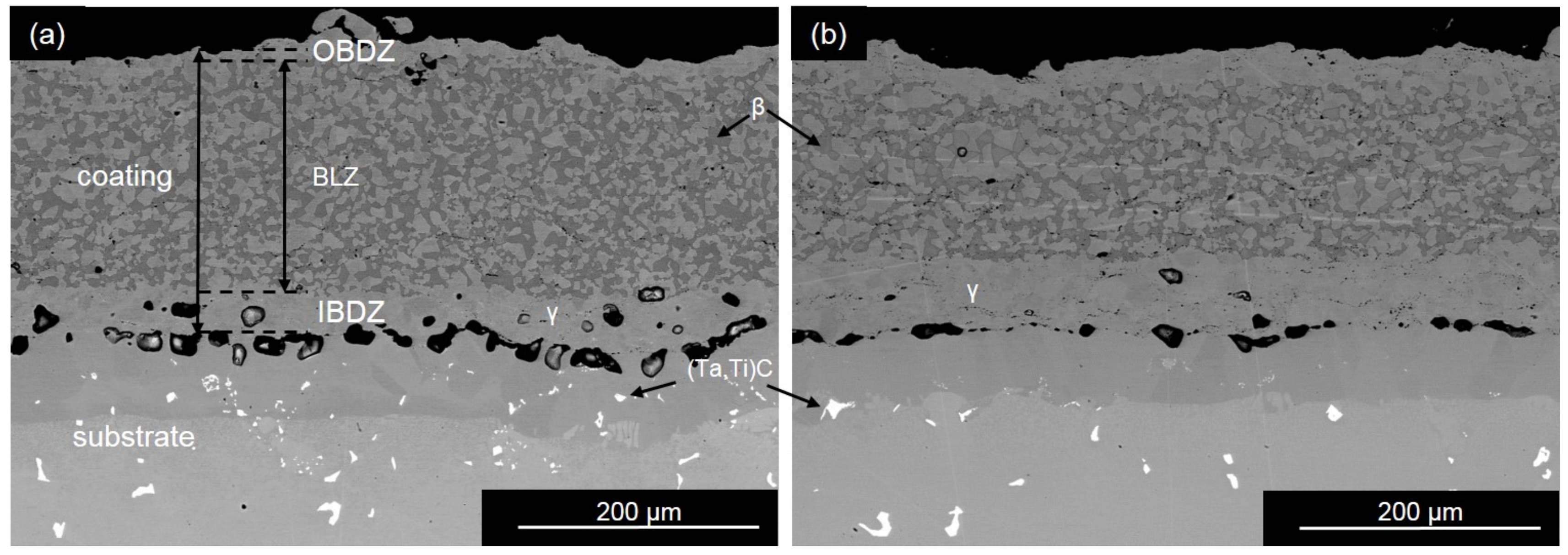
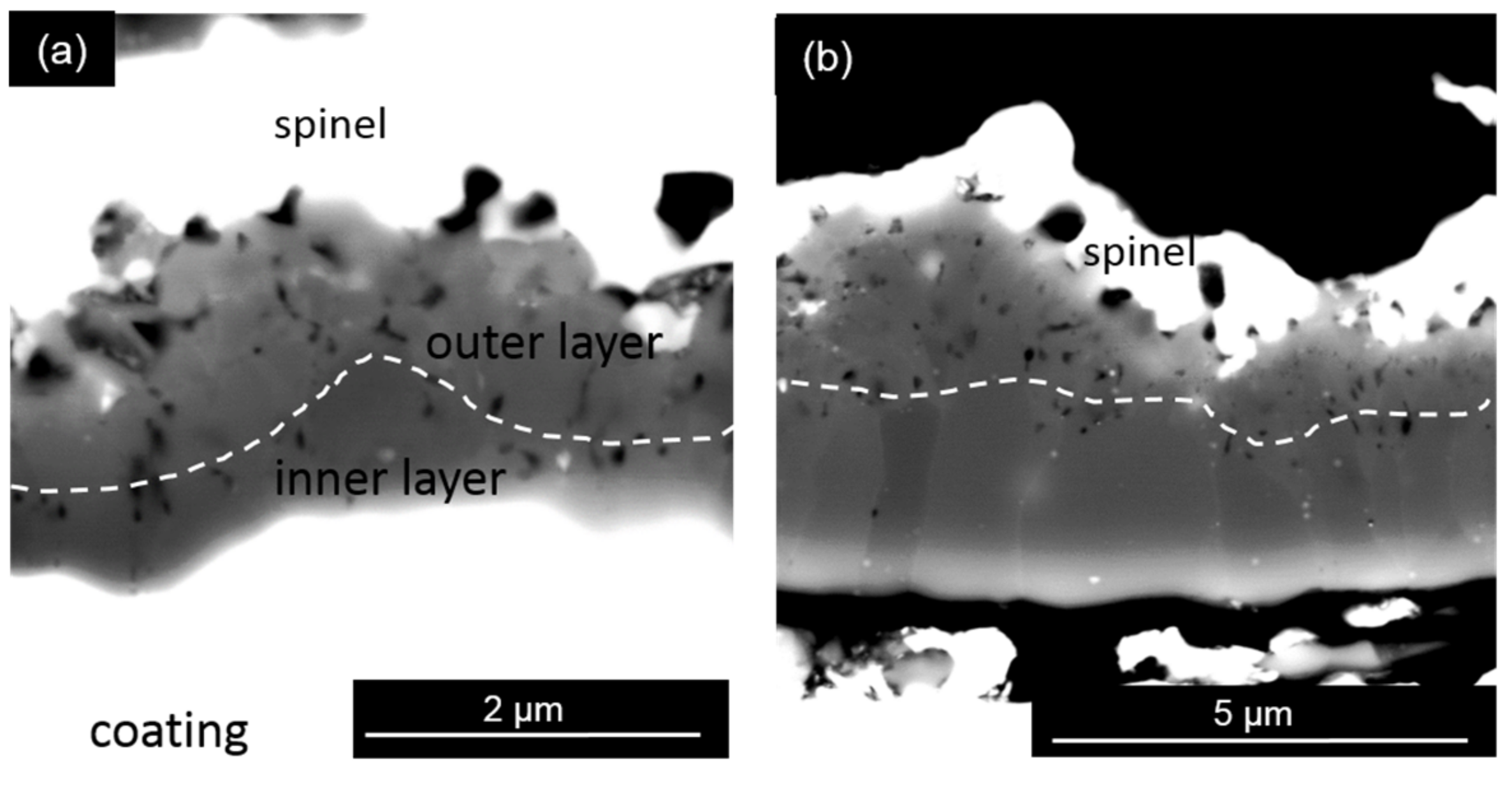
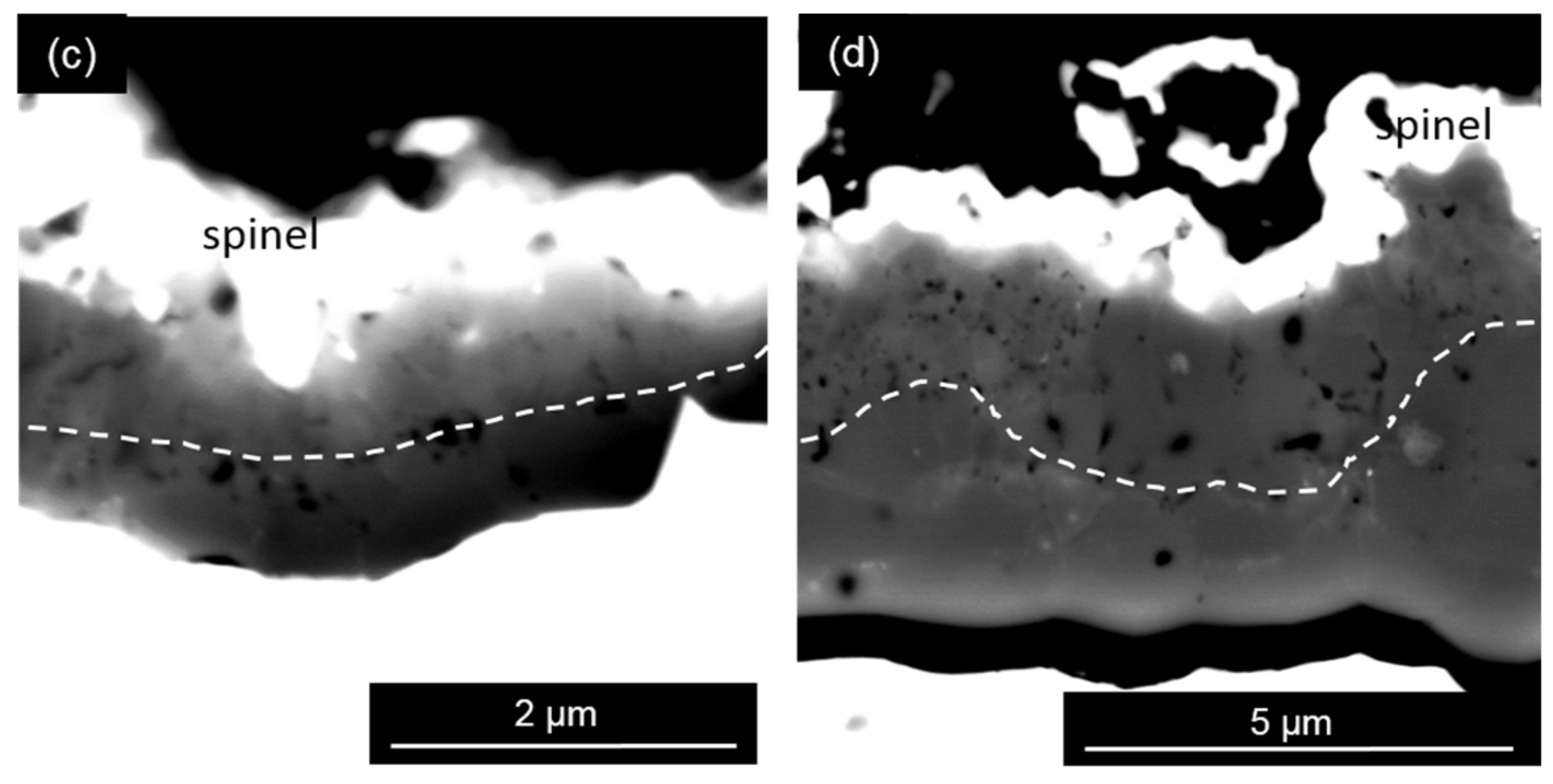
4.1.2. Thermally Grown Oxide Microstructure
4.1.3. Oxidation Kinetics
4.1.4. Cyclic Oxidation Resistance
4.2. Simulation Results
4.2.1. Phase Equilibrium Calculation
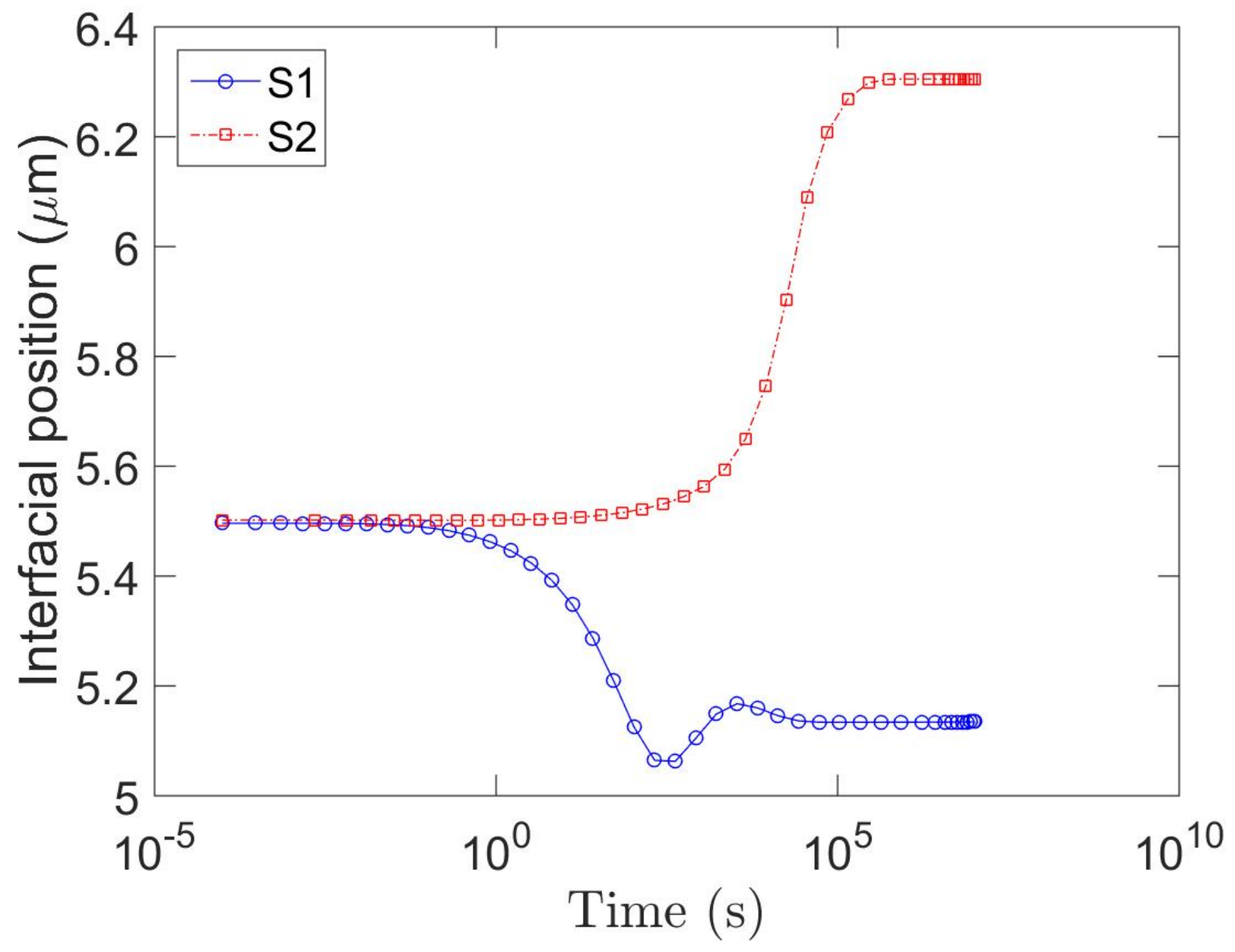
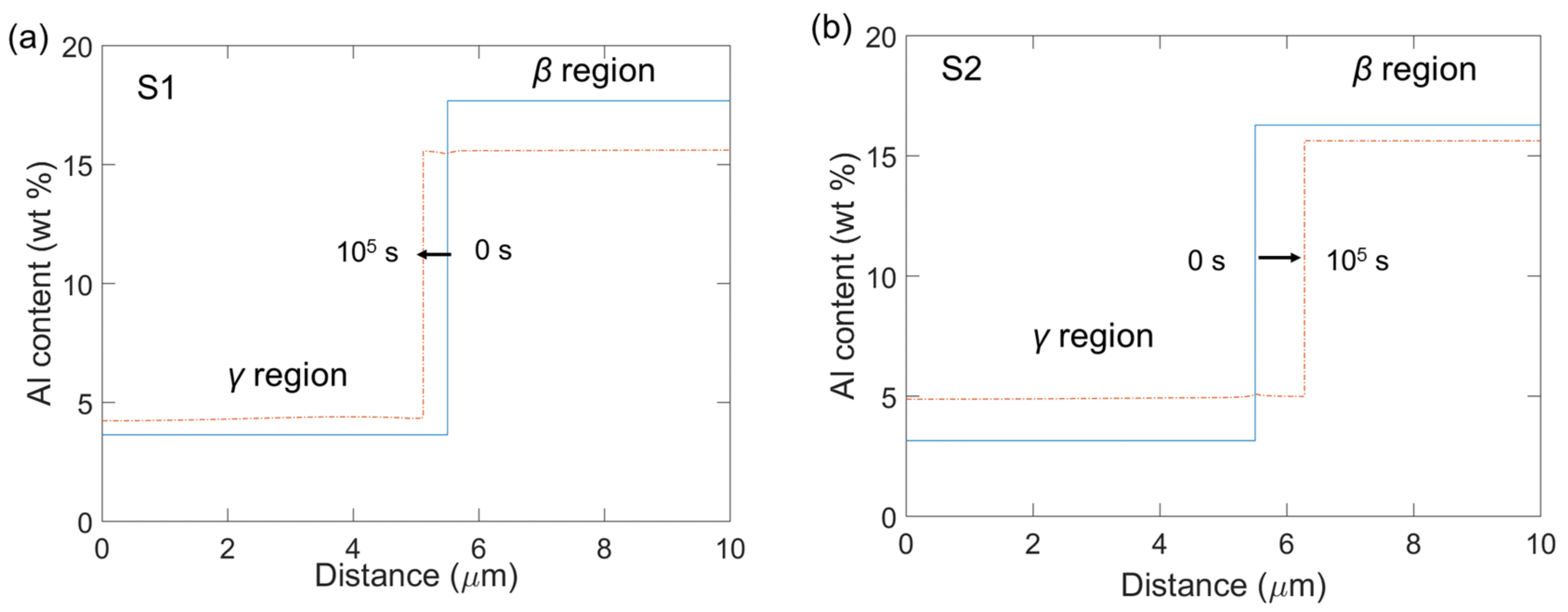
4.2.2. Short-Range Diffusion
4.2.3. Long-Range Diffusion
5. Conclusions
- Comparison of the activation energy for the OBDZ growth with that for the Al lattice diffusion in Al2O3 indicates that the growth of the alumina scale in the Y + Ru-doped coating is dominated by grain boundary diffusion of oxygen with the positive effect of Y to increase the growth kinetics of the alumina scale. However, the Ce-doped coating shows a different alumina scale growth kinetics.
- In comparison with the Ce-doped coating, the Y + Ru-doped coating showed a lower growth rate of the outer β-depletion zone (beneath the protective scale) in both isothermal 1100 °C and cyclic oxidation tests, indicating a better resistance to oxide growth and a stronger adhesion of the TGO. The spallation of the alumina scale in the Ce-doped coating at 1100 °C accelerated the consumption of the Al in the coating.
- The addition of Y in MCrAlX coatings plays an important role during coating oxidation, including modifying alumina growth mechanisms, aiding the columnar alumina microstructure formation, and increasing alumina scale adhesion against spallation. However, none of such positive effects can be observed by the addition of 0.2 wt % Ce in the MCrAlX coating. Therefore, it is reasonable to assume that the addition of 0.2 wt % Ce cannot replace the important role of Y in MCrAlX coatings.
- The Y + Ru-doped coating exhibited a lower growth rate of inner β-depletion zone (near the coating-substrate interface) induced by interdiffusion. The simulation results show that the addition of Ru increases the solubility of the Al in the β phase and the β phase fraction, which reduces the loss of the Al due to interdiffusion and stabilizes the β phase.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brandl, W.; Toma, D.; Krüger, J.; Grabke, H.J.; Matthäus, G. The oxidation behaviour of HVOF thermal-sprayed MCrAlY coatings. Surf. Coat. Technol. 1997, 94–95, 21–26. [Google Scholar] [CrossRef]
- Chen, L.C.; Zhang, C.; Yang, Z.G. Effect of pre-oxidation on the hot corrosion of CoNiCrAlYRe alloy. Corros. Sci. 2011, 53, 374–380. [Google Scholar] [CrossRef]
- Smeggil, J.G. Some comments on the role of yttrium in protective oxide scale adherence. Mater. Sci. Eng. 1987, 87, 261–265. [Google Scholar] [CrossRef]
- Stott, F.H.; Wood, G.C. Growth and adhesion of oxide scales on Al2O3-forming alloys and coatings. Mater. Sci. Eng. 1987, 87, 267–274. [Google Scholar] [CrossRef]
- Pint, B.A.; More, K.L.; Wright, I.G. Effect of quaternary additions on the oxidation behavior of Hf-doped NiAl. Oxid. Met. 2003, 59, 257–283. [Google Scholar] [CrossRef]
- Guo, H.; Cui, Y.; Peng, H.; Gong, S. Improved cyclic oxidation resistance of electron beam physical vapor deposited nano-oxide dispersed β-NiAl coatings for Hf-containing superalloy. Corros. Sci. 2010, 52, 1440–1446. [Google Scholar] [CrossRef]
- Taylor, T.A.; Bettridge, D.F. Development of alloyed and dispersion-strengthened MCrAlY coatings. Surf. Coat. Technol. 1996, 86–87, 9–14. [Google Scholar] [CrossRef]
- Czech, N.; Schmitz, F.; Stamm, W. Improvement of MCrAlY coatings by addition of rhenium. Surf. Coat. Technol. 1994, 68–69, 17–21. [Google Scholar] [CrossRef]
- Mevrel, R. Cyclic oxidation of high-temperature alloys. Mater. Sci. Technol. 1987, 3, 531–535. [Google Scholar] [CrossRef]
- Tanno, M.; Ogawa, K.; Shoji, T. Effect of cerium and silicon additions to MCrAlY on the high-temperature oxidation behavior and bond strength of thermal barrier coatings. Key Eng. Mater. 2004, 261–263, 1061–1066. [Google Scholar] [CrossRef]
- Amano, T.; Taguchi, T. Spalling of the surface oxide formed on Ni-(20,40,60,80)Cr alloys with small additions of Ce and Si. J. Alloys Compd. 1993, 193, 20–22. [Google Scholar] [CrossRef]
- Itoh, Y.; Tamura, M. Reaction diffusion behaviors for interface between Ni-based super alloys and vacuum plasma sprayed MCrAlY coatings. Eng. Gas Turbines Power 1999, 121, 476–483. [Google Scholar] [CrossRef]
- Yuan, K.; Eriksson, R.; Peng, R.L.; Li, X.H.; Johansson, S.; Wang, Y.D. MCrAlY coating design based on oxidation-diffusion modelling. Part I: Microstructural evolution. Surf. Coat. Technol. 2014, 254, 79–96. [Google Scholar] [CrossRef]
- Salam, S.; Hou, P.; Zhang, Y.-D.; Wang, H.-F.; Zhang, C.; Yang, Z.-G. Compositional effects on the high-temperature oxidation lifetime of MCrAlY type coating alloys. Corros. Sci. 2015, 95, 143–151. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Peng, H.; Peng, L.; Gong, S. Diffusion barrier behaviors of (Ru,Ni)Al/NiAl coatings on Ni-based superalloy substrate. Intermetallics 2011, 19, 191–195. [Google Scholar] [CrossRef]
- Sato, A.; Harada, H.; Yokokawa, T.; Murakumo, T.; Koizumi, Y.; Kobayashi, T.; Imai, H. The effects of ruthenium on the phase stability of fourth generation Ni-base single crystal superalloys. Scr. Mater. 2006, 54, 1679–1684. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Sloof, W.G. Effect of reactive element oxide inclusions on the growth kinetics of protective oxide scales. Acta Mater. 2007, 55, 5980–5987. [Google Scholar] [CrossRef]
- Luthra, K.L.; Briant, C.L. Mechanism of adhesion of alumina on MCrAlY alloys. Oxid. Met. 1986, 26, 397–416. [Google Scholar] [CrossRef]
- Yuan, K.; Peng, R.L.; Li, X.H.; Johansson, S.; Wang, Y.D. Some aspects of elemental behaviour in HVOF MCrAlY coatings in high-temperature oxidation. Surf. Coat. Technol. 2015, 261, 86–101. [Google Scholar] [CrossRef]
- Yuan, K.; Eriksson, R.; Peng, R.L.; Li, X.H.; Johansson, S.; Wang, Y.D. Modeling of microstructural evolution and lifetime prediction of MCrAlY coatings on nickel based superalloys during high temperature oxidation. Surf. Coat. Technol. 2013, 232, 204–215. [Google Scholar] [CrossRef]
- Eriksson, R.; Yuan, K.; Li, X.; Peng, R.L. MCrAlY coating design based on oxidation–diffusion modelling. Part II: Lifing aspects. Surf. Coat. Technol. 2014, 253, 27–37. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Q.; Zhang, H.; Sun, X.; Guan, H.; Hu, Z. Effects of heat treatments on the microstructure of IN792 alloy. Mater. Sci. Eng. A 2010, 527, 1016–1021. [Google Scholar] [CrossRef]
- Saeidi, S.; Voisey, K.T.; McCartney, D.G. The effect of heat treatment on the oxidation behavior of HVOF and VPS CoNiCrAlY coatings. J. Therm. Spray Technol. 2009, 18, 209–216. [Google Scholar] [CrossRef]
- TCS Ni-Based Superalloys Database, version 8; Thermo-Calc Software: Solna, Sweden, 2015.
- TCS Ni-Alloys Mobility Database, version 4; Thermo-Calc Software: Solna, Sweden, 2015.
- Andersson, J.O.; Helander, T.; Hoglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Larsson, H.; Reed, R.C. On the numerical simulation of diffusion-controlled reactions under local equilibrium conditions. Acta Mater. 2008, 56, 3754–3760. [Google Scholar] [CrossRef]
- Larsson, H. A model for 1D multiphase moving phase boundary simulations under local equilibrium conditions. Calphad 2014, 47, 1–8. [Google Scholar] [CrossRef]
- Toscano, J.; Vaben, R.; Gil, A.; Subanovic, M.; Naumenko, D.; Singheiser, L.; Quadakkersa, W.J. Parameters affecting TGO growth and adherence on MCrAlY-bond coats for TBC’s. Surf. Coat. Technol. 2006, 201, 3906–3910. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Bao, Z.B.; Wei, H.; Sun, X.F.; Guan, H.R.; Hu, Z.Q. Oxidation behavior of graded NiCrAlYRe coatings at 900, 1000 and 1100 °C. Oxid. Met. 2009, 71, 125–142. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The oxidation behaviour of NiAl-I. Phase transformations in the alumina scale during oxidation of NiAl and NiAl-Cr alloys. Corros. Sci. 1992, 33, 1677–1690. [Google Scholar] [CrossRef]
- Rybicki, G.C.; Smialek, J.L. Effect of the theta-alpha-Al2O3 transformation on the oxidation behavior of beta-NiAl + Zr. Oxid. Met. 1989, 31, 275–304. [Google Scholar] [CrossRef]
- Doychak, J.; Smialek, J.L.; Barrett, C.A. The oxidation of Ni-rich Ni-Al intermetallics. In Oxidation of High-Temperature Intermetallics, Proceedings of the Workshop on the Oxidation of High-Temperature Intermetallics, Cleveland, OH, USA, 22–23 September 1988; Grobstein, T., Doychak, J.K., Eds.; TMS: Pittsburgh, PA, USA, 1989; pp. 41–55. [Google Scholar]
- Pint, B.A. On the Formation of Interfacial and Internal Voids in a-Al2O3 Scales. Oxid. Met. 1997, 48, 24–27. [Google Scholar] [CrossRef]
- Brady, M.P.; Pint, B.A.; Tortorelli, P.F.; Wright, I.G.; Hanrahan, R.J., Jr. High-Temperature Oxidation and Corrosion of Metals. In Materials Science and Technology: A Comprehensive Treatment Materials Science and Technology: A Comprehensive Treatment: Corrosion and Environmental Degradation, Volumes I+II, 1; Cahn, R.W., Haasen, P., Kramer, E.J., Eds.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2008; pp. 229–325. [Google Scholar]
- Naumenko, D.; Gleeson, B.; Wessel, E.; Singheiser, L.; Quadakkers, W.J. Correlation between the microstructure, growth mechanism, and growth kinetics of alumina scales on a FeCrAlY alloy. Metall. Mater. Trans. A 2007, 38, 2974–2983. [Google Scholar] [CrossRef]
- Burtin, P.; Brunelle, J.P.; Pijolat, M.; Soustelle, M. Influence of surface area and additives on the thermal stability of transition alumina catalyst supports. I: Kinetic data. Appl. Catal. 1987, 34, 225–238. [Google Scholar] [CrossRef]
- Lelait, L.; Alpérine, S.; Mévrel, R. Alumina scale growth at zirconia-MCrAlY interface: A microstructural study. J. Mater. Sci. 1992, 27, 5–12. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Sun, X.; Li, J.; Yin, F.; Hu, W.; Guan, H.; Hu, Z. Oxidation behavior of sputter-deposited NiCrAlY coating. Surf. Coat. Technol. 2003, 165, 241–247. [Google Scholar] [CrossRef]
- Paladino, A.E.; Kingery, W.D. Aluminum ion diffusion in aluminum oxide. J. Chem. Phys. 1962, 37, 957. [Google Scholar] [CrossRef]
- Ramanarayanan, T.A.; Raghavan, M.; Petkovic-Luton, R. Metallic yttrium additions to high temperature alloys: Influence on Al2O3 scale properties. Oxid. Met. 1984, 22, 83–100. [Google Scholar] [CrossRef]
- Ogawa, K.; Ito, K.; Shoji, T.; Seo, D.W.; Tezuka, H.; Kato, H. Effects of Ce and Si additions to CoNiCrAlY bond coat materials on oxidation behavior and crack propagation of thermal barrier coatings. J. Therm. Spray Technol. 2006, 15, 640–651. [Google Scholar] [CrossRef]










| MCrAlX | X = Ru | X = Ce | X = Y | X = Others |
|---|---|---|---|---|
| C1 | 3.3 | – | 0.3 | 0.3 Si, (Mo,Ta) < 0.6 |
| C2 | – | 0.2 | – |
| Condition | Time/Cycle |
|---|---|
| Isothermal oxidation-900 °C | 500, 1500, 5000 h |
| Isothermal oxidation-1000 °C | 500, 1500, 5000 h |
| Isothermal oxidation-1100 °C | 50, 300, 500, 800 h |
| Cyclic oxidation | 50, 300, 500, 800 cycles |
| Coating | Phase | Ni | Co | Cr | Al | Ru |
|---|---|---|---|---|---|---|
| S1 | γ | 36.3 | 33.3 | 24.7 | 3.8 | 1.9 |
| β | 51.5 | 17.3 | 7.4 | 17.5 | 6.3 | |
| S2 | γ | 41.5 | 32.4 | 22 | 4.1 | – |
| β | 56.8 | 18.6 | 6.8 | 17.8 | – |
| Element | Average Content Measured in the Coatings | γ | β | ||
|---|---|---|---|---|---|
| wt % | Ratio | wt % | Ratio | ||
| Al (in C1) | 11.5 | 3.8 | 0.33 | 17.5 | 1.52 |
| Al (in C2) | 12 | 4.1 | 0.34 | 17.8 | 1.48 |
| Ru (in C1) | 3.7 | 1.9 | 0.41 | 6.3 | 1.62 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Lin Peng, R.; Li, X.-H.; Johansson, S. Investigation of Element Effect on High-Temperature Oxidation of HVOF NiCoCrAlX Coatings. Coatings 2018, 8, 129. https://doi.org/10.3390/coatings8040129
Zhang P, Lin Peng R, Li X-H, Johansson S. Investigation of Element Effect on High-Temperature Oxidation of HVOF NiCoCrAlX Coatings. Coatings. 2018; 8(4):129. https://doi.org/10.3390/coatings8040129
Chicago/Turabian StyleZhang, Pimin, Ru Lin Peng, Xin-Hai Li, and Sten Johansson. 2018. "Investigation of Element Effect on High-Temperature Oxidation of HVOF NiCoCrAlX Coatings" Coatings 8, no. 4: 129. https://doi.org/10.3390/coatings8040129
APA StyleZhang, P., Lin Peng, R., Li, X.-H., & Johansson, S. (2018). Investigation of Element Effect on High-Temperature Oxidation of HVOF NiCoCrAlX Coatings. Coatings, 8(4), 129. https://doi.org/10.3390/coatings8040129





