Effects of Graphene-Oxide-Modified Coating on the Properties of Carbon-Fiber-Reinforced Polypropylene Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
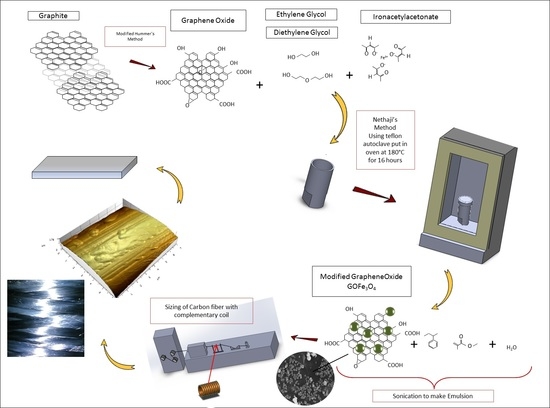
2.2. Preparation of GO and Iron Oxide Functionalized GO (GO@Fe3O4)
2.3. Sizing of the Tows
2.4. Making of the Composite Panels
2.5. Characterization
3. Results and Discussion
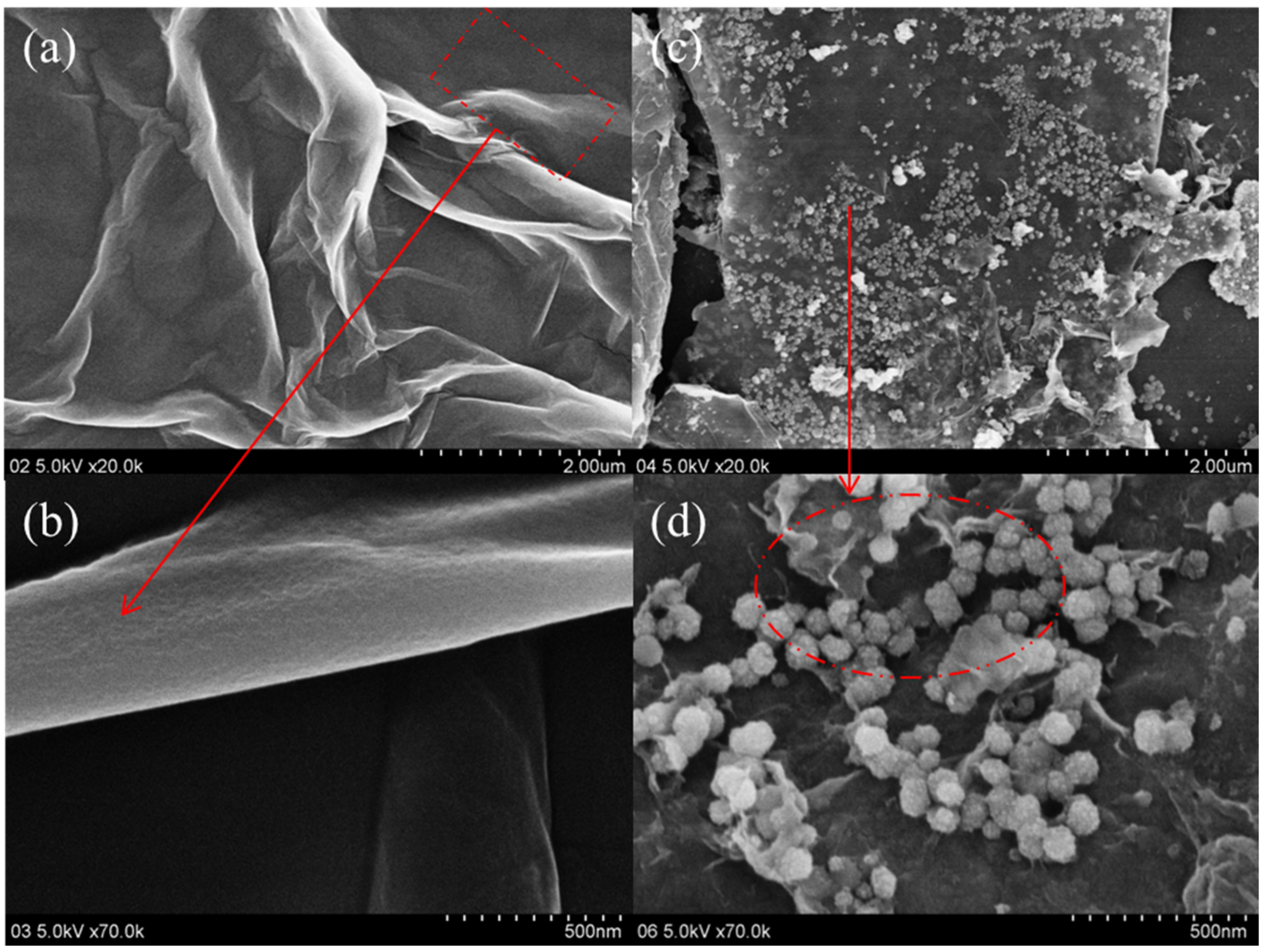
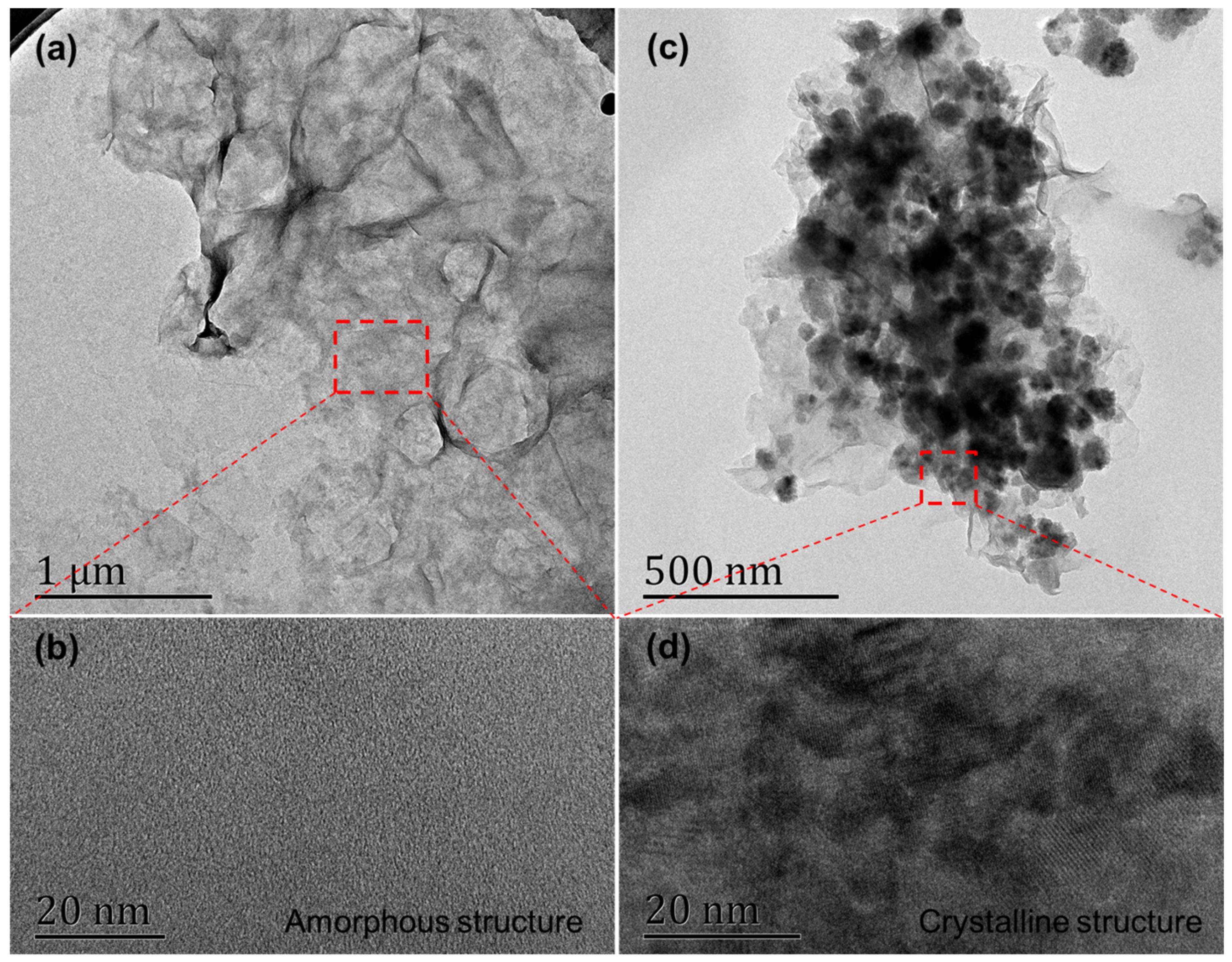
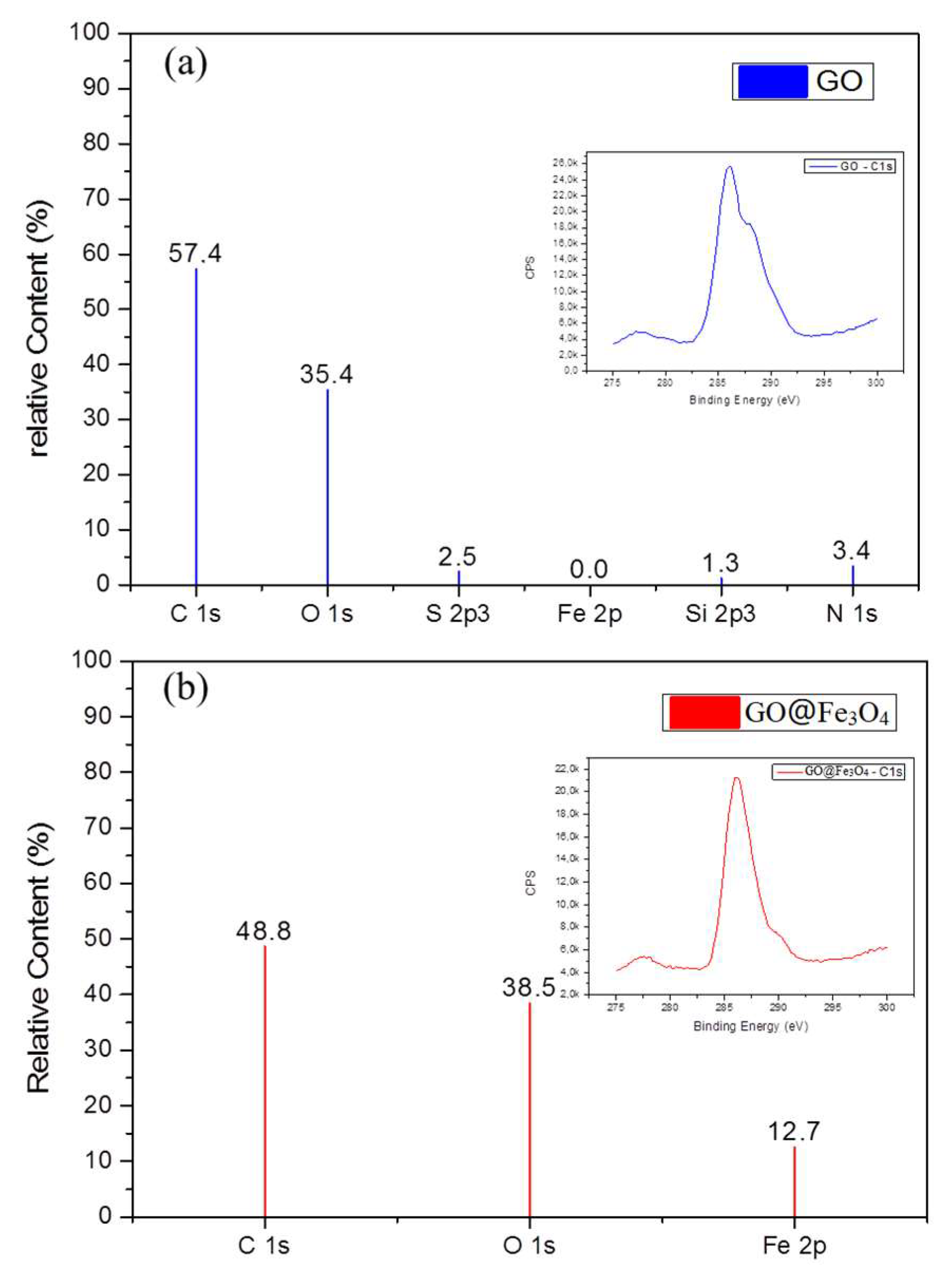
3.1. Morphology and Composition of GO@Fe3O4
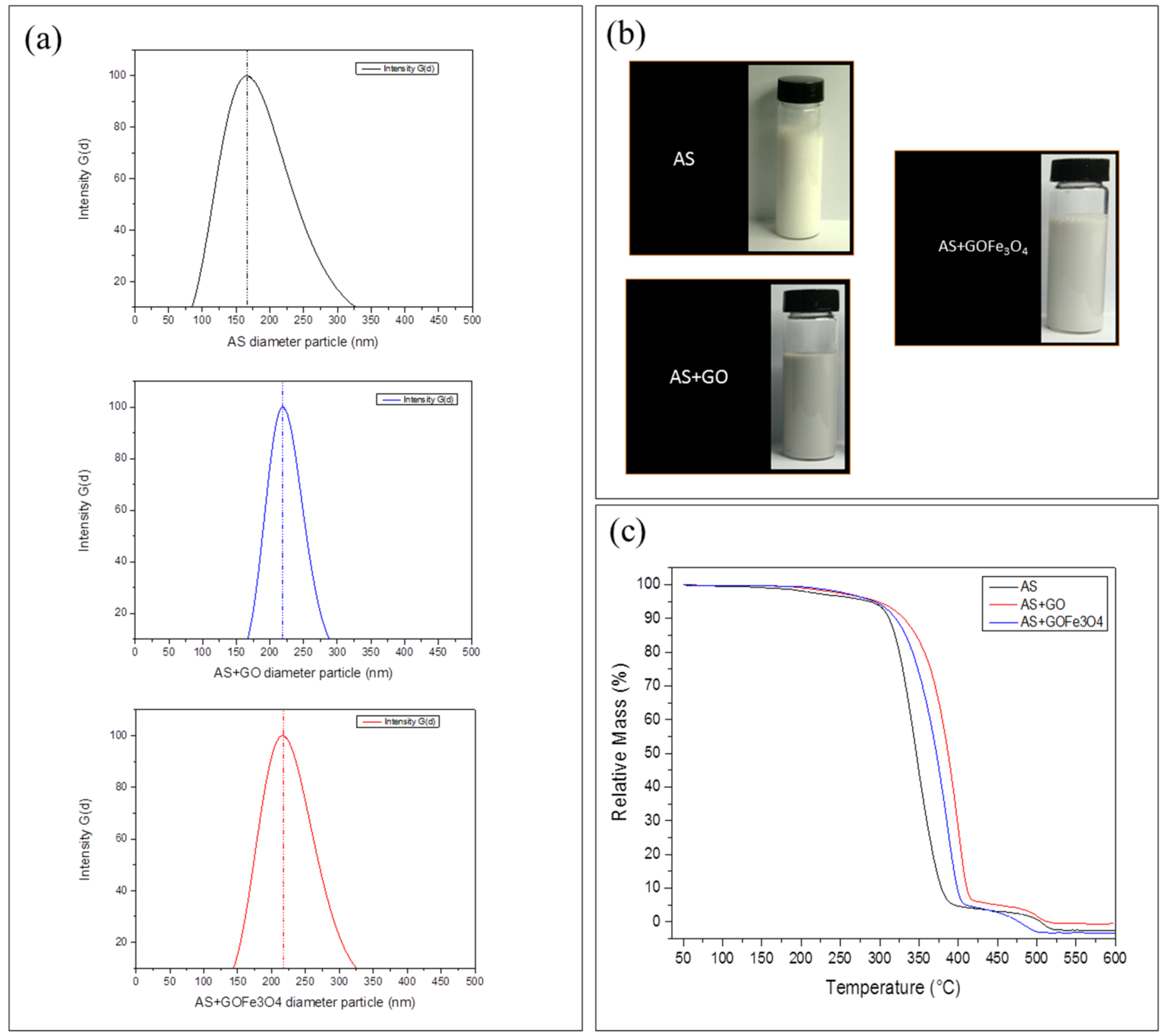
3.2. Effect on the Emulsions and Thermal Stability of TP Sizing Films
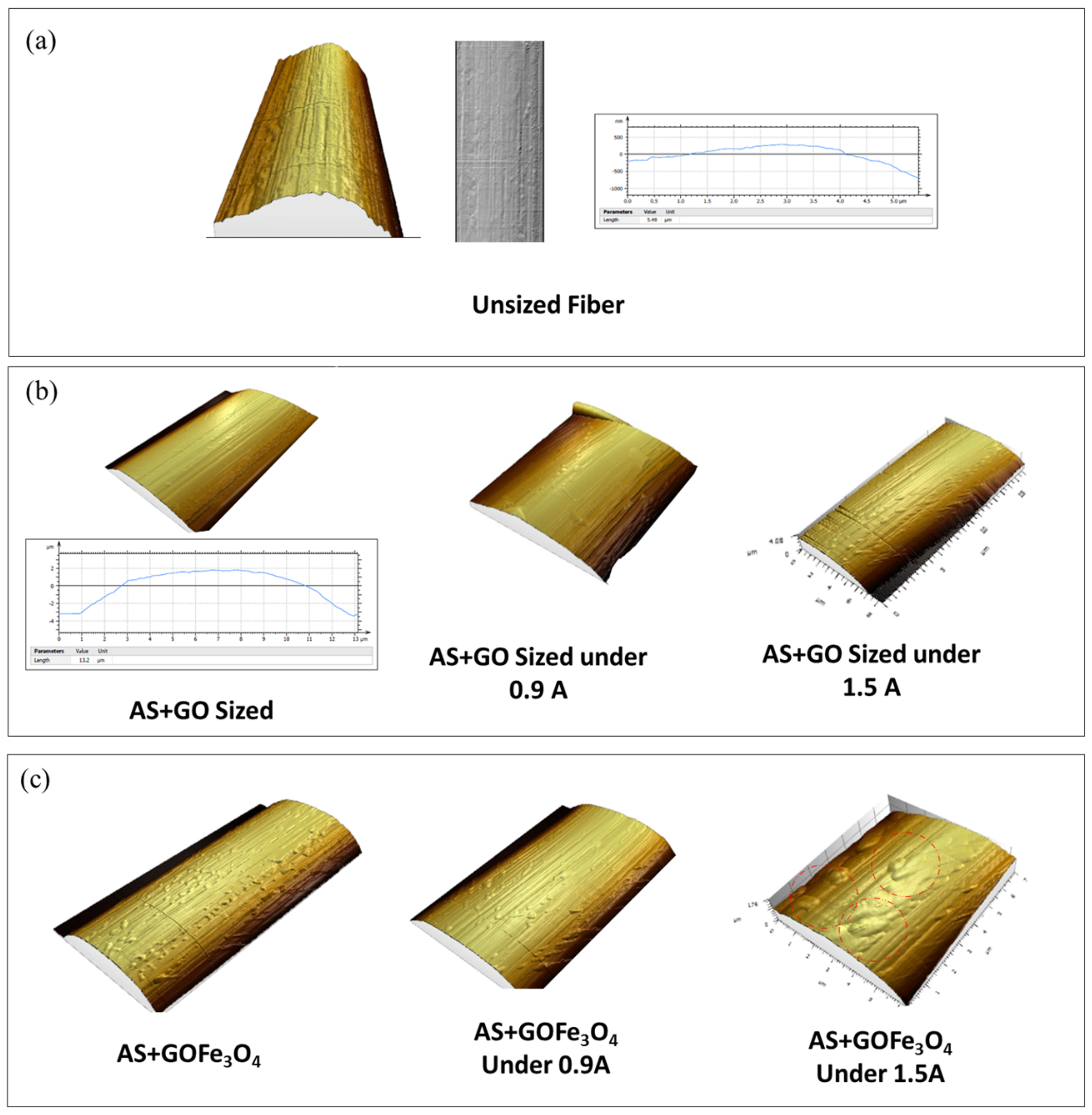
3.3. Effect of the Induced Magnetic Field on the Quality of the Sizing
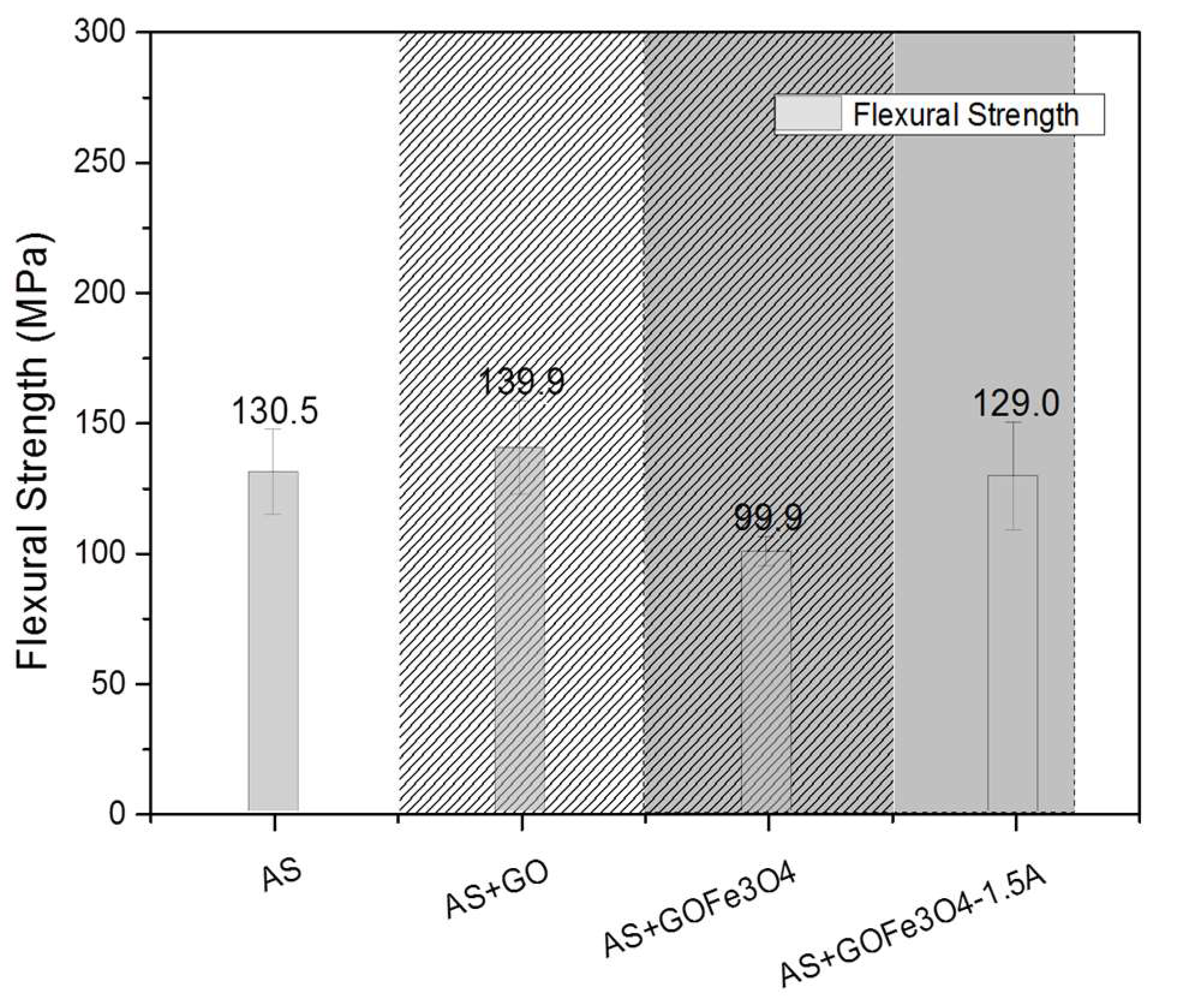
3.4. Effect of the Ferrites and Induced Magnetic Field on the Final Mechanical Properties
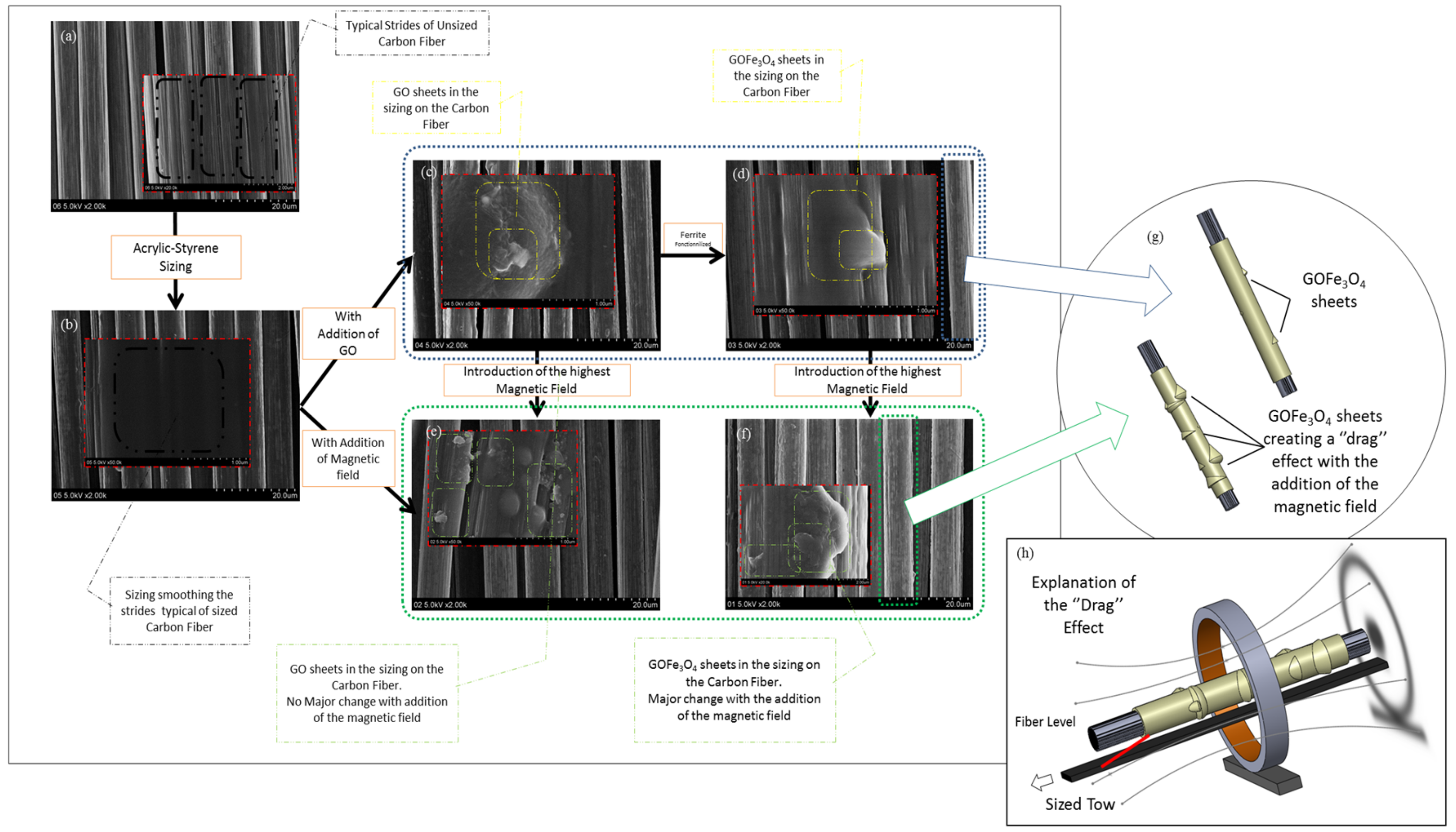
3.5. Effect of the Ferrites and Induced Magnetic Field on the Fiber/Matrix Interphase
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chung, D.D.L. Composite Materials Science and Applications, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Donnet, J.B.; Ransal, R.C. Carbon Fibers; CRC Press: Bocca Raton, FL, USA, 1990. [Google Scholar]
- Kandola, B.; Sarker, F.; Luangtriratana, P.; Myler, P. Thermal protection of carbon fiber-reinforced composites by ceramic particles. Coatings 2016, 6, 22. [Google Scholar] [CrossRef]
- Plastics, R. New Developments Help Composites Compete; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003; Volume 47, pp. 27–31. [Google Scholar]
- Brandt, J. The use of high-performance thermoplastic composites for structural aerospace applications. In Proceedings of the 9th Int Conference on Composite Materials, Madrid, Spain, 12–16 July 1993; pp. 143–150. [Google Scholar]
- Nairn, J.A.; Liu, Y.C.; Galiotis, C. Analysis of stress transfer from the matrix to the fiber through an imperfect interface: Application to raman data and the single-fiber fragmentation test. In Fiber, Matrix, and Interface Properties, ASTM STP 1290; Spragg, C.J., Drzal, L.T., Eds.; ASTM International: West Conshohocken, PA, USA, 1996; pp. 47–65. [Google Scholar]
- Yokobori, A.T.; Takeda, H.; Adachi, T.; Ha, J.C.; Yokobori, T. Characteristics of fatigue life and damage accumulation of shory-fiber reinforced polymer composites. In Fiber, Matrix, and Interface Properties, ASTM STP 1290; Spragg, C.J., Drzal, L.T., Eds.; ASTM International: West Conshohocken, PA, USA, 1996; pp. 152–167. [Google Scholar]
- Pitkethly, M.J. The use of interfacial test methods in composite materials development. In Fiber, Matrix, and Interface Properties, ASTM STP 1290; Spragg, C.J., Drzal, L.T., Eds.; ASTM International: West Conshohocken, PA, USA, 1996; pp. 34–46. [Google Scholar]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon fiber surfaces and composite interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, S.; Lu, C. Surface modification of carbon fibers by a polyether sulfone emulsion sizing for increased interfacial adhesion with polyether sulfone. Appl. Surf. Sci. 2014, 317, 737–744. [Google Scholar] [CrossRef]
- Zhang, R.L.; Huang, Y.D.; Liu, L.; Tang, Y.R.; Su, D.; Xu, L.W. Effect of the molecular weight of sizing agent on the surface of carbon fibres and interface of its composites. Appl. Surf. Sci. 2011, 257, 1840–1844. [Google Scholar] [CrossRef]
- Han, S.H.; Oh, H.J.; Kim, S.S. Evaluation of fiber surface treatment on the interfacial behavior of carbon fiber-reinforced polypropylene composites. Compos. Part B Eng. 2014, 60, 98–105. [Google Scholar] [CrossRef]
- Yao, T.-T.; Wu, G.-P.; Song, C. Interfacial adhesion properties of carbon fiber/polycarbonate composites by using a single-filament fragmentation test. Compos. Sci. Technol. 2017, 149, 108–115. [Google Scholar] [CrossRef]
- Woodhead, A.L.; de Souza, M.L.; Church, J.S. An investigation into the surface heterogeneity of nitric acid oxidized carbon fiber. Appl. Surf. Sci. 2017, 401, 79–88. [Google Scholar] [CrossRef]
- Andideh, M.; Esfandeh, M. Statistical optimization of treatment conditions for the electrochemical oxidation of PAN-based carbon fiber by response surface methodology: Application to carbon fiber/epoxy composite. Compos. Sci. Technol. 2016, 134, 132–143. [Google Scholar] [CrossRef]
- Askeland, P.A.; Fukushima, H.; Rich, M.; Drzal, L.T. UV Ozone Surface Modification of Carbon Based Reinforcements for Composite Materials; Composite Materials and Structures Center, Michigan State University: East Lansing, MI, USA, 2004; p. 9. [Google Scholar]
- Fan, W.; Wang, Y.; Wang, C.; Chen, J.; Wang, Q.; Yuan, Y.; Niu, F. High efficient preparation of carbon nanotube-grafted carbon fibers with the improved tensile strength. Appl. Surf. Sci. 2016, 364, 539–551. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Qu, C.-B.; Xiao, H.-M.; Hua, Y.; Sui, G.-X.; Fu, S. Enhanced mechanical properties of short carbon fiber reinforced polyethersulfone composites by graphene oxide coating. Polymer 2015, 59, 155–165. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.B.; Hao, L.F.; Jiao, W.C.; Yang, F.; Wang, R.G. Preparation of carbon nanotube/carbon fiber hybrid fiber by combining electrophoretic deposition and sizing process for enhancing interfacial strength in carbon fiber composites. Compos. Sci. Technol. 2013, 88, 120–125. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Yan, C.; Li, H.; Zhu, Y.; Li, X.; Yu, L. Interfacial microstructure and properties of carbon fiber composites modified with graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A. Graphene oxide coated with porous iron oxide ribbons for 2,4-Dichlorophenoxyacetic acid (2,4-D) removal. Ecotoxicol. Environ. Saf. 2017, 138, 292–297. [Google Scholar] [CrossRef] [PubMed]
- ASTM D790-17 Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2344 Standard Test Method for Short-Beam Strength of Polymer Matrix Composite Materials and Their Laminates; ASTM International: West Conshohocken, PA, USA, 2016.
- He, F.; Fan, J.; Ma, D.; Zhang, L.; Leung, C.; Chan, H.L. The attachment of Fe3O4 nanoparticles to graphene oxide by covalent bonding. Carbon 2010, 48, 3139–3144. [Google Scholar] [CrossRef]
- Luan, V.H.; Bae, D.; Han, J.H.; Lee, W. Mussel-inspired dopamine-mediated graphene hybrid with silver nanoparticles for high performance electrochemical energy storage electrodes. Compos. Part B Eng. 2018, 134, 141–150. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Wu, T.; Sun, D.; Chen, W.; Zhou, X. Magnetic iron oxide/graphene oxide nanocomposites: Formation and interaction mechanism for efficient removal of methylene blue and p-tert-butylphenol from aqueous solution. Mater. Chem. Phys. 2018, 205, 240–252. [Google Scholar] [CrossRef]
- Dong, R.; Liu, L. Preparation and properties of acrylic resin coating modified byfunctional graphene oxide. Appl. Surf. Sci. 2016, 368, 378–387. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowman, S.; Hu, X.; Jiang, Q.; Qiu, Y.; Liu, W.; Wei, Y. Effects of Graphene-Oxide-Modified Coating on the Properties of Carbon-Fiber-Reinforced Polypropylene Composites. Coatings 2018, 8, 149. https://doi.org/10.3390/coatings8040149
Bowman S, Hu X, Jiang Q, Qiu Y, Liu W, Wei Y. Effects of Graphene-Oxide-Modified Coating on the Properties of Carbon-Fiber-Reinforced Polypropylene Composites. Coatings. 2018; 8(4):149. https://doi.org/10.3390/coatings8040149
Chicago/Turabian StyleBowman, Sean, Xiaoyu Hu, Qiuran Jiang, Yiping Qiu, Wanshuang Liu, and Yi Wei. 2018. "Effects of Graphene-Oxide-Modified Coating on the Properties of Carbon-Fiber-Reinforced Polypropylene Composites" Coatings 8, no. 4: 149. https://doi.org/10.3390/coatings8040149