Approaches for Detecting Madder Lake in Multi-Layered Coating Systems of Historical Bowed String Instruments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Mock-Ups
2.2. Exposure to Artificial Solar Radiation
2.3. Analytical Techniques
3. Results
3.1. Visible and UV Light Imaging
3.2. Colourimetric Characterization of the Mock-Ups
3.3. FORS Analyses
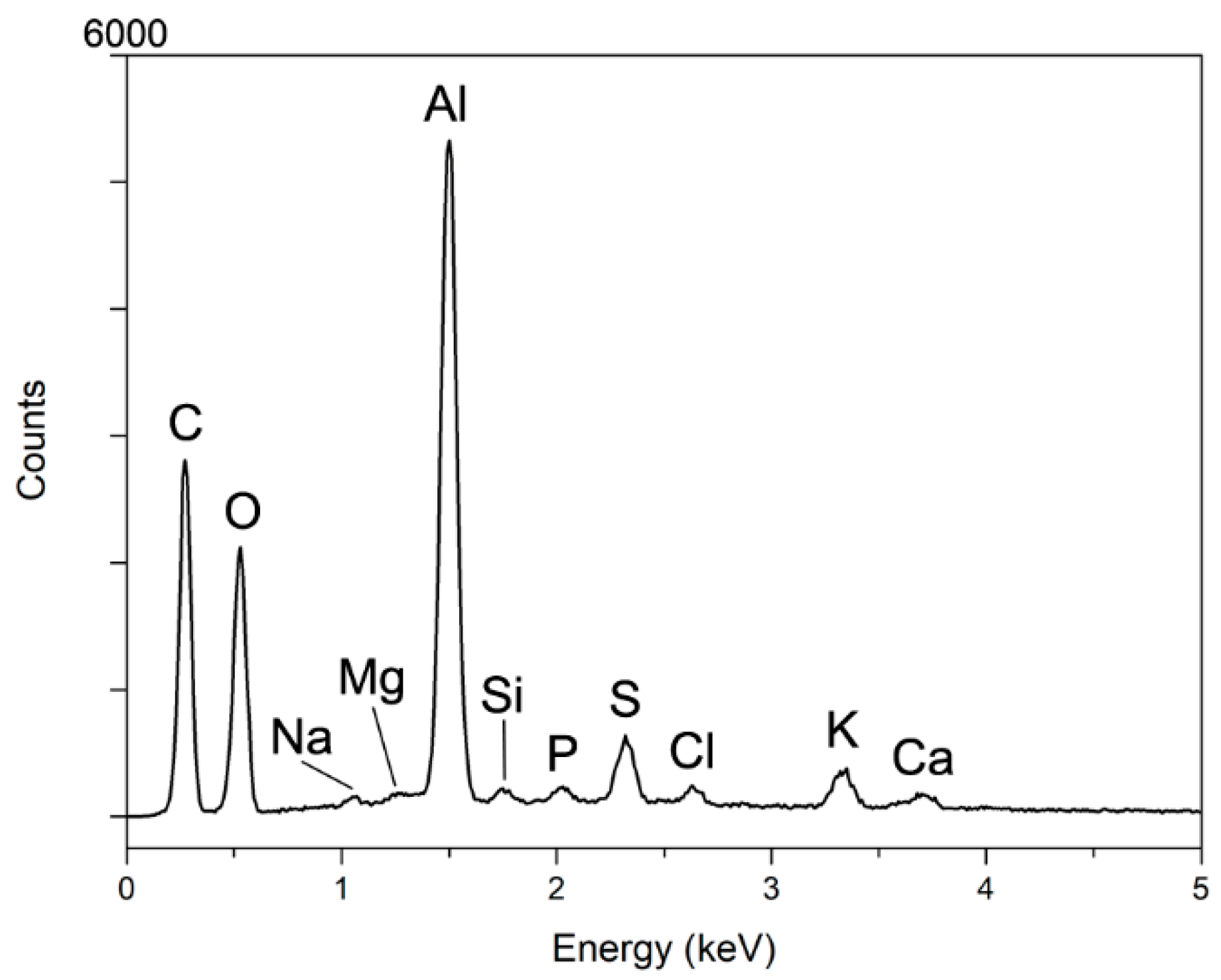
3.4. XRF and EDX Analyses
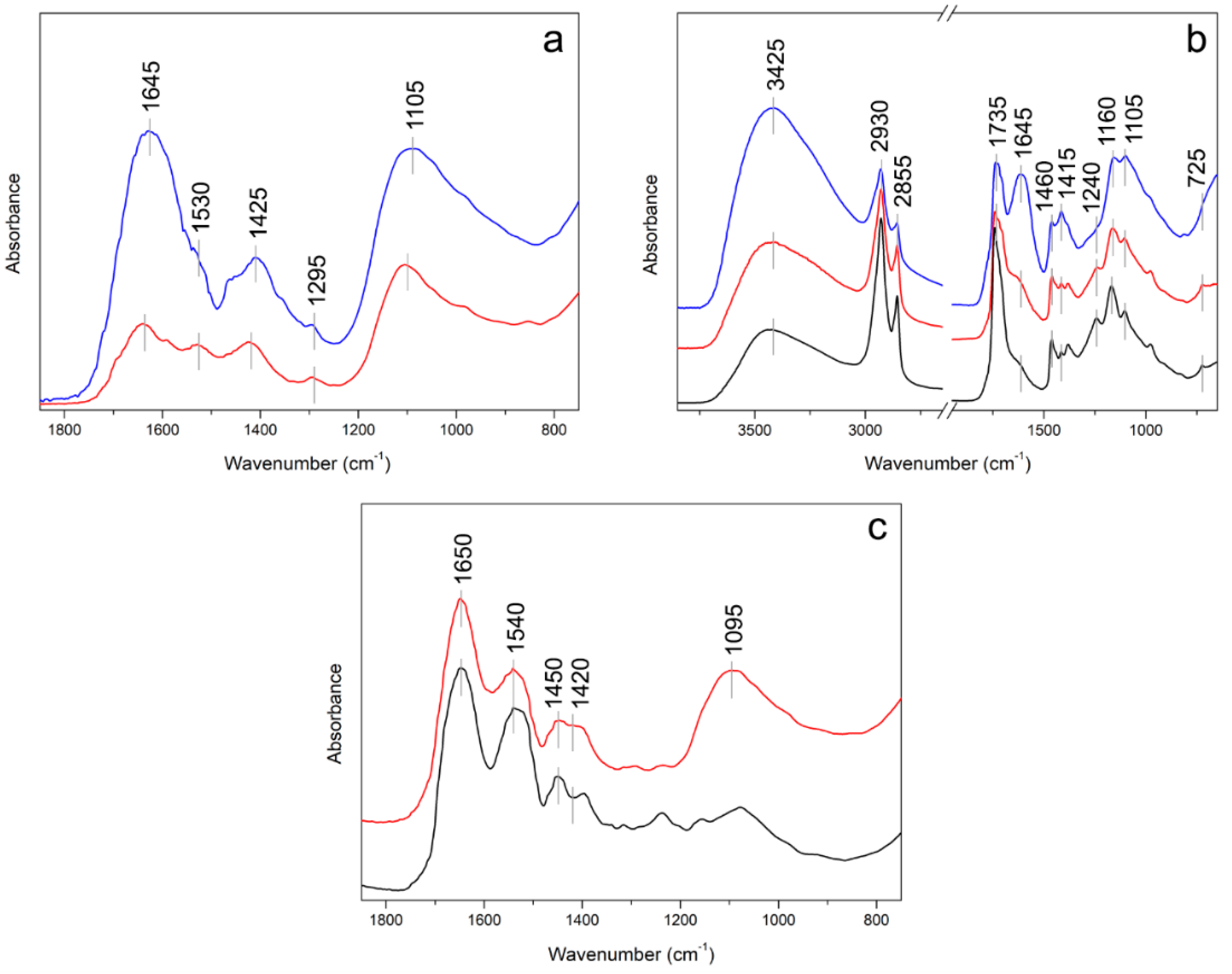
3.5. Infrared Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Echard, J.P.; Lavedrine, B. Review on the characterisation of ancient stringed musical instruments varnishes and implementation of an analytical strategy. J. Cult. Herit. 2008, 9, 420–429. [Google Scholar] [CrossRef]
- Invernizzi, C.; Fichera, G.V.; Licchelli, M.; Malagodi, M. A non-invasive stratigraphic study by reflection FT-IR spectroscopy and UV-induced fluorescence technique: The case of historical violins. Microchem. J. 2018, 138, 273–281. [Google Scholar] [CrossRef]
- Fiocco, G.; Rovetta, T.; Gulmini, M.; Piccirillo, A.; Licchelli, M.; Malagodi, M. Spectroscopic analysis to characterize finishing treatments of ancient bowed string instruments. Appl. Spectrosc. 2017, 71, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Tai, B.H. Stradivari’s Varnish: A review of scientific findings—Part I. J. Violin Soc. Am. 2007, 21, 119–144. [Google Scholar]
- Tai, B.H. Stradivari’s Varnish: A review of scientific findings—Part II. J. Violin Soc. Am. 2009, 22, 1–31. [Google Scholar]
- Bucur, V. The varnish. In Handbook of Materials for String Musical Instruments, 1st ed.; Springer International Publishing: Basel, Switzerland, 2016; pp. 373–453. [Google Scholar]
- Rovetta, T.; Invernizzi, C.; Licchelli, M.; Cacciatori, F.; Malagodi, M. The elemental composition of Stradivari’s musical instruments: New results through non-invasive EDXRF analysis. X-Ray Spectrom. 2018, 47, 159–170. [Google Scholar] [CrossRef]
- Fichera, G.V.; Rovetta, T.; Fiocco, G.; Alberti, G.; Invernizzi, C.; Licchelli, M.; Malagodi, M. Elemental analysis as statistical preliminary study of historical musical instruments. Microchem. J. 2018, 137, 309–317. [Google Scholar] [CrossRef]
- Invernizzi, C.; Daveri, A.; Rovetta, T.; Vagnini, M.; Licchelli, M.; Cacciatori, F.; Malagodi, M. A multi-analytical non-invasive approach to violin materials: The case of Antonio Stradivari “Hellier” (1679). Microchem. J. 2016, 124, 743–750. [Google Scholar] [CrossRef]
- Echard, J.P.; Bertrand, L.; von Bohlen, A.; Le Hô, A.S.; Paris, C.; Bellot-Gurlet, L.; Soulier, B.; Lattuati-Derieux, A.; Thao, S.; Robinet, L.; et al. The nature of the extraordinary finish of Stradivari’s instruments. Angew. Chem. 2010, 49, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Martino, D.F.C.; Saverwyns, S.; Van Bos, M.; Burgio, L.; Di Stefano, C.; Peschke, G.; Caponetti, E. Micro-analytical identification of the components of varnishes from South Italian historical musical instruments by PLM, ESEM-EDX, microFTIR, GC–MS, and Py–GC–MS. Microchem. J. 2014, 116, 31–40. [Google Scholar] [CrossRef]
- Gulmini, M.; Idone, A.; Diana, E.; Gastaldi, D.; Vaudanc, D.; Aceto, M. Identification of dyestuffs in historical textiles: Strong and weak points of a non-invasive approach. Dyes Pigment. 2013, 98, 136–145. [Google Scholar] [CrossRef]
- Casadio, F.; Leona, M.; Lombardi, J.R.; Van Duyne, R. Identification of organic colorants in fibers, paints, and glazes by surface enhanced Raman spectroscopy. Acc. Chem. Res. 2010, 43, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.J.; Claro, A. Bright light: Microspectrofluorimetry for the characterization of lake pigments and dyes in works of art. Acc. Chem. Res. 2010, 43, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Idone, A.; Aceto, M.; Diana, E.; Appolonia, L.; Gulmini, M. Surface-enhanced Raman scattering for the analysis of red lake pigments in painting layers mounted in cross sections. J. Raman Spectrosc. 2014, 45, 1127–1132. [Google Scholar] [CrossRef]
- Bisulca, C.; Picollo, M.; Bacci, M.; Kunzelman, D. UV-Vis-NIR reflectance spectroscopy of red lakes in paintings. In Proceedings of the 9th International Conference of NDT of Art, Jerusalem, Israel, 25–30 May 2008. [Google Scholar]
- Meyer, M.; Huthwelker, T.; Borca, C.N.; Meßlinger, K.; Bieber, M.; Finka, R.H.; Späth, A. In-situ spectroscopic analysis of the traditional dyeing pigment Turkey red inside textile matrix. J. Instrum. 2018, 13, C03007. [Google Scholar] [CrossRef]
- Campanella, B.; Grifoni, E.; Hidalgob, M.; Legnaioli, S.; Lorenzetti, G.; Pagnotta, S.; Poggialini, F.; Ripoll-Seguer, L.; Palleschi, V. Multi-technique characterization of madder lakes: A comparison between non- and micro-destructive methods. J. Cult. Herit. 2018, in press. [Google Scholar] [CrossRef]
- Daniels, V.; Devièse, T.; Hacke, M.; Higgitt, C. Technological insights into madder pigment production in antiquity. Br. Mus. Tech. Res. Bull. 2014, 8, 13–28. [Google Scholar]
- Blackburn, R.S. Natural dyes in madder (Rubia spp.) and their extraction and analysis in historical textiles. Color. Technol. 2017, 133, 449–462. [Google Scholar] [CrossRef]
- Schweppe, H.; Winter, J. Madder and alizarin. In Artists’ Pigments: A Handbook of Their History and Characteristics, 1st ed.; Fitzhugh, E.W., Ed.; National Gallery of Art: Washington, DC, USA; Archetype Publications: London, UK, 1997; Volume 3, pp. 109–142. [Google Scholar]
- Michelman, J. Violin Varnish: A Plausible Re-Creation of the Varnish Used by the Italian Violin Makers Between the Years 1550 and 1750, A.D, 1st ed.; Joseph Michelman: Cincinnati, OH, USA, 1946; pp. 58–154. [Google Scholar]
- Barlow, C.Y.; Woodhouse, J. Of old wood and varnish: Peering into the can of worms. Catgut. Acoust. Soc. J. Ser. II 1989, 1, 2–9. [Google Scholar]
- Tirat, S.; Degano, I.; Echard, J.P.; Lattuati-Derieux, A.; Lluveras-Tenorio, A.; Marie, A.; Serfaty, S.; Le Huerou, J.Y. Historical linseed oil/colophony varnishes formulations: Study of their molecular composition with micro-chemical chromatographic techniques. Microchem. J. 2016, 126, 200–213. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Canevari, C.; Legnani, A.; Licchelli, M.; Malagodi, M.; Ricca, M.; Zeffiro, A. Experimental characterization of oil-colophony varnishes: A preliminary study. Int. J. Conserv. Sci. 2016, 7, 813–826. [Google Scholar]
- UNI EN 15886. Conservation of Cultural Property—Test Methods-Colour Measurement of Surfaces; Czech Office for Standards, Metrology and Testing: Praha, Czech Republic, 2010. [Google Scholar]
- Claro, A.; Melo, M.J.; Schäfer, S.; Seixas de Melo, J.S.; Pina, F.; van den Berg, K.J.; Burnstock, A. The use of microspectrofluorimetry for the characterization of lake pigments. Talanta 2008, 74, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Farke, M.; Binetti, M.; Hahn, O. Light damage to selected organic materials in display cases: A study of different light sources. Stud. Conserv. 2016, 61, 83–93. [Google Scholar] [CrossRef]
- Saunders, D.; Kirby, J. Light-induced colour changes in red and yellow lake pigments. In National Gallery Technical Bulletin, 1st ed.; Roy, A., Ed.; National Gallery Publications Limited: London, UK, 1994; Volume 15, pp. 79–97. [Google Scholar]
- Clementi, C.; Doherty, B.; Gentili, P.L.; Miliani, C.; Romani, A.; Brunetti, B.G.; Sgamellotti, A. Vibrational and electronic properties of painting lakes. Appl. Phys. A 2008, 92, 25–33. [Google Scholar] [CrossRef]
- Mantler, M.; Willis, J.P.; Lachance, G.R.; Vrebos, B.A.R.; Mauser, K.E.; Kawahara, N.; Rousseau, R.M.; Brouwer, P.N. Quantitative analysis in Handbook of Practical X-ray Fluorescence Analysis, 1st ed.; Beckhoff, B., Kanngießer, B., Langhoff, N., Wedell, R., Wolff, H., Eds.; Springer: Berlin, Germany, 2006; pp. 309–410. [Google Scholar]
- Glinsman, L.D. The practical application of air-path X-ray fluorescence spectrometry in the analysis of museum objects. Stud. Conserv. 2005, 50, 3–17. [Google Scholar] [CrossRef]
- Kirby, J.; Spring, M.; Higgitt, M. The technology of red lake pigment manufacture: Study of the dyestuff substrate. In National Gallery Technical Bulletin, 1st ed.; Roy, A., Ed.; National Gallery Company Limited: London, UK, 2005; Volume 26, pp. 71–87. [Google Scholar]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science. Scientific Tools for Conservation, 1st ed.; The Getty Conservation Institute: Los Angeles, CA, USA, 1999; pp. 182–185. [Google Scholar]
- Koperska, M.; Łojewski, T.; Łojewska, J. Vibrational spectroscopy to study degradation of natural dyes. Assessment of oxygen-free cassette for safe exposition of artefacts. Anal. Bioanal. Chem. 2011, 399, 3271–3283. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Obenborf, S.K. Dyes on archaeological textiles: Analyzing alizarin and its degradation products. Text. Res. J. 2004, 74, 949–954. [Google Scholar] [CrossRef]
- Azémard, C.; Vieillescazes, C.; Ménager, M. Effect of photodegradation on the identification of natural varnishes by FT-IR spectroscopy. Microchem. J. 2014, 112, 137–149. [Google Scholar] [CrossRef]
- Invernizzi, C.; Daveri, A.; Vagnini, M.; Malagodi, M. Non-invasive identification of organic materials in historical stringed musical instruments by reflection infrared spectroscopy: A methodological approach. Anal. Bioanal. Chem. 2017, 409, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Degani, L.; Gulmini, M.; Piccablotto, G.; Iacomussi, P.; Gastaldi, D.; Dal Bello, F.; Chiantore, O. Stability of natural dyes under light emitting diode lamps. J. Cult. Herit. 2017, 26, 12–21. [Google Scholar] [CrossRef]
- Fichera, G.V.; Albano, M.; Fiocco, G.; Invernizzi, C.; Licchelli, M.; Malagodi, M.; Rovetta, T. Innovative monitoring plan for the preventive conservation of historical musical instruments. Stud. Conserv. 2017, accepted. [Google Scholar]
- Crews, P.C. The fading rates of some natural dyes. Stud. Conserv. 1987, 32, 65–72. [Google Scholar]
- Echard, J.P. In situ multi-element analyses by Energy-Dispersive X-ray Fluorescence on varnishes of historical violins. Spectrochim. Acta B 2004, 59, 1663–1667. [Google Scholar] [CrossRef]
- Bruni, S.; De Luca, E.; Guglielmi, V.; Pozzi, F. Identification of natural dyes on laboratory-dyed wool and ancient wool, silk, and cotton fibers using attenuated total reflection (ATR) Fourier transform infrared (FT-IR) spectroscopy and Fourier transform Raman spectroscopy. Appl. Spectrosc. 2011, 65, 1017–1023. [Google Scholar] [CrossRef] [PubMed]






| Model Code | Number of Coats | Sequence of the Layers on the Maple Wood |
|---|---|---|
| P1 | – | AC10% |
| P2 | – | AC10% AC15% |
| P3 | 2 | AC10% AC15% ML (7%) in OCV |
| P4 | 2 | AC10% AC15% ML (20%) in OCV |
| P5 | 3 | AC10% ML (1%) in AC15% |
| P6 | 2 | AC10% ML (4%) in AC15% |
| P7 | 3 | AC10% ML (1%) in AC15% OCV |
| P8 | 2 | AC10% ML (4%) in AC15% OCV |
| Suggested Assignment | IR Bands for t = 0 h (cm−1) | IR Bands for t = 520 h (cm−1) | Ref. | ||
|---|---|---|---|---|---|
| νO–H | 3400 | s | 3395 | s | [34,35] |
| νC=O | 1695 | sh | 1695 | sh | [34] |
| νC=O, νC=C | 1640 | m | 1651 | s | [35] |
| νC=C | 1590 | sh | – | – | [34,35] |
| νC=C | 1530 | w | 1530 | sh | [34,35] |
| δC–H2 | 1425 | m | 1425 | m | [34,35] |
| δC–O | 1295 | w | 1300 | w | [34] |
| νS–O | 1105 | s | 1105 | s | [34,35] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiocco, G.; Rovetta, T.; Gulmini, M.; Piccirillo, A.; Canevari, C.; Licchelli, M.; Malagodi, M. Approaches for Detecting Madder Lake in Multi-Layered Coating Systems of Historical Bowed String Instruments. Coatings 2018, 8, 171. https://doi.org/10.3390/coatings8050171
Fiocco G, Rovetta T, Gulmini M, Piccirillo A, Canevari C, Licchelli M, Malagodi M. Approaches for Detecting Madder Lake in Multi-Layered Coating Systems of Historical Bowed String Instruments. Coatings. 2018; 8(5):171. https://doi.org/10.3390/coatings8050171
Chicago/Turabian StyleFiocco, Giacomo, Tommaso Rovetta, Monica Gulmini, Anna Piccirillo, Claudio Canevari, Maurizio Licchelli, and Marco Malagodi. 2018. "Approaches for Detecting Madder Lake in Multi-Layered Coating Systems of Historical Bowed String Instruments" Coatings 8, no. 5: 171. https://doi.org/10.3390/coatings8050171








