Tribological, Tribocorrosion and Wear Mechanism Studies of TaZrN Coatings Deposited by Magnetron Sputtering on TiAlV Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of Films (SEM, XRD, Raman, and Corrosion Studies)
3.2. Tribological Studies in Dry and Corrosive Conditions
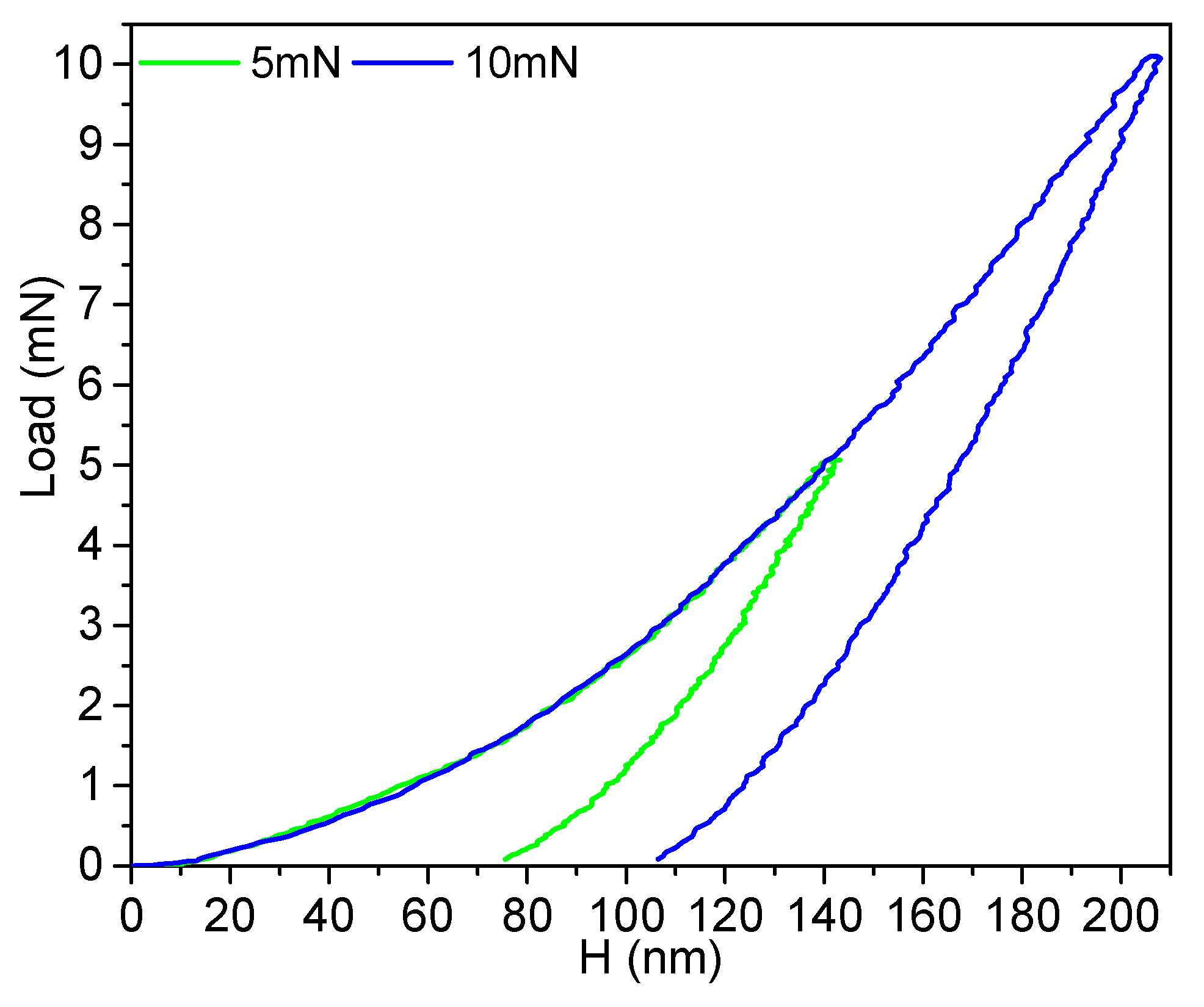
3.2.1 Friction and Wear
3.2.2. Wear Mechanism Analysis at Dry Conditions
3.3. Tribological and Corrosion Studies in Corrosive Media
3.3.1. Friction, Tribocorrosion, and Corrosion Results
3.3.2. Wear Mechanisms in Wet Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inspektor, A.; Salvador, P.A. Architecture of PVD coatings for metalcutting applications: A review. Surf. Coat. Technol. 2014, 257, 138–153. [Google Scholar] [CrossRef]
- Bemporad, E.; Pecchio, C.; De Rossi, S.; Carassiti, F. Characterization and hardness modelling of alternate tin/ticn multilayer cathodic arc PVD coating on tool steel. Surf. Coat. Technol. 2001, 146, 363–370. [Google Scholar] [CrossRef]
- Gautier, C.; Moussaoui, H.; Elstner, F.; Machet, J. Comparative study of mechanical and structural properties of CrN films deposited by DC Magnetron sputtering and vacuum arc evaporation. Surf. Coat. Technol. 1996, 86–87, 254–262. [Google Scholar] [CrossRef]
- Ma, K.; Chao, C.; Liu, D.; Chen, Y.; Shieh, M.B. Friction and wear behaviour of TiN/Au, TiN/MoS2 and TiN/TiCN/a-C:H coatings. J. Mater. Process. Technol. 2002, 127, 182–186. [Google Scholar] [CrossRef]
- Chhowalla, M.; Unalan, H.E. Thin films of hard cubic Zr3N4 stabilized by stress. Nat. Mater. 2005, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Jehn, H.A. Multicomponent and multiphase hard coatings for tribological applications. Surf. Coat. Technol. 2000, 131, 433–440. [Google Scholar] [CrossRef]
- Jozwik, J. Evaluation of tribological properties and condition of Ti6Al4V titanium alloy surface. Tech. Gazette 2018, 25, 170–175. [Google Scholar]
- Münz, W.D. Titanium aluminum nitride films: A new alternative to tin coatings. J. Vac. Sci. Technol. A 1986, 4, 2717–2725. [Google Scholar] [CrossRef]
- Sun, X.; Kolawa, E.; Chen, J.-S.; Reid, J.S.; Nicolet, M.-A. Properties of reactively sputter-deposited TaN thin films. Thin Solid Films 1993, 236, 347–351. [Google Scholar] [CrossRef]
- Alishahi, M.; Mahboubi, F.; Mousavi Khoie, S.M.; Aparicio, M.; Lopez-Elvira, E.; Mendez, J.; Gago, R. Structural properties and corrosion resistance of tantalum nitride coatings produced by reactive DC magnetron sputtering. RSC Adv. 2016, 6, 89061–89072. [Google Scholar] [CrossRef]
- Lee, G.; Kim, H.; Choi, H.; Lee, J. Superhard tantalum-nitride films formed by inductively coupled plasma-assisted sputtering. Surf. Coat. Technol. 2007, 201, 5207–5210. [Google Scholar] [CrossRef]
- Aouadi, S.M. Structural and mechanical properties of tazrn films: Experimental and ab initio studies. J. Appl. Phys. 2006, 99, 053507. [Google Scholar] [CrossRef]
- Yildiz, F.; Yetim, A.F.; Alsaran, A.; Çelik, A. Plasma nitriding behavior of Ti6Al4V orthopedic alloy. Surf. Coat. Technol. 2008, 202, 2471–2476. [Google Scholar] [CrossRef]
- Yetim, A.F.; Alsaran, A.; Efeoglu, I.; Çelik, A. A comparative study: The effect of surface treatments on the tribological properties of Ti–6Al–4V alloy. Surf. Coat. Technol. 2008, 202, 2428–2432. [Google Scholar] [CrossRef]
- Benea, L.; Wenger, F.; Ponthiaux, P.; Celis, J.P. Tribocorrosion behaviour of Ni–SiC nano-structured composite coatings obtained by electrodeposition. Wear 2009, 266, 398–405. [Google Scholar] [CrossRef]
- Azzi, M.; Paquette, M.; Szpunar, J.A.; Klemberg-Sapieha, J.E.; Martinu, L. Tribocorrosion behaviour of DLC-coated 316l stainless steel. Wear 2009, 267, 860–866. [Google Scholar] [CrossRef]
- Probst, J.; Gbureck, U.; Thull, R. Binary nitride and oxynitride PVD coatings on titanium for biomedical applications. Surf. Coat. Technol. 2001, 148, 226–233. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Tepsanongsuk, K.; Tsuji, Y.; Kajikawa, Y.; Ogawa, Y.; Komiyama, H. Preferred orientation and film structure of TaN films deposited by reactive magnetron sputtering. J. Vac. Sci. Technol. A Vac. 2004, 22, 332–338. [Google Scholar] [CrossRef]
- Chang, C.-C.; Jeng, J.S.; Chen, J.S. Microstructural and electrical characteristics of reactively sputtered Ta–N thin films. Thin Solid Films 2002, 413, 46–51. [Google Scholar] [CrossRef]
- Shen, H.; Ramanathan, R. Fabrication of a low resistivity tantalum nitride thin film. Microelectron. Eng. 2006, 83, 206–212. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Geok Ing, N.; Gopalakrishnan, R. Reactive sputter deposition and characterization of tantalum nitride thin films. Mater. Sci. Eng. B 1999, 57, 224–227. [Google Scholar] [CrossRef]
- Tsukimoto, S.; Moriyama, M.; Murakami, M. Microstructure of amorphous tantalum nitride thin films. Thin Solid Films 2004, 460, 222–226. [Google Scholar] [CrossRef]
- Lee, W.-H.; Lin, J.-C.; Lee, C. Characterization of tantalum nitride films deposited by reactive sputtering of Ta in N2/Ar gas mixtures. Mater. Chem. Phys. 2001, 68, 266–271. [Google Scholar] [CrossRef]
- Nie, H.; Xu, S.; Wang, S.; You, L.; Yang, Z.; Ong, C.K.; Li, J.; Liew, T.Y.F. Structural and electrical properties of tantalum nitride thin films fabricated by using reactive radio-frequency magnetron sputtering. Appl. Phys. A 2001, 73, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Cha, B.C. Deposition of tantalum nitride thin films by DC Magnetron sputtering. Thin Solid Films 2005, 475, 202–207. [Google Scholar] [CrossRef]
- Abadias, G. Stress and preferred orientation in nitride-based PVD coatings. Surf. Coat. Technol. 2008, 202, 2223–2235. [Google Scholar] [CrossRef]
- Linder, C.; Dommann, A.; Staufert, G.; Nicolet, M.A. Ternary Ta–Si–N films for sensors and actuators. Sens. Actuators A Phys. 1997, 61, 387–391. [Google Scholar] [CrossRef]
- Sandu, C.S.; Sanjinés, R.; Benkahoul, M.; Medjani, F.; Lévy, F. Formation of composite ternary nitride thin films by magnetron sputtering co-deposition. Surf. Coat. Technol. 2006, 201, 4083–4089. [Google Scholar] [CrossRef]
- Abadias, G.; Kanoun, M.B.; Goumri-Said, S.; Koutsokeras, L.; Dub, S.N.; Djemia, P. Electronic structure and mechanical properties of ternary ZrTaN alloys studied by ab initio calculations and thin-film growth experiments. Phys. Rev. B 2014, 90, 144107. [Google Scholar] [CrossRef]
- Valleti, K. Studies on hard tan thin film deposition by R C-Mag technique. J. Vac. Sci. Technol. A 2009, 27, 626–630. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, C.; Lee, J. Effects of processing variables on the mechanical properties of Ta/TaN multilayer coatings. Mater. Sci. Eng. B 2000, 75, 17–23. [Google Scholar] [CrossRef]
- Kim, D.-k.; Lee, H.; Kim, D.; Keun Kim, Y. Electrical and mechanical properties of tantalum nitride thin films deposited by reactive sputtering. J. Cryst. Growth 2005, 283, 404–408. [Google Scholar] [CrossRef]
- Mody, N.R.; Hwang, R.Q.; Venka-taraman, S.; Angelo, J.E.; Norwood, D.P.; Gerberich, W.W. Adhesion and fracture of tantalum nitride films. Acta Mater. 1998, 46, 585–597. [Google Scholar] [CrossRef]
- Westergård, R.; Bromark, M.; Larsson, M.; Hedenqvist, P.; Hogmark, S. Mechanical and tribological characterization of DC magnetron sputtered tantalum nitride thin films. Surf. Coat. Technol. 1997, 97, 779–784. [Google Scholar] [CrossRef]
- Musil, J. Hard nanocomposite coatings: Thermal stability, oxidation resistance and toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Constable, C.P.; Yarwood, J.; Münz, W.D. Raman microscopic studies of PVD hard coatings. Surf. Coat. Technol. 1999, 116–119, 155–159. [Google Scholar] [CrossRef]
- Constable, C.P.; Lewis, D.B.; Yarwood, J.; Münz, W.D. Raman microscopic studies of residual and applied stress in PVD hard ceramic coatings and correlation with X-ray diffraction (XRD) measurements. Surf. Coat. Technol. 2004, 184, 291–297. [Google Scholar] [CrossRef]
- Blanpain, B.; Franck, M.; Mohrbacher, H.; Vancoille, E.; Celis, J.P.; Roos, J.R. Micro-Raman spectroscopy for the characterization of wear induced surface modifications on hard coatings. Tribol. Ser. 1993, 25, 623–630. [Google Scholar]
- Jallad, K.N.; Ben-Amotz, D. Raman chemical imaging of tribological nitride coated (TiN, TiAlN) surfaces. Wear 2002, 252, 956–969. [Google Scholar] [CrossRef]
- Biswas, S.K.; Arvind Singh, R. Wear of metals—Influence of some primary material properties. Tribol. Lett. 2002, 13, 203–207. [Google Scholar] [CrossRef]
- Holmberg, K.; Ronkainen, H.; Matthews, A. Tribology of thin coatings. Ceram. Int. 2000, 26, 787–795. [Google Scholar] [CrossRef]
- Sánchez-López, J.C.; Martínez-Martínez, D.; Abad, M.D.; Fernández, A. Metal carbide/amorphous C-based nanocomposite coatings for tribological applications. Surf. Coat. Technol. 2009, 204, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Aouadi, S.M.; Luster, B.; Kohli, P.; Muratore, C.; Voevodin, A.A. Progress in the development of adaptive nitride-based coatings for high temperature tribological applications. Surf. Coat. Technol. 2009, 204, 962–968. [Google Scholar] [CrossRef]
- Neuhaeuser, M.; Hilgers, H.; Joeris, P.; White, R.; Windeln, J. Raman spectroscopy measurements of DC-magnetron sputtered carbon nitride (a-C:N) thin films for magnetic hard disk coatings. Diam. Relat. Mater. 2000, 9, 1500–1505. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Liao, Y.; Pourzal, R.; Wimmer, M.A.; Jacobs, J.J.; Fischer, A.; Marks, L.D. Graphitic tribological layers in metal-on-metal hip replacements. Science 2011, 334, 1687–1690. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.E.; Marks, L.D. Graphitic carbon films across systems. Tribol. Lett. 2016, 63, 32. [Google Scholar] [CrossRef]
- Rivera-Tello, C.D.; Broitman, E.; Flores-Ruiz, F.J.; Jiménez, O.; Flores, M. Mechanical properties and tribological behavior at micro and macro-scale of WC/WCN/W hierarchical multilayer coatings. Tribol. Int. 2016, 101, 194–203. [Google Scholar] [CrossRef]
- Álvarez, R.; Palmero, A.; Prieto-López, L.O.; Yubero, F.; Cotrino, J.; de la Cruz, W.; Rudolph, H.; Habraken, F.H.P.M.; Gonzalez-Elipe, A.R. Morphological evolution of pulsed laser deposited ZrO2 thin films. J. Appl. Phys. 2010, 107, 054311. [Google Scholar] [CrossRef]
- Barbéris, P.; Corolleur-Thomas, G.; Guinebretière, R.; Merle-Mejean, T.; Mirgorodsky, A.; Quintard, P. Raman spectra of tetragonal zirconia: Powder to zircaloy oxide frequency shift. J. Nucl. Mater. 2001, 288, 241–247. [Google Scholar] [CrossRef]
- Dobal, P.S.; Katiyar, R.S.; Jiang, Y.; Guo, R.; Bhalla, A.S. Raman scattering study of a phase transition in tantalum pentoxide. J. Raman Spectrosc. 2000, 31, 1061–1065. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kim, H.-M.; Kokubo, T.; Kato, H.; Nakamura, T. Induction and acceleration of bonelike apatite formation on tantalum oxide gel in simulated body fluid. J. Sol-Gel Sci. Technol. 2001, 21, 83–88. [Google Scholar] [CrossRef]
- Mathew, M.T.; Runa, M.J.; Laurent, M.; Jacobs, J.J.; Rocha, L.A.; Wimmer, M.A. Tribocorrosion behavior of cocrmo alloy for hip prosthesis as a function of loads: A comparison between two testing systems. Wear 2011, 271, 1210–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso Gil, R.; Igual Muñoz, A. Influence of the sliding velocity and the applied potential on the corrosion and wear behavior of HC CoCrMo biomedical alloy in simulated body fluids. J. Mech. Behav. Biomed. Mater. 2011, 4, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Mendizabal, L.; Lopez, A.; Bayón, R.; Herrero-Fernandez, P.; Barriga, J.; Gonzalez, J.J. Tribocorrosion response in biological environments of multilayer TaN films deposited by HPPMS. Surf. Coat. Technol. 2016, 295, 60–69. [Google Scholar] [CrossRef]
- Musil, J. Hard and superhard nanocomposite coatings. Surf. Coat. Technol. 2000, 125, 322–330. [Google Scholar] [CrossRef]













© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, E.; Flores, M.; Rodríguez, E.; Rivera, L.P.; Camps, E.; Muhl, S. Tribological, Tribocorrosion and Wear Mechanism Studies of TaZrN Coatings Deposited by Magnetron Sputtering on TiAlV Alloy. Coatings 2018, 8, 295. https://doi.org/10.3390/coatings8090295
García E, Flores M, Rodríguez E, Rivera LP, Camps E, Muhl S. Tribological, Tribocorrosion and Wear Mechanism Studies of TaZrN Coatings Deposited by Magnetron Sputtering on TiAlV Alloy. Coatings. 2018; 8(9):295. https://doi.org/10.3390/coatings8090295
Chicago/Turabian StyleGarcía, Ernesto, Martín Flores, Eduardo Rodríguez, Laura P. Rivera, Enrique Camps, and Stephen Muhl. 2018. "Tribological, Tribocorrosion and Wear Mechanism Studies of TaZrN Coatings Deposited by Magnetron Sputtering on TiAlV Alloy" Coatings 8, no. 9: 295. https://doi.org/10.3390/coatings8090295
APA StyleGarcía, E., Flores, M., Rodríguez, E., Rivera, L. P., Camps, E., & Muhl, S. (2018). Tribological, Tribocorrosion and Wear Mechanism Studies of TaZrN Coatings Deposited by Magnetron Sputtering on TiAlV Alloy. Coatings, 8(9), 295. https://doi.org/10.3390/coatings8090295




