3.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Chlorophyllins
Antimicrobial activity of E-140 and E-141 chlorophyllins was tested against
L. monocytogenes and
E. coli using two types of irradiation: halogen and LED lights. The results obtained are shown in
Table 1 (halogen light) and
Table 2 (LED light).
The first important data were that the MIC values were higher against
E. coli than against
L. monocytogenes. In general, Gram-positive bacteria are more sensitive than Gram-negative bacteria to chemical compounds owing to the protection of the outer membrane surrounding the Gram-negative cell wall, which restricts the diffusion of antimicrobial substances [
12,
13]. Caires et al. [
14] reported on the antimicrobial activity of two photosensitizers—eosin methylene blue and chlorophyllin sodium copper salt (E-141)—and showed that eosin methylene blue was able to photoinactivate
E. coli and
S. aureus, while E-141 was only active against
S. aureus. In that test, the maximum concentration tested was 50 µM, which was much lower than the 4.15 mM obtained in this study. However, it is difficult to compare the published results because the important parameters of the photoactivation process—intensity, time, and type of radiation—were either different or were not provided. In this test, 15 min was enough to induce photoactivation, much less than the 120 min employed elsewhere [
14].
It is also important to note that although both chlorophyllins presented high antimicrobial activity, porphyrin E-140 was much more effective (with much lower MIC and MBC values) than E-141. With regard to the type of photoactivation, halogen lights were more effective than LED lights. The use of LEDs slightly reduced the efficacy of E-140 against
L. monocytogenes and
E. coli. Other authors have reported the antimicrobial effectiveness of zinc complexes of tetrakis (
N-methylpyridinium-4-yl) tetraiodide porphyrin and tetrakis (
N-methylpyridinium-4-yl) tetraiodide phthalocyanine impregnated in paper using an inexpensive consumer LED lamp as activation mechanism [
9].
In the case of E-141, porphyrin appeared to be inactive when exposed to the radiation of LED lamps, and neither the MBC nor the MIC of the two tested bacteria was found. Our hypothesis is that the LED lamps employed did not emit radiation of sufficient intensity in the wavelength range necessary for E-141 activation. Moreover, copper chlorophyillins have been reported to present low yield of singlet oxygen [
15].
Finally, two control tests were carried out. In one, the two microorganisms were exposed to the chlorophyllins in darkness; in the other, they were exposed to radiation without porphyrin. The two microorganisms tested reflected the absence of antimicrobial effect, indicating that chlorophyllins were nontoxic for the two model bacteria, and light radiation alone did not produce antimicrobial activity.
3.2. Development of Coated Films
G, PE, PVOH, and HPMC coatings on PET film with and without 1% of E-140 or E-141 were successfully obtained. They were homogeneous, without discontinuities, flexible, transparent, had a light green color when the chlorophyllins were added, and with thicknesses that are shown in
Table 3. As can be seen, the coating thicknesses varied according to the polymer material as a consequence of the different solid content of the film-forming solution and the polymer density; the PE-based materials were the thickest, while PVOH and HPMC were the thinnest. The incorporation of chlorophyllins in the coatings did not significantly affect thickness.
The most significant effect of the addition of the chlorophyllins to the coatings was the yellowish (E-140) or greenish (E-141) color induced.
Table 4 shows the color coordinates in the CIELAB system for different coatings, including chromaticity (
C*) and tone (
h). As can be seen, in general, the coated films presented high luminosity as revealed by the
L* values ranging between 83 and 90. The only exceptions were the coatings based on PE-incorporated chlorophyllins, which presented a considerable reduction in luminosity. The addition of E-140 provided coatings with a yellowish color, which is characterized in CIELAB coordinates by positive
b* values and low negative
a* values. As a consequence of this, the tone of the coatings was characterized by values ranging between 95 and 110°. The incorporation of E-141 provided a greener color, with higher negative
a* values and positive
b* and tone values ranging between 107 and 135°. With respect to saturation or chromaticity, the values were greater for E-141 samples than for E-140. Comparing the polymeric materials,
C* values were greatest for the thickest material (PE).
Figure 2 shows, as examples, photos of the different coatings. As can be seen, in the PE coatings there was a clearly visible alteration of the color of the material after incorporation of both chlorophyllins. On the other hand, the color of the HPMC coatings with E-140 or E-141 was hardly distinguishable from the control.
3.4. Migration of Chlorophyllins from Coatings to the Food Simulant
The release of chlorophyllin E-140 was studied in 50% ethanol as a fatty food simulant in accordance with Directive 85/572/EEC prior to application on bologna slices. The regulation stipulates that the results of overall migration obtained must be in accordance with the overall migration limit of 10 mg/dm
2 established in Directive 2002/72/EC and in Royal Decree 866/2008, which are both related to materials and plastic objects in contact with food products. The results are shown in
Figure 4.
The release of chlorophyllins from G, PVOH, and HPMC coatings on PET was very low—practically zero—indicating that there was no substantial migration. This means the antimicrobial effect was produced by the generation of free radicals and it was not necessary for the chlorophyllins to migrate to the simulating medium. On the other hand, the PE coatings presented a maximum release of 6.4 mg/dm2 (according to the surface of each coating, which was 0.125 dm2), which was less than the overall migration limit set by legislation (10 mg/dm2).
In the case of the other coatings, the low migration obtained makes it unnecessary to calculate migration limits, and therefore they can be used as packaging systems for fatty food. In this case, they were tested with bologna slices. The fact that the greatest release was observed in PE could be due to several factors. First, the thickness of the PE coating—well above 25 µm—results in a large amount of porphyrin in absolute values. Second, the poor affinity of LDPE for chlorophyllins might result in their separation, forming a two-phase matrix in which the agent is isolated in small regions dispersed in the pure LDPE matrix. When the material comes into contact with a solvent medium, the porphyrin located close to the surface is immediately released, as observed in
Figure 4. In the other matrices there would be specific interactions between E-140 and the polymers, keeping the agent in the package where porphyrin molecules have a compatible chemical environment. Similar release results were observed for films containing E-141 (data not shown).
3.5. Application to Food
Once the antimicrobial and self-sanitizing capacity of the films had been determined, their effectiveness when applied to a real food was studied. The experiment was carried out with G, HPMC, PE, and PVOH coatings on PET films used as bologna slice separators.
This food product was chosen to test the coatings developed because it has a high water activity (
aw = 0.970) and is therefore very susceptible to spoilage by microorganisms. The antimicrobial effect of the films was studied against
L. monocytogenes, which was inoculated on the bologna surface and against the usual microbial load of this meat product. The results are shown in
Figure 5.
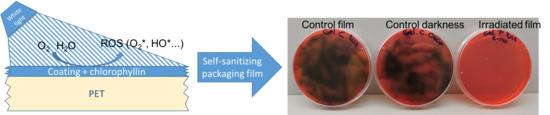
There was no microbial growth of
Salmonella spp., enterobacteria, or coliform bacteria on any sample. Control coatings and coatings kept in darkness showed no antimicrobial effect, confirming the nontoxicity of the active agents without photoactivation. However, coatings with E-140 exposed to LED lights inhibited microbial growth of
L. monocytogenes successfully. The greatest antimicrobial effect was observed with the G coating and the least with the PE coating. The HPMC and PVOH coatings were slightly less effective than G (
Figure 5).
Growth of the microbial load was also inhibited in irradiated samples with chlorophyllin. Lactic, mesophilic, and psychrophilic bacteria and Pseudomonas were significantly reduced. Once again, the G coatings presented the greatest antimicrobial effect against all the microorganisms tested. The highest efficiency was observed against psychrophiles and mesophiles, with reductions of 2.87 and 2.30 log, respectively. In the case of lactic acid bacteria and Pseudomonas, although the degree of inhibition was lower, it was also considerable. The HPMC and PVOH coatings with chlorophyllin presented similar degrees of inhibition against all the microorganisms studied, ranging between 0.76 and 1 log. Finally, the antimicrobial effect of the PE coatings was lower, ranging between approximately 0.24 and 0.69 log.
Results showed that all the coatings developed in this study had antimicrobial activity, with gelatin being the most effective matrix. This is possibly due to its morphology that facilitates the release of free radicals produced by the porphyrin molecule, which is responsible for the antimicrobial action. It should also be taken into account that the G coatings were thicker, and therefore there was a greater amount of agent in absolute values than in the HPMC and PVOH coatings. The application of the gelatin coating would be very useful given gelatin is a component that is usually present in many foods and does not constitute a risk when it forms part of the surface of a package intended for food use.
To confirm the absence of effects on the organoleptic properties of the product and because the release of chlorophyllin could produce a green color, the color of the bologna slices was measured before and after the photoactivation treatment. The parameters
L* [black (0) to white (100)],
a* [green (−) to red (+)], and
b* [blue (−) to yellow (+)] were obtained, and the polar coordinates, chroma
C*, and hue angle
h that were calculated are shown in
Table 6.
The bologna slices were displaced towards the + a coordinate, i.e., towards red and slightly towards the + b coordinate (yellow), which is logical given their rosy hue.
In general, irradiation produced a slight decrease in the luminosity of the bologna slices and a slight shift to the left of the
b* coordinates. However, these color modifications were not distinguishable to the naked eye. On the other hand, no color difference was observed between samples with and without porphyrin submitted to the same treatment (darkness or light). Therefore, from the data shown above, it can be confirmed that no substantial release of porphyrin to the bologna took place. Similar results were reported in a study of chlorophyllin gelatin films applied as wraps to frankfurter sausages, which found no substantial differences between uncoated and coated products [
2].
Finally, it can be concluded that chlorophyllin-based photosensitization of coated films is an effective way of reducing the population of microorganisms naturally present in meat and poultry products and as a way of improving asepsis of packaging materials through their self-sanitizing function. Moreover, this efficiency has also been demonstrated when light is applied through the supporting material (in this case, PET) if the material is transparent to visible light. This aspect is of great importance because it would greatly facilitate carrying out its antimicrobial or self-sanitizing function in food products that have already been packaged. The process that has been developed in this study, which requires only white light, could become an alternative food process that is nonchemical, nonthermal, inexpensive, and environmentally friendly.
The films developed could be applied as part of a package intended for meat derivatives—either as an external protective cover or as separators of cold cuts—or in some dairy products, such as fresh cheese. In addition, the films developed could be used to wrap food that are sensitive to microbial contamination in daily use, such as pieces of cooked ham that are not immediately consumed completely but are rather manipulated (cut) on a number of occasions before being completely consumed, therefore making them potential vectors for the transmission of microorganisms. These films could be used to cover the cut surface and protect it from microbial spoilage thanks to the photoactivation induced by the white lights of refrigerated displays in grocery stores. Furthermore, this active packaging does not require any agent release, thereby increasing food shelf life without substantial changes in aroma, flavor, or color.