Investigation of Using Sol-Gel Technology for Corrosion Protection Coating Systems Incorporating Colours and Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Inhibitor Loading Process
2.3. Coating Preparation
2.4. Characterizations
3. Results
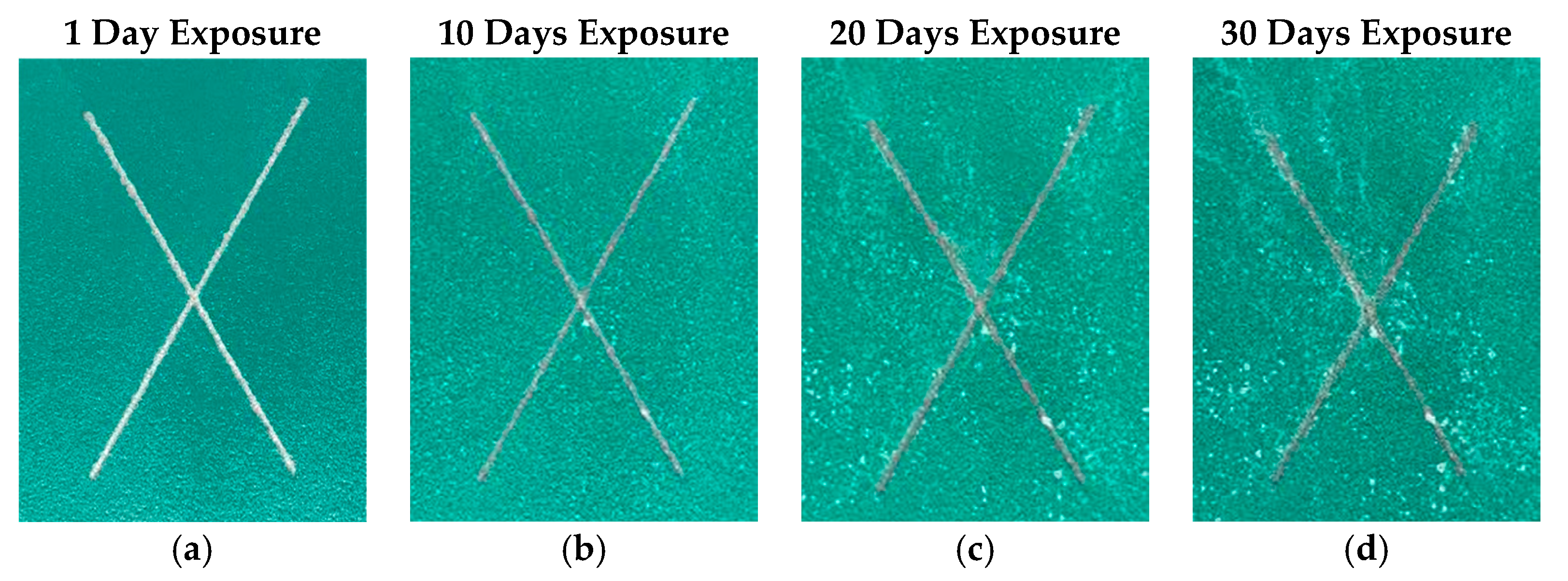
3.1. Evaluation of Commercial Zinc Rich Coating System
3.2. Two-Coat System with Sol-Gel Topcoat
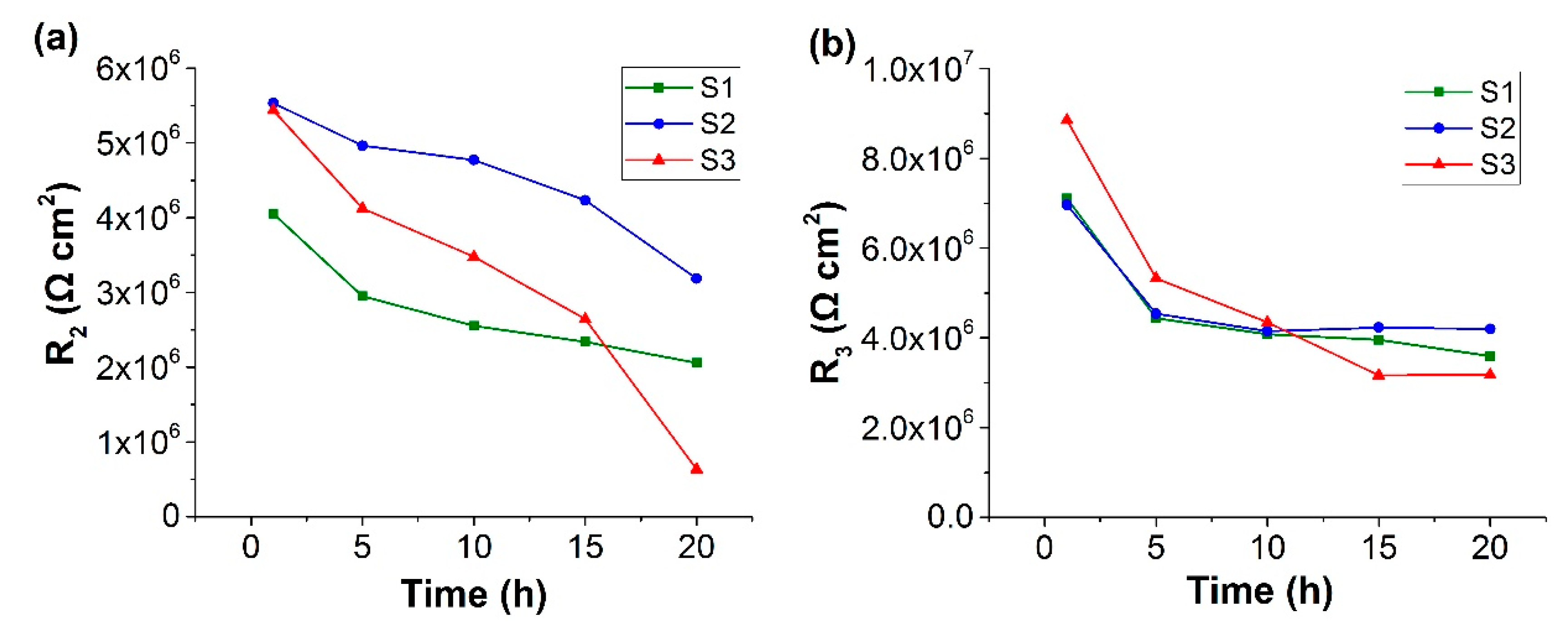
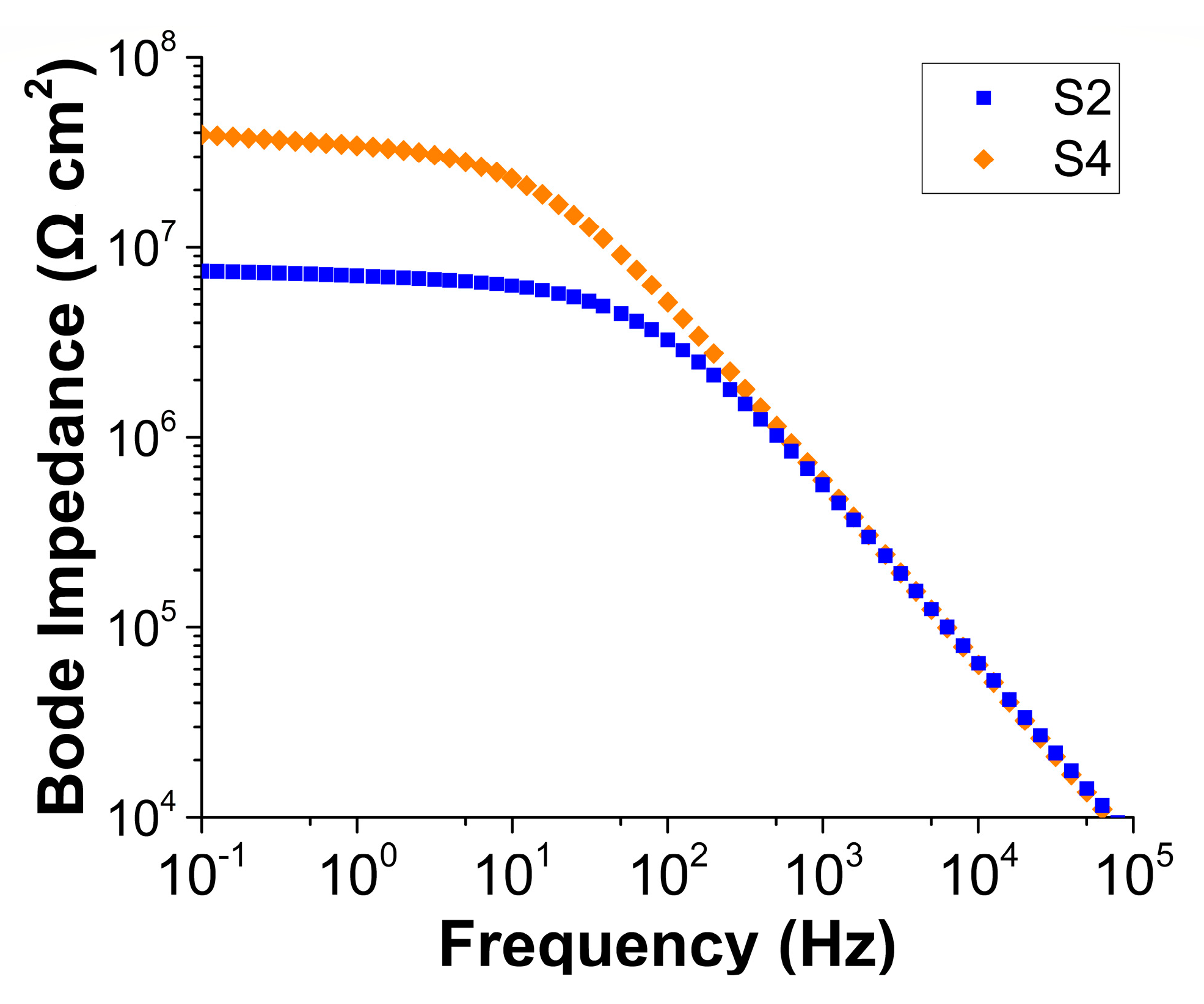
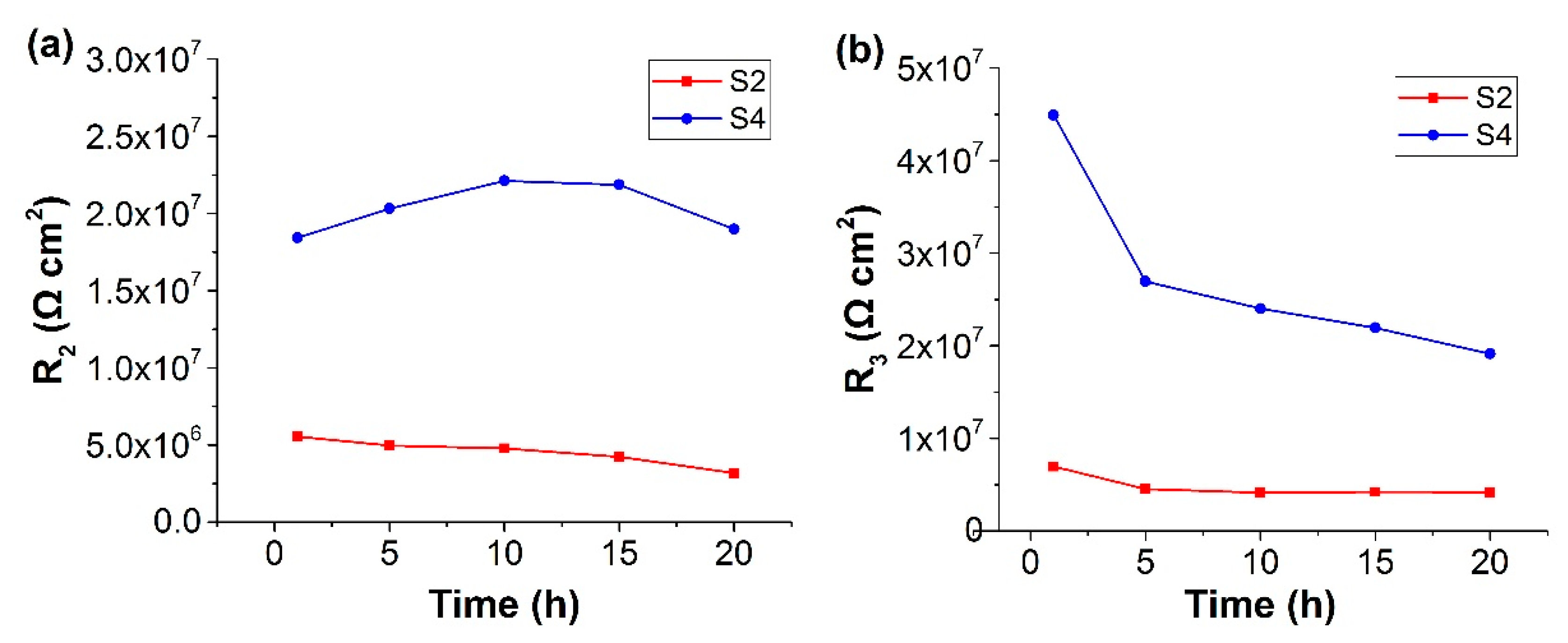
3.3. Electrochemical Impedance Spectroscopy (EIS) Study on Ce-BTN/Mo-HT Incorporated Sol-Gel Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured sol–gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3: Corrosion protection performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Joshua Du, Y.; Damron, M.; Tang, G.; Zheng, H.; Chu, C.-J.; Osborne, J.H. Inorganic/organic hybrid coatings for aircraft aluminum alloy substrates. Prog. Org. Coat. 2001, 41, 226–232. [Google Scholar] [CrossRef]
- Hofacker, S.; Mechtel, M.; Mager, M.; Kraus, H. Sol–gel: A new tool for coatings chemistry. Prog. Org. Coat. 2002, 45, 159–164. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R.; Gobara, M. Scratch-resistant anticorrosion sol–gel coating for the protection of AZ31 magnesium alloy via a low temperature sol-gel route. Corros. Sci. 2010, 52, 2565–2570. [Google Scholar] [CrossRef]
- Prado, R.; Beobide, G.; Marcaide, A.; Goikoetxea, J.; Aranzabe, A. Development of multifunctional sol-gel coatings: Anti-reflection coatings with enhanced self-cleaning capacity. Sol. Energy Mater. Sol. Cells 2010, 94, 1081–1088. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Guglielmi, M. Sol-gel coatings on metals. J. Sol-Gel Sci. Technol. 1997, 8, 443–449. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Mahadik, S.A.; Kappenstein, C. Mechanically stable and corrosion resistant superhydrophobic sol-gel coatings on copper substrate. Appl. Surf. Sci. 2011, 257, 5772–5776. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Möhwald, H.; Ferreira, M.G.S. Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Akbarian, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. Evaluation of the corrosion protection performance of mild steel coated with hybrid sol-gel silane coating in 3.5 wt.% NaCl solution. Prog. Org. Coat. 2018, 123, 190–200. [Google Scholar] [CrossRef]
- Tan, A.L.K.; Soutar, A.M.; Annergren, I.F.; Liu, Y.N. Multilayer sol-gel coatings for corrosion protection of magnesium. Surf. Coat. Technol. 2005, 198, 478–482. [Google Scholar] [CrossRef]
- Shen, G.X.; Chen, Y.C.; Lin, C.J. Corrosion protection of 316L stainless steel by a TiO2 nanoparticle coating prepared by sol-gel method. Thin Solid Films 2005, 489, 130–136. [Google Scholar] [CrossRef]
- Vijayalakshmi, U.; Rajeswari, S. Synthesis and characterization of sol-gel derived glass-ceramic and its corrosion protection on 316L SS. J. Sol-Gel Sci. Technol. 2007, 43, 251–258. [Google Scholar] [CrossRef]
- Jianguo, L.; Gaoping, G.; Chuanwei, Y. Enhancement of the erosion–corrosion resistance of Dacromet with hybrid SiO2 sol-gel. Surf. Coat. Technol. 2006, 200, 4967–4975. [Google Scholar] [CrossRef]
- Fedel, M.; Poelman, M.; Zago, M.; Vandermiers, C.; Cossement, D.; Olivier, M.-G.; Deflorian, F. Influence of formulation and application parameters on the performances of a sol-gel/clay nanocomposite on the corrosion resistance of hot-dip galvanized steel. Part II. Effect of curing temperature and time. Surf. Coat. Technol. 2015, 274, 9–17. [Google Scholar] [CrossRef]
- Mrad, M.; Dhouibi, L.; Montemor, M.F. Elaboration of γ-glycidoxypropyltrimethoxysilane coating on AA2024-T3 aluminum alloy: Influence of synthesis route on physicochemical and anticorrosion properties. Prog. Org. Coat. 2018, 121, 1–12. [Google Scholar] [CrossRef]
- Mrad, M.; Ben Amor, Y.; Dhouibi, L.; Montemor, M.F. Effect of AA2024-T3 surface pretreatment on the physicochemical properties and the anticorrosion performance of poly(γ-glycidoxypropyltrimethoxysilane) sol-gel coating. Surf. Interface Anal. 2017, 50, 335–345. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, M.; Liu, J.; Li, S.; Xue, B.; Liang, M. Effect of surface roughness on corrosion resistance of sol-gel coatings on AA2024-T3 alloy. J. Electrochem. Soc. 2015, 162, C718–C724. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Carneiro, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Influence of sol-gel process parameters on the protection properties of sol–gel coatings applied on AA2024. Surf. Coat. Technol. 2014, 246, 6–16. [Google Scholar] [CrossRef]
- Barranco, V.; Feliu, S., Jr.; Galvan, J.C.; Carmona, N.; Sanchez-Majado, S.; Jimenez-Morales, A. Cerium doped hybrid silica sol-gel coatings with selfhealing properties for corrosion protection of mild steel. In Proceedings of the 17th International Corrosion Congress 2008: Corrosion Control in the Service of Society, Las Vegas, NV, USA, 6–10 October 2008; Volume 5, pp. 3197–3229. [Google Scholar]
- Baldin, E.K.K.; Kunst, S.R.; Beltrami, L.V.R.; Lemos, T.M.; Quevedo, M.C.; Bastos, A.C.; Ferreira, M.G.S.; Santos, P.R.R.; Sarmento, V.H.V.; Malfatti, C.D.F. Ammonium molybdate added in hybrid films applied on tinplate: Effect of the concentration in the corrosion inhibition action. Thin Solid Films 2016, 600, 146–156. [Google Scholar] [CrossRef]
- Maia, F.; Yasakau, K.A.; Carneiro, J.; Kallip, S.; Tedim, J.; Henriques, T.; Cabral, A.; Venâncio, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion protection of AA2024 by sol-gel coatings modified with MBT-loaded polyurea microcapsules. Chem. Eng. J. 2016, 283, 1108–1117. [Google Scholar] [CrossRef]
- Dias, S.A.S.; Lamaka, S.V.; Diamantino, T.C.; Ferreira, M.G.S. Synergistic protection against corrosion of aa2024-t3 by sol-gel coating modified with La and Mo-enriched zeolites. J. Electrochem. Soc. 2014, 161, C215–C222. [Google Scholar] [CrossRef]
- Trabelsi, W.; Cecilio, P.; Ferreira, M.G.S.; Montemor, M.F. Electrochemical assessment of the self-healing properties of Ce-doped silane solutions for the pre-treatment of galvanised steel substrates. Prog. Org. Coat. 2005, 54, 276–284. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Montemor, M.F. “SMART” protective ability of water based epoxy coatings loaded with CaCO3 microbeads impregnated with corrosion inhibitors applied on AA2024 substrates. Electrochim. Acta 2012, 83, 439–447. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Carneiro, J.; Caetano, A.F.; Kuznetsova, A.; Maia, F.; Salak, A.N.; Tedim, J.; Scharnagl, N.; Zheludkevich, M.L.; Ferreira, M.G.S. Polyelectrolyte-modified layered double hydroxide nanocontainers as vehicles for combined inhibitors. RSC Adv. 2015, 5, 39916–39929. [Google Scholar] [CrossRef] [Green Version]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S.; Montemor, M.F. Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Figiel, P.; Jedrzejewski, R.; Biedunkiewicz, A.; Castro, Y.; Aparicio, M.; Pellice, S.A.; Durán, A. Influence of cerium concentration on the structure and properties of silica-methacrylate sol-gel coatings. J. Sol-Gel Sci. Technol. 2010, 54, 301–311. [Google Scholar] [CrossRef]
- Trenado, C.; Wittmar, M.; Veith, M.; Rosero-Navarro, N.C.; Aparicio, M.; Durán, A.; Castro, Y.; Strauss, D.J. Multiscale numerical modeling of Ce3+-inhibitor release from novel corrosion protection coatings. Model. Simul. Mater. Sci. Eng. 2011, 19, 25009. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Pellice, S.A.; Durán, A.; Aparicio, M. Effects of Ce-containing sol-gel coatings reinforced with SiO2 nanoparticles on the protection of AA2024. Corros. Sci. 2008, 50, 1283–1291. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Pellice, S.A.; Durán, A.; Ceré, S.; Aparicio, M. Corrosion protection of aluminium alloy AA2024 with cerium doped methacrylate-silica coatings. J. Sol-Gel Sci. Technol. 2009, 52, 31–40. [Google Scholar] [CrossRef]
- Bohm, S.; McMurray, H.N.; Worsley, D.A.; Powell, S.M. Novel environment friendly corrosion inhibitor pigments based on naturally occurring clay minerals. Mater. Corros. 2001, 52, 896–903. [Google Scholar] [CrossRef]
- ISO 8501-1:2007 Preparation of Steel Substrates before Application of Paints and Related Products—Visual Assessment of Surface Cleanliness—Part 1: Rust Grades and Preparation Grades of Uncoated Steel Substrates and of Steel Substrates after Overall Removal of Previous Coatings; ISO: Geneva, Switzerland, 2007; Available online: https://www.iso.org/obp/ui/#iso:std:iso:8501:-1:ed-2:v1:en (accessed on 30 November 2018).
- Wu, L.Y.L.; Chwa, E.; Chen, Z.; Zeng, X.T. A study towards improving mechanical properties of sol-gel coatings for polycarbonate. Thin Solid Films 2008, 516, 1056–1062. [Google Scholar] [CrossRef]
- ASTM D3363 - 05(2011)e2 Standard Test Method for Film Hardness by Pencil Test; ASTM International: West Conshohocken, PA, USA, 2011; Available online: https://www.astm.org/Standards/D3363.htm (accessed on 30 November 2018).
- ASTM D3359-17 Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2017; Available online: https://www.astm.org/Standards/D3359.htm (accessed on 30 November 2018).
- Mackenzie, J.D.; Bescher, E.P. Physical properties of sol-gel coatings. J. Sol-Gel Sci. Technol. 2000, 19, 23–29. [Google Scholar] [CrossRef]
- Moutarlier, V.; Neveu, B.; Gigandet, M.P. Evolution of corrosion protection for sol-gel coatings doped with inorganic inhibitors. Surf. Coat. Technol. 2008, 202, 2052–2058. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Karavai, O.V.; Ferreira, M.G.S. Influence of inhibitor addition on the corrosion protection performance of sol-gel coatings on AA2024. Prog. Org. Coat. 2008, 63, 352–361. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R. Encapsulated cerium nitrate inhibitors to provide high-performance anti-corrosion sol-gel coatings on mild steel. Corros. Sci. 2008, 50, 1142–1148. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; Tan, Q.; Jiang, L. Chloride absorption by nitrate, nitrite and aminobenzoate intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 5908–5916. [Google Scholar] [CrossRef]
- Mahajanam, S.P.V. Application of Hydrotalcites as Corrosion-Inhibiting Pigments in Organic Coatings. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2005. [Google Scholar]
- Garcia-Heras, M.; Jimenez-Morales, A.; Casal, B.; Galvan, J.C.; Radzki, S.; Villegas, M.A. Preparation and electrochemical study of cerium–silica sol–gel thin films. J. Alloys Compd. 2004, 380, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Arenas, M.A.; de Damborenea, J.J. Growth mechanisms of cerium layers on galvanised steel. Electrochim. Acta 2003, 48, 3693–3698. [Google Scholar] [CrossRef] [Green Version]
- Fedel, M.; Callone, E.; Fabbian, M.; Deflorian, F.; Dirè, S. Influence of Ce3+ doping on molecular organization of Si-based organic/inorganic sol-gel layers for corrosion protection. Appl. Surf. Sci. 2017, 414, 82–91. [Google Scholar] [CrossRef]
- Santana, I.; Pepe, A.; Jimenez-Pique, E.; Pellice, S.; Milošev, I.; Ceré, S. Corrosion protection of carbon steel by silica-based hybrid coatings containing cerium salts: Effect of silica nanoparticle content. Surf. Coat. Technol. 2015, 265, 106–116. [Google Scholar] [CrossRef]
- Wang, J.-X.; Wang, Z.-H.; Chen, J.-F.; Yun, J. Direct encapsulation of water-soluble drug into silica microcapsules for sustained release applications. Mater. Res. Bull. 2008, 43, 3374–3381. [Google Scholar] [CrossRef]
- Hinton, B.R.W. Corrosion inhibition with rare earth metal salts. J. Alloys Compd. 1992, 180, 15–25. [Google Scholar] [CrossRef]
- Kakaroglou, A.; Domini, M.; De Graeve, I. Encapsulation and incorporation of sodium molybdate in polyurethane coatings and study of its corrosion inhibition on mild steel. Surf. Coat. Technol. 2016, 303, 330–341. [Google Scholar] [CrossRef]
- Mekeridis, E.D.; Kartsonakis, I.A.; Kordas, G.C. Multilayer organic–inorganic coating incorporating TiO2 nanocontainers loaded with inhibitors for corrosion protection of AA2024-T3. Prog. Org. Coat. 2012, 73, 142–148. [Google Scholar] [CrossRef]
- Vukasovich, M.S.; Farr, J.P.G. Molybdate in corrosion inhibition—A review. Polyhedron 1986, 5, 551–559. [Google Scholar] [CrossRef]
- Ilevbare, G.O.; Burstein, G.T. The inhibition of pitting corrosion of stainless steels by chromate and molybdate ions. Corros. Sci. 2003, 45, 1545–1569. [Google Scholar] [CrossRef]
- Shams El Din, A.M.; Wang, L. Mechanism of corrosion inhibition by sodium molybdate. Desalination 1996, 107, 29–43. [Google Scholar] [CrossRef]
- Tan, Y.T.; Wijesinghe, S.L.; Blackwood, D.J. Effect of Molybdate on the Passivation of Carbon Steel in Alkaline Solutions under Open-Circuit Conditions. J. Electrochem. Soc. 2016, 163, C649–C658. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, S.; Surber, B.; Nylund, P. Influence of MoO42− anion in the electrolyte on passivity breakdown of iron. Corros. Sci. 2001, 43, 1165–1177. [Google Scholar] [CrossRef]
- Voevodin, N.N.; Grebasch, N.T.; Soto, W.S.; Arnold, F.E.; Donley, M.S. Potentiodynamic evaluation of sol-gel coatings with inorganic inhibitors. Surf. Coat. Technol. 2001, 140, 24–28. [Google Scholar] [CrossRef]






| Description | Property |
|---|---|
| pencil hardness test | 2H |
| cross hatch adhesion test | 4B |
| topcoat thickness | 20–30 µm |
| Sample ID | Curing Conditions | Thickness |
|---|---|---|
| TC1 | 150 °C, 30 min | 20 µm |
| TC2 | 150 °C, 120 min | 20 µm |
| TC3 | 160 °C, 30 min | 20 µm |
| TC4 | 150 °C, 30 min | 30 µm |
| Sample ID | Coating Description (Directly on Carbon Steel) |
|---|---|
| S1 | Original sol-gel coating |
| S2 | Sol-gel coating incorporated with 0.2 wt.% Ce-BTN |
| S3 | Sol-gel coating incorporated with 0.2 wt.% Mo-HT |
| S4 | Sol-gel coating incorporated with 0.5 wt.% Ce-BTN |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Ong, W.K.; Wu, L.Y.; Wijesinghe, S.L. Investigation of Using Sol-Gel Technology for Corrosion Protection Coating Systems Incorporating Colours and Inhibitors. Coatings 2019, 9, 52. https://doi.org/10.3390/coatings9010052
Yan W, Ong WK, Wu LY, Wijesinghe SL. Investigation of Using Sol-Gel Technology for Corrosion Protection Coating Systems Incorporating Colours and Inhibitors. Coatings. 2019; 9(1):52. https://doi.org/10.3390/coatings9010052
Chicago/Turabian StyleYan, Wenjin, Wee Kit Ong, Linda Yongling Wu, and Sudesh L. Wijesinghe. 2019. "Investigation of Using Sol-Gel Technology for Corrosion Protection Coating Systems Incorporating Colours and Inhibitors" Coatings 9, no. 1: 52. https://doi.org/10.3390/coatings9010052
APA StyleYan, W., Ong, W. K., Wu, L. Y., & Wijesinghe, S. L. (2019). Investigation of Using Sol-Gel Technology for Corrosion Protection Coating Systems Incorporating Colours and Inhibitors. Coatings, 9(1), 52. https://doi.org/10.3390/coatings9010052






