Influences of Oxygen Ion Beam on the Properties of Magnesium Fluoride Thin Film Deposited Using Electron Beam Evaporation Deposition
Abstract
:1. Introduction
2. Experiment
2.1. Thin Film Preparation
2.2. Film Characterization
2.3. Some Background for Characterizations
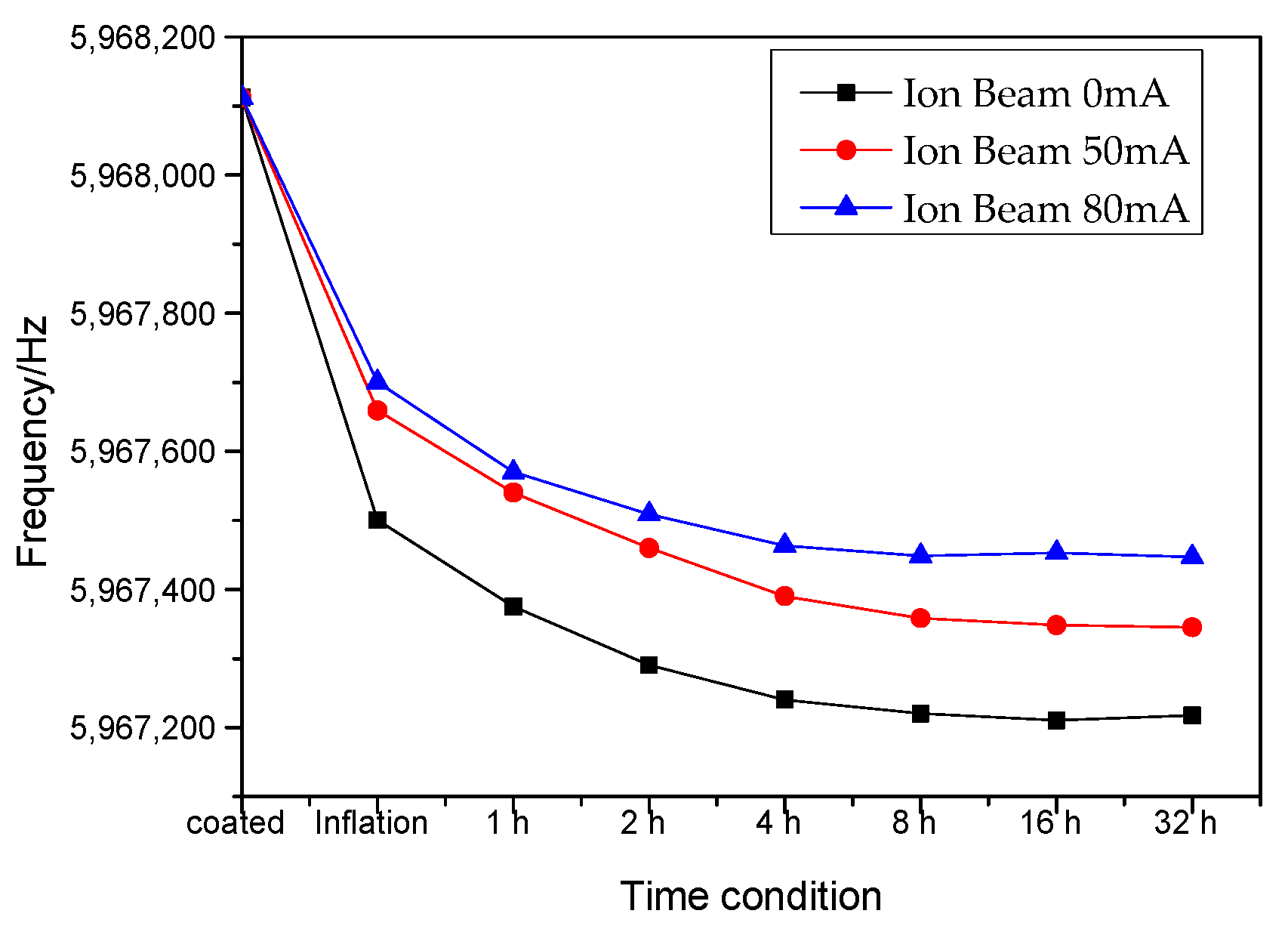
2.3.1. Quartz Frequency Measurement of Density
2.3.2. Zygo Interferometry for Measuring Film Stress
3. Result and Analysis
3.1. Packing Density Character
3.2. Optical Properties
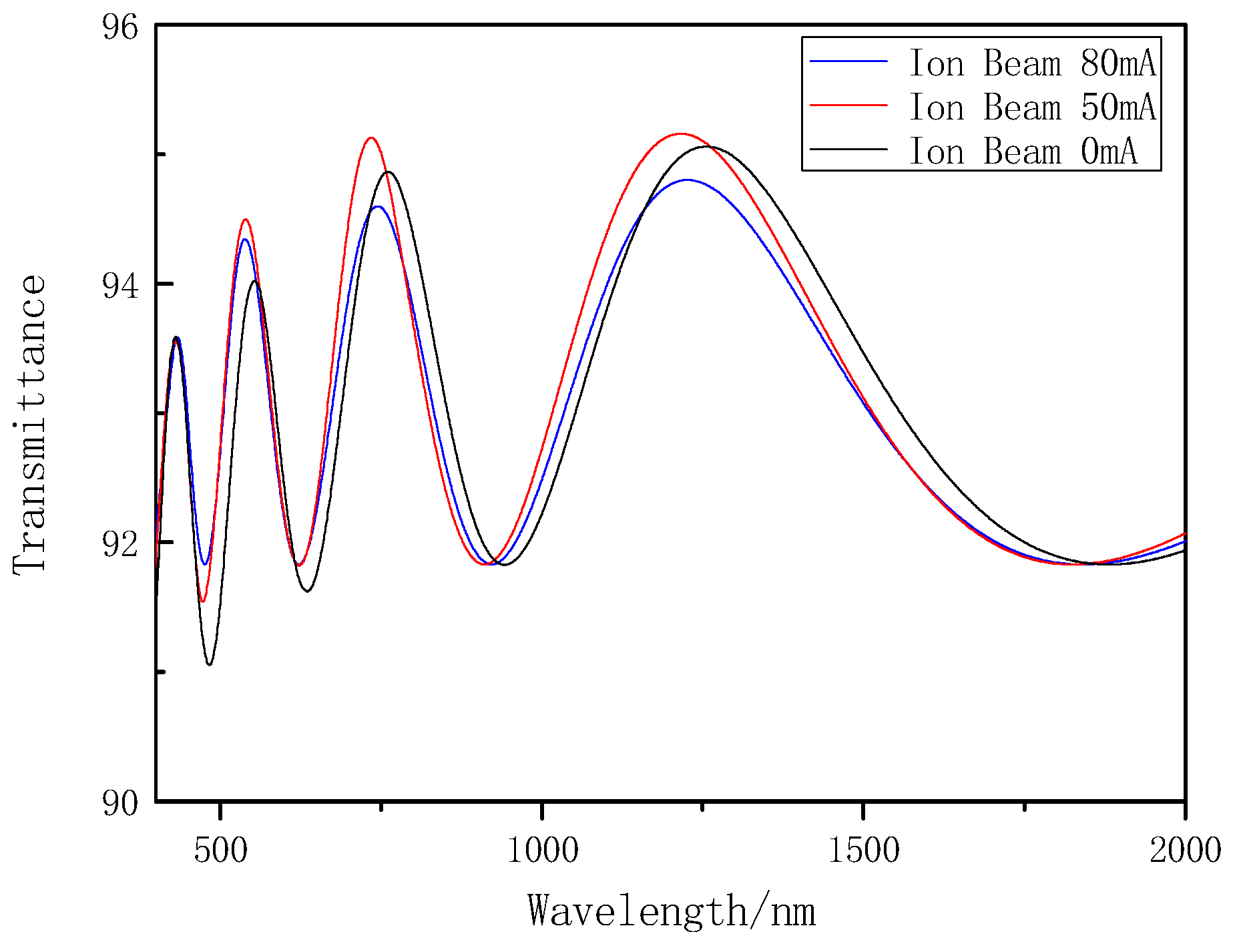
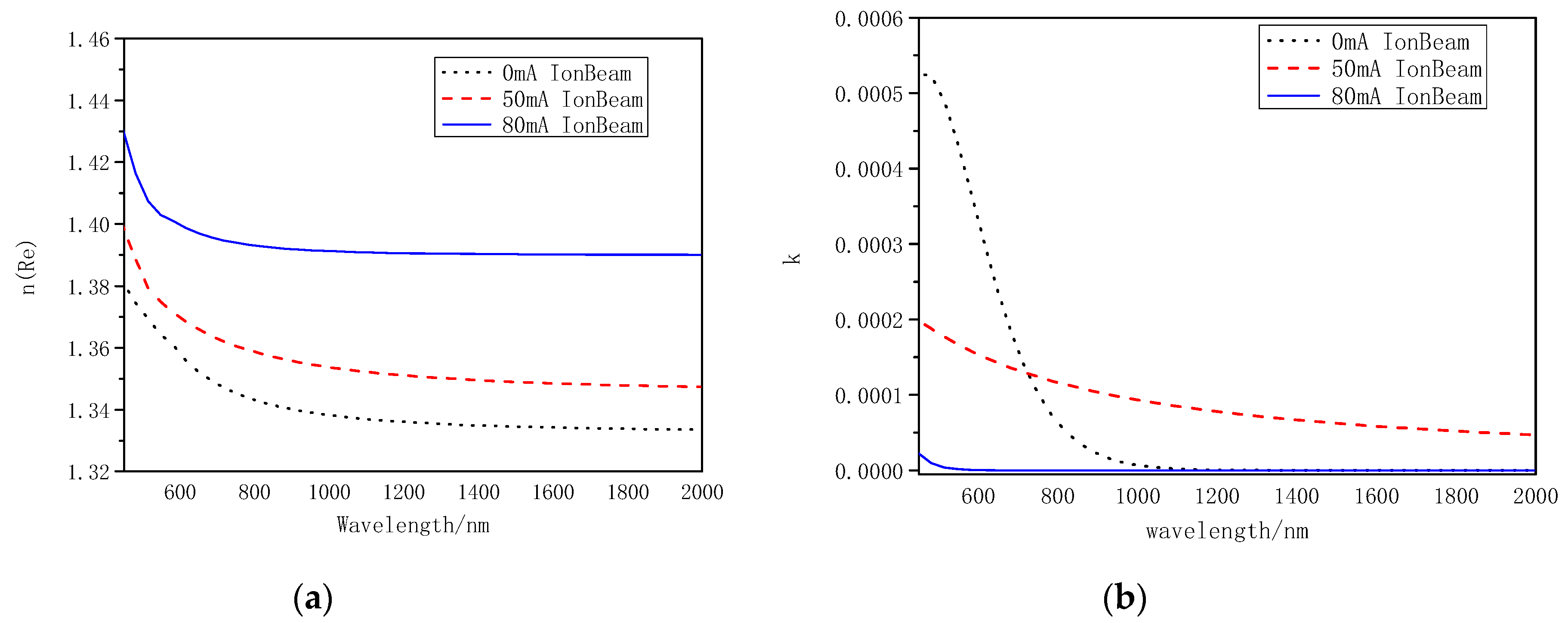
3.2.1. The Visible/Near Infrared Analysis
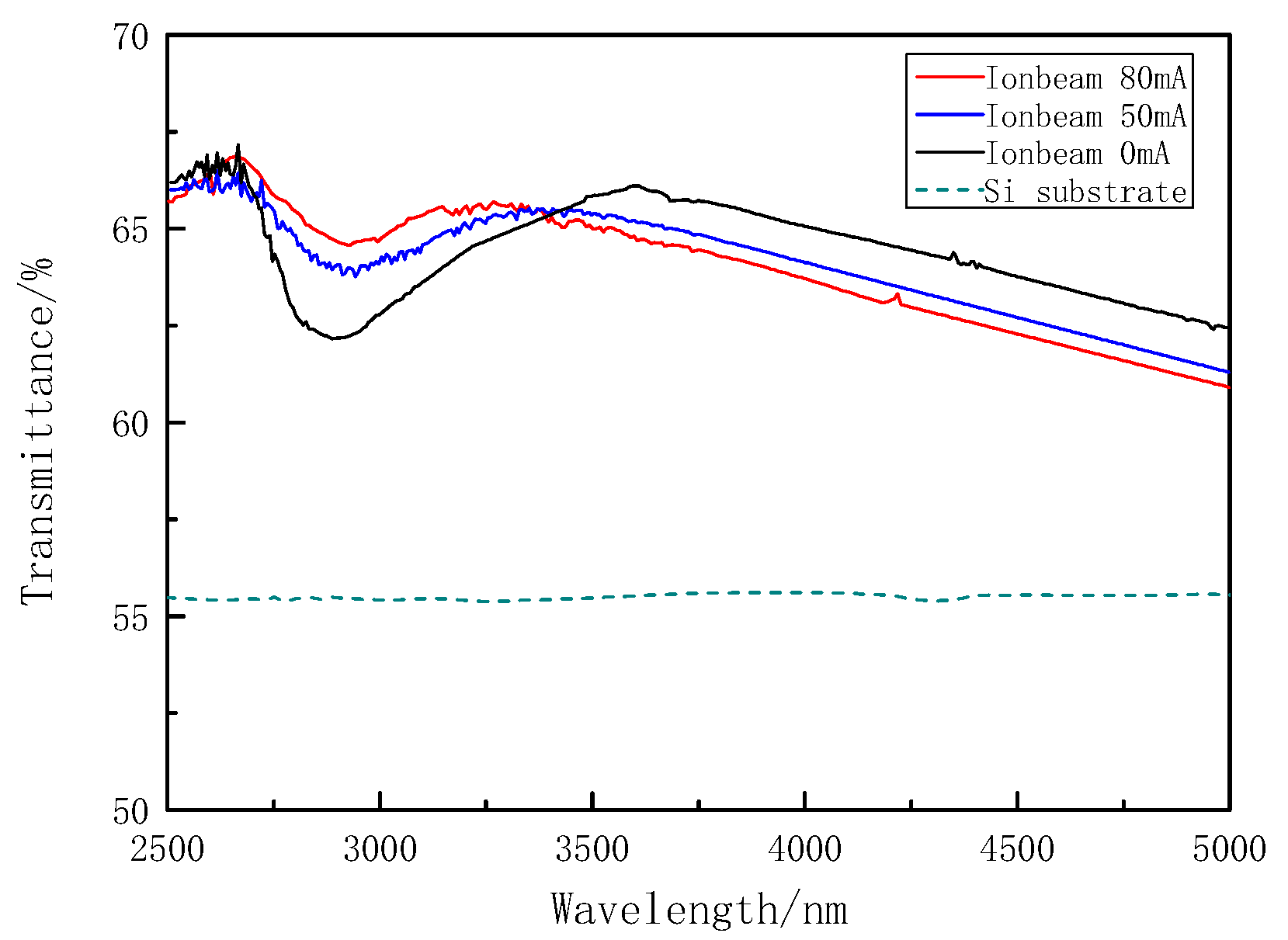
3.2.2. Hydroxyl Vibration Absorption
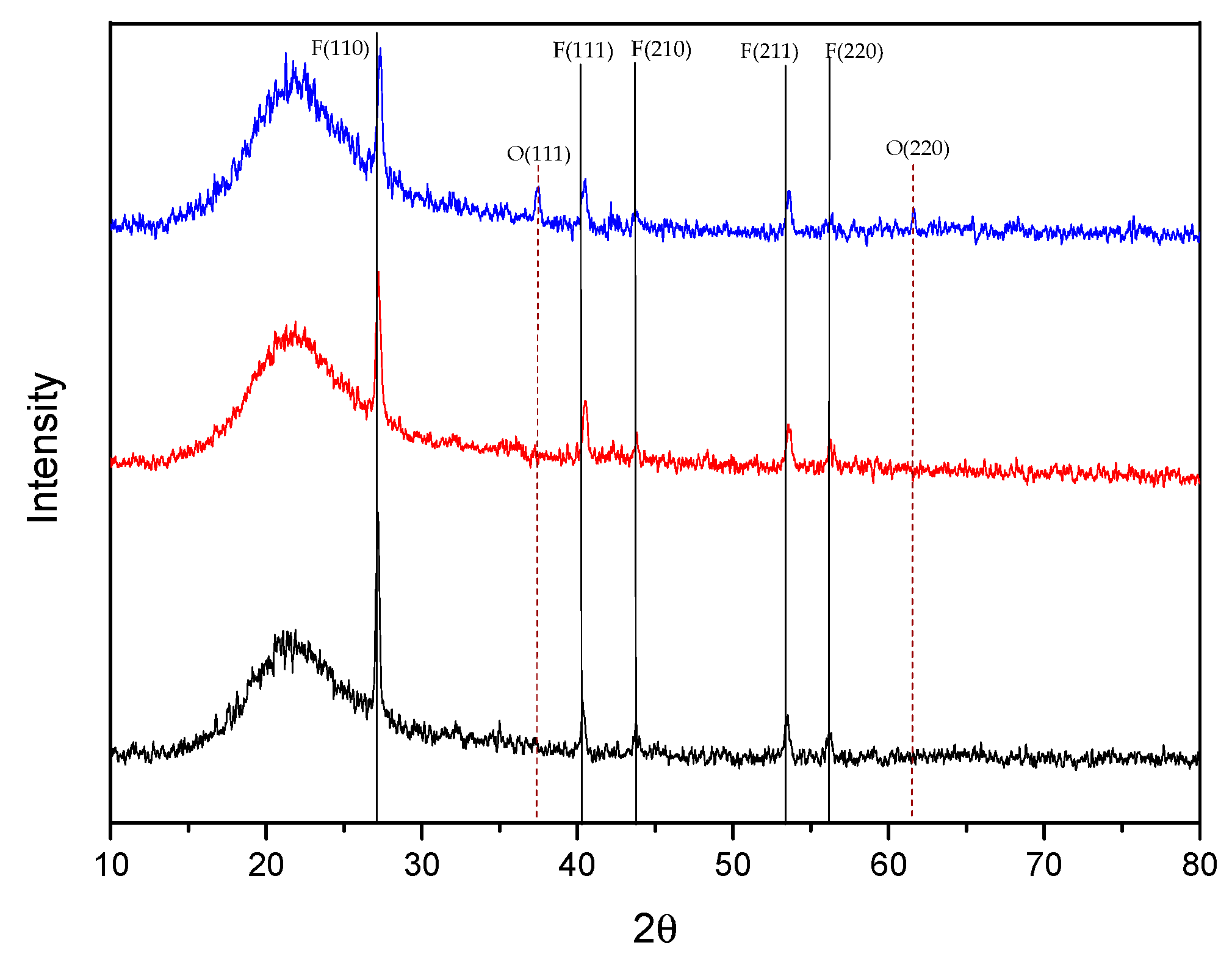
3.3. XRD
3.4. Adhesion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pham, T.; Du, W.; Tran, H.; Margetis, J.; Tolle, J.; Sun, G.; Soref, R.A.; Naseem, H.A.; Li, B.; Yu, S.-Q. Systematic study of Si-based GeSn photodiodes with 26 µm detector cutoff for short-wave infrared detection. Opt. Express 2016, 47, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Kim, H.; Cheng, J.; Lo, Y.-H. Ultrahigh responsivity visible and infrared detection using silicon nanowire photo transistion. Nano Lett. 2010, 10, 2117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, C.; Chen, H.; Sun, C.; Lin, J.; Wang, X. MTF measurement and analysis of linear array HgCdTe infrared detectors. Infrared Phys. Technol. 2018, 8, 123–127. [Google Scholar] [CrossRef]
- Dodge, M.J. Refractive properties of magnesium fluoride. Appl. Opt. 1984, 23, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.W. Optical properties of magnesium fluoride in the vacuum ultraviolet. J. Appl. Phys. 1967, 38, 1701. [Google Scholar] [CrossRef]
- Al-Douri, A.A.J.; Heavens, O.S. The role of rate of deposition and substrate temperature on the structure and loss of zinc sulphide and magnesium fluoride films. J. Phys. D 1983, 16, 927–935. [Google Scholar] [CrossRef]
- Zhang, Y. Research on Preparation of low absorption infrared coatings and its environmental stability and reliability. Ph.D. Thesis, Institute of Optics and Electronics, Chinese Academy of Sciences, Beijing, China, June 2019. (In Chinese). [Google Scholar]
- Bischoff, M.; Stenzel, O.; Friedrich, K. Plasma-assisted deposition of metal fluoride coatings and modeling the extinction coefficient of as-deposited single layers. Appl. Opt. 2011, 50, 232–238. [Google Scholar] [CrossRef]
- Guenther, K.H.; Pulker, H.K. Electron microscopic investigations of cross sections of optical thin films. Appl. Opt. 1976, 15, 2992–2997. [Google Scholar] [CrossRef]
- Targove, J.D. The ion-assisted deposition of optical thin films. Ph.D. Thesis, The University of Arizona, Arizona, Tucson, AZ, USA, 1987. [Google Scholar]
- Kennedy, M.; Ristau, D.; Niederwald, H.S. Ion beam-assisted deposition of MgF2 and YbF3 films. Thin Solid Film. 1998, 333, 1–2. [Google Scholar] [CrossRef]
- Dumas, L.; Quesnel, E. Characterization of magnesium fluoride thin films produced by argon ion beam-assisted deposition. Thin Solid Film. 2001, 382, 61–68. [Google Scholar] [CrossRef]
- Wilbrandt, S.; Stenzel, O. Combined in situ and ex situ optical data analysis of magnesium fluoride coatings deposited by plasma ion-assisted deposition. Appl. Opt. 2011, 50, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Booster, J.L.; Voncken, J.H.L. Characterization of hydroxyl-bearing magnesium fluoride containing physically bound water. Powder Diffr. 2002, 17, 112–118. [Google Scholar] [CrossRef]
- Sun, J.; Shao, J. Effects of substrate temperatures on the characterization of magnesium fluoride thin films in deep-ultraviolet region. Appl. Opt. 2014, 53, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Sugawara, N.; Hiraga, R. Refractive index and packing density for MgF2 films: Correlation of temperature dependence with water sorption. Thin Solid Film. 1975, 3, 3–10. [Google Scholar] [CrossRef]
- Forman, P.F. The Zygo Interferometer System; Hopkins, G.W., Ed.; SPIE: Bellingham, WA, USA, 1979; Volume 192. [Google Scholar]
- Wang, J.; Shrotriya, P.; Kim, K.S. Surface residual stress measurement using curvature interferometry. Exp. Mech. 2006, 46, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Ottermann, C.; Otto, J.; Jeschkowski, U. Stress of TiO2 thin films produced by different deposition techniques. MRS Proc. 1993, 308, 69. [Google Scholar] [CrossRef]
- Daniel, C. Durable 3–5 micron transmitting infrared window materials. Infrared Phys. Technol. 1998, 1998 39, 185–201. [Google Scholar]
- Coker, D.; Reimers, J.; Watts, R. The infrared absorption spectrum of water. Aust. J. Phys. 1982, 35, 62. [Google Scholar] [CrossRef] [Green Version]
- Lu, N.H. Adhesion test for dry film photoresist on copper by the peeling method. Circuit World 1989, 15, 47–49. [Google Scholar] [CrossRef]
- Chapman, B.N. Thin-film adhesion. J. Vac. Sci. Technol. 1974, 11, 106–113. [Google Scholar] [CrossRef]
- Bikerman, J.J. Cause of poor adhesion boundary: Weak boundary layers. Ind. Eng. Chem. 1967, 59, 40–44. [Google Scholar] [CrossRef]
- Bikerman, J.J. The fundamentals of tackiness and adhesion. J. Colloid Sci. 1947, 2, 163–175. [Google Scholar] [CrossRef]


| Group | Equipment and Accuracy | Purpose | Process |
|---|---|---|---|
| 1 | Inficon golden crystaluency (Δf < 0.004657 Hz) | Peaking density | 1. Recorded the crystal frequency after coating at pressure of 1.0 × 10−3 Pa, recorded the crystal frequency, vented and recorded again. 2. The crystal was taken out and soaked in the ionized water for 36 h to make the crystal oscillator fully absorb water and then the frequency of the crystal oscillator was displayed in the XTC-3100 quartz monitoring system until the frequency stabilized. 3. Thereafter, the crystal was placed in the chamber in which it was pumped to 1.0 × 10−3 Pa again at a baking temperature of 300 °C, and the vibration frequency of the crystal piece was recorded. |
| 2 | SHIMADZU UV-3150 (ΔT < 0.0002% T) | UV–Visible spectrum | After deposition, the film deposited on BK7 substrate was measured, optichar software (version 9.51) was used to fit the transmittance curve and calculate the optical constant. The Cauchy dispersion module homogeneous layer with a smooth surface was used to fit the optical constant. |
| 3 | Agilent VR610 (ΔT < 0.05% T) | Mid-infrared spectrum | After deposition, the film transmittance deposited on Si substrate was measured, and the –OH absorption of different samples were analyzed. |
| 4 | Bruker D8 Advance XRD (Δθ <± 0.001°) | Chemistry phase of film | X-ray diffraction studies were performed with a Bruker D8 Advance XRD instrument equipped with Cu Ka source, the scan type was “absolute” and scan mode was continuous |
| 5 | Zygo interferometer (Δλ < 1/2000λ) | The films’ stress | The Zygo interferometer tested the Δpower value then calculated the stress of film. |
| 6 | Adhesive experiments | 3M adhesive tape | Test was done using 3M scotch 610 test tape: Stickiness of (10 ± 1) N/25mm, and keeping tape pull angle at 90° to the film surface. |
| Ion Beam | Density |
|---|---|
| 0 | 0.89 |
| 50 | 0.905 |
| 80 | 0.92 |
| Oxygen Ion Current | 0 mA | 50 mA | 80 mA |
|---|---|---|---|
| Before water immersion (A) | 5,968,113.2 | 5,968,115.3 | 5,968,110.7 |
| After water immersion (B) | 5,967,217.3 | 5,967,345.3 | 5,967,447.3 |
| Under vacuum at 300 °C (C) | 5,967,982.4 | 5,968,003.2 | 5,968,092.6 |
| Frequency differences (C–B) | 761.5 | 657.9 | 645.3 |
| Frequency differences (C–A) | −130.8 | –112.1 | –18.1 |
| Film Condition | Ion Beam 0 mA | Ion Beam 50 mA | Ion Beam 80 mA |
|---|---|---|---|
| After coated | 3.22% | 1.74% | 0.83% |
| 32 h dipped | 5.65% | 2.64% | 1.52% |
| After vacuum baked | 5.2% | 1.98% | 0.96% |
| Spacing (d) <hkl> | Literature ICCD41-1443 | Sample 1 Ion Beam 0 mA | Sample 2 Ion Beam 50 mA | Sample 3 Ion Beam 80 mA |
|---|---|---|---|---|
| <110> | 3.2670 | 3.2668 | 3.2683 | 3.2686 |
| <111> | 2.2309 | 2.2299 | 2.2312 | 2.2315 |
| <210> | 2.0672 | 2.0670 | 2.0673 | 2.0674 |
| <211> | 1.7112 | 1.7113 | 1.7120 | 1.7124 |
| <220> | 1.6335 | 1.6338 | 1.6343 | 1.6345 |
| Lattice | Literature ICCD41-1443 | Sample 1 Ion Beam 0 mA | Sample 2 Ion Beam 50 mA | Sample 3 Ion Beam 80 mA |
|---|---|---|---|---|
| a (nm) | 0.46200 | 0.46220 | 0.4295 | 0.4635 |
| C (nm) | 0.30509 | 0.30622 | 0.3041 | 0.3032 |
| Ion Current | Power before Coated | Power after Coated |
|---|---|---|
| Ion Beam 0 mA | 0.091 | 0.866 |
| Ion Beam 50 mA | 0.091 | 0.425 |
| Ion Beam 80 mA | 0.091 | 0.365 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Fu, X.; Song, S.; Guo, K.; Zhang, J. Influences of Oxygen Ion Beam on the Properties of Magnesium Fluoride Thin Film Deposited Using Electron Beam Evaporation Deposition. Coatings 2019, 9, 834. https://doi.org/10.3390/coatings9120834
Zhang G, Fu X, Song S, Guo K, Zhang J. Influences of Oxygen Ion Beam on the Properties of Magnesium Fluoride Thin Film Deposited Using Electron Beam Evaporation Deposition. Coatings. 2019; 9(12):834. https://doi.org/10.3390/coatings9120834
Chicago/Turabian StyleZhang, Gong, Xiuhua Fu, Shigeng Song, Kai Guo, and Jing Zhang. 2019. "Influences of Oxygen Ion Beam on the Properties of Magnesium Fluoride Thin Film Deposited Using Electron Beam Evaporation Deposition" Coatings 9, no. 12: 834. https://doi.org/10.3390/coatings9120834





