Life under Continuous Streaming: Recrystallization of Low Concentrations of Bacterial SbpA in Dynamic Flow Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Quartz Crystal Microbalance with Dissipation (QCMD)
2.2.2. Atomic Force Microscopy
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tjong, S.C.; Chen, H. Nanocrystalline materials and coatings. Mater. Sci. Eng. R Rep. 2004, 45, 1–88. [Google Scholar] [CrossRef] [Green Version]
- Buchan, I.; Ryder, M.R.; Tan, J.C. Micromechanical behavior of polycrystalline metal–organic framework thin films synthesized by electrochemical reaction. Cryst. Growth Des. 2015, 15, 1991–1999. [Google Scholar] [CrossRef]
- Grimberg, A.; Avni, R.; Grill, A. Preparation of polycrystalline silicon coatings from trichlorosilane. Thin Solid Films 1982, 96, 163–167. [Google Scholar] [CrossRef]
- Qiao, C.; Zhao, J.; Jiang, S.; Ji, X.; An, L.; Jiang, B. Crystalline morphology evolution in PCL thin films. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1303–1309. [Google Scholar] [CrossRef]
- Reddy, H.; Guler, U.; Chaudhuri, K.; Dutta, A.; Kildishev, A.V.; Shalaev, V.M.; Boltasseva, A. Temperature-dependent optical properties of single crystalline and polycrystalline silver thin films. ACS Photonics 2017, 4, 1083–1091. [Google Scholar] [CrossRef]
- Saju, K.K.; Reshmi, R.; Jayadas, N.H.; James, J.; Jayaraj, M.K. Polycrystalline coating of hydroxyapatite on TiAl6V4 implant material grown at lower substrate temperatures by hydrothermal annealing after pulsed laser deposition. Proc. Inst. Mech. Eng. Part H 2009, 223, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, L.; Fryda, M.; Stolley, T.; Xiang, L.; Klages, C.P. Chemical vapour deposition of polycrystalline diamond films on high-speed steel. Surf. Coat. Technol. 1999, 116–119, 447–451. [Google Scholar] [CrossRef]
- Hamley, I.W. Nanotechnologie mit weichen materialien. Angew. Chem. 2003, 115, 1730–1752. [Google Scholar] [CrossRef]
- McManus, J.J.; Charbonneau, P.; Zaccarelli, E.; Asherie, N. The physics of protein self-assembly. Curr. Opin. Colloid Interface Sci. 2016, 22, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Szilvay, G.R.; Paananen, A.; Laurikainen, K.; Vuorimaa, E.; Lemmetyinen, H.; Peltonen, J.; Linder, M.B. Self-assembled hydrophobin protein films at the air-water interface: Structural analysis and molecular engineering. Biochemistry 2007, 46, 2345–2354. [Google Scholar] [CrossRef]
- Oling, F.; Bergsma-Schutter, W.; Brisson, A. Trimers, dimers of trimers, and trimers of trimers are common building blocks of Annexin A5 two-dimensional crystals. J. Struct. Biol. 2001, 133, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.P.; Him, J.L.K.; Tessier, B.; Tessier, C.; Brisson, A.R. On the kinetics of adsorption and two-dimensional self-assembly of Annexin A5 on supported lipid bilayers. Biophys. J. 2005, 89, 3372–3385. [Google Scholar] [CrossRef] [PubMed]
- Sleytr, U.B.; Schuster, B.; Egelseer, E.M.; Pum, D. S-layers: Principles and applications. FEMS Microbiol. Rev. 2014, 38, 823–864. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Möhwald, H. Protein multilayer formation on colloids through a stepwise self-assembly technique. J. Am. Chem. Soc. 1999, 121, 6039–6046. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Iturri, J.; Moreno-Cencerrado, A.; Toca-Herrera, J.L. Cation-chelation and pH induced controlled switching of the non-fouling properties of bacterial crystalline films. Colloids Surf. B 2017, 158, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Cencerrado, A.; Iturri, J.; Pecorari, I.; DM Vivanco, M.; Sbaizero, O.; Toca-Herrera, J.L. Investigating cell-substrate and cell-cell interactions by means of single-cell-probe force spectroscopy. Microsc. Res. Tech. 2017, 80, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Flores, S.; Küpcü, S. 2D protein arrays induce 3D in vivo-like assemblies of cells. Soft Matter 2015, 11, 1259–1264. [Google Scholar] [CrossRef]
- Iturri, J.; Vianna, A.C.; Moreno-Cencerrado, A.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. Impact of surface wettability on S-layer recrystallization: A real-time characterization by QCMD. Beilstein J. Nanotechnol. 2017, 8, 91–98. [Google Scholar] [CrossRef]
- Lopez, A.E.; Moreno-Flores, S.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. Surface dependence of protein nanocrystal formation. Small 2010, 6, 396–403. [Google Scholar] [CrossRef]
- Iturri, J.; Breitwieser, A.; Pum, D.; Sleytr, U.; Toca-Herrera, J. Electrochemical-QCMD control over S-layer (SbpA) recrystallization with Fe2+ as specific ion for self-assembly induction. Appl. Sci. 2018, 8, 1460. [Google Scholar] [CrossRef]
- Moreno-Cencerrado, A.; Iturri, J.; Toca-Herrera, J.L. In-situ 2D bacterial crystal growth as a function of protein concentration: An atomic force microscopy study. Microsc. Res. Tech. 2018, 81, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Stel, B.; Cometto, F.; Rad, B.; De Yoreo, J.J.; Lingenfelder, M. Dynamically resolved self-assembly of S-layer proteins on solid surfaces. Chem. Commun. 2018, 54, 10264–10267. [Google Scholar] [CrossRef] [PubMed]
- López, A.E.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. Influence of surface chemistry and protein concentration on the adsorption rate and S-layer crystal formation. Phys. Chem. Chem. Phys. 2011, 13, 11905–11913. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, E.; Grey, M.; Ainalem, M.L.; Ankner, J.; Forsyth, V.T.; Fragneto, G.; Haertlein, M.; Dauvergne, M.T.; Nilsson, H.; Brundin, P.; et al. Adsorption of α-synuclein to supported lipid bilayers: Positioning and role of electrostatics. ACS Chem. Neurosci. 2013, 4, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Iturri, J.; Moreno-Cencerrado, A.; Toca-Herrera, J.L. Polyelectrolyte brushes as supportive substrate for bacterial S-layer recrystallization: Polymer charge and chain extension factors. Colloids Surf. A 2017, 526, 56–63. [Google Scholar] [CrossRef]

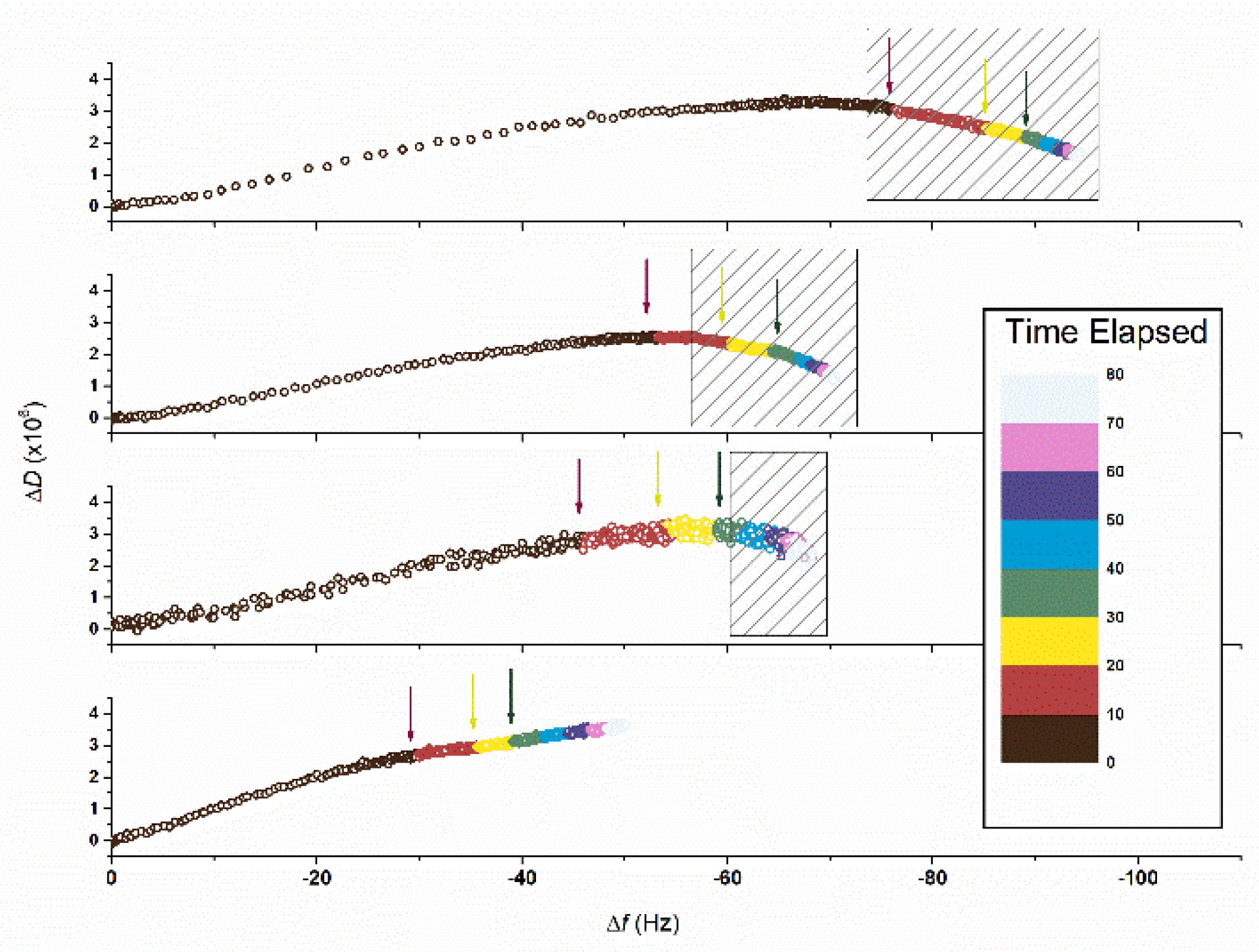

| SbpA Concentration (µg/mL) | Slope (Hz/min) | |
|---|---|---|
| Stopped-Flow | Continuous Flow | |
| 100 | −150 | −45 |
| 50 | −101 | −35 |
| 25 | −45 | −17 |
| 10 | −16 | −8 |
| 5 | −7 | −5 |
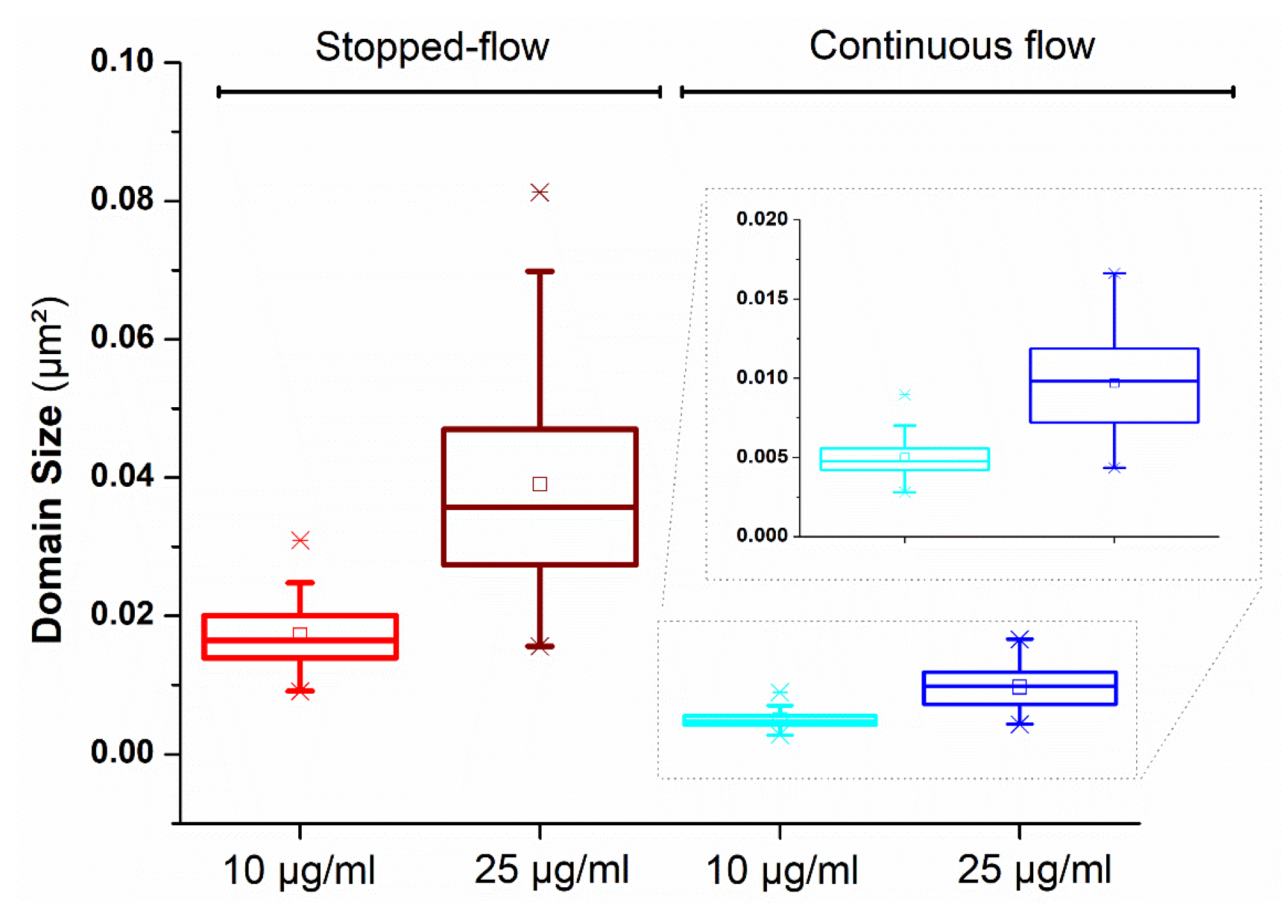
| SbpA (µg/mL) | Stopped-Flow | Continuous Flow | ||
|---|---|---|---|---|
| Size (µm2) | SE | Size (µm2) | SE | |
| 25 | 0.0390 | 7 × 10−4 | 0.0097 | 4 × 10−4 |
| 10 | 0.0172 | 2 × 10−3 | 0.0049 | 2 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iturri, J.; Moreno-Cencerrado, A.; Toca-Herrera, J.L. Life under Continuous Streaming: Recrystallization of Low Concentrations of Bacterial SbpA in Dynamic Flow Conditions. Coatings 2019, 9, 76. https://doi.org/10.3390/coatings9020076
Iturri J, Moreno-Cencerrado A, Toca-Herrera JL. Life under Continuous Streaming: Recrystallization of Low Concentrations of Bacterial SbpA in Dynamic Flow Conditions. Coatings. 2019; 9(2):76. https://doi.org/10.3390/coatings9020076
Chicago/Turabian StyleIturri, Jagoba, Alberto Moreno-Cencerrado, and José Luis Toca-Herrera. 2019. "Life under Continuous Streaming: Recrystallization of Low Concentrations of Bacterial SbpA in Dynamic Flow Conditions" Coatings 9, no. 2: 76. https://doi.org/10.3390/coatings9020076





