Synthesis of Iron Oxide Nanostructures via Carbothermal Reaction of Fe Microspheres Generated by Infrared Pulsed Laser Ablation
Abstract
:1. Introduction
2. Materials and Methods
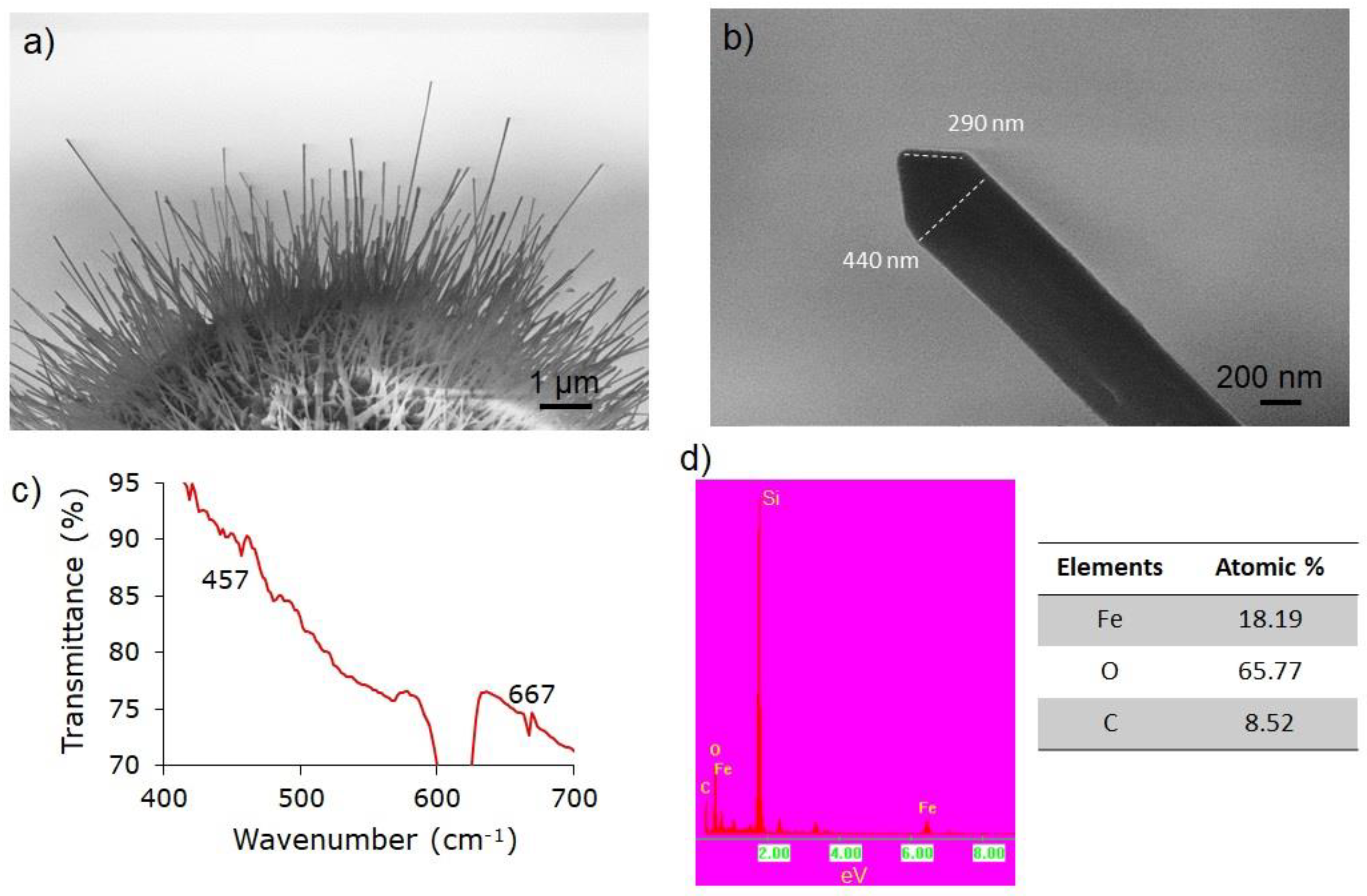
3. Results
4. Discussion
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bery, C. Progress in functionalization of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224003. [Google Scholar] [CrossRef]
- Xie, Y.; Ju, Y.; Toku, Y.; Morita, Y. Synthesis of single-crystal Fe2O3 nanowire array based on stress-induced atomic diffusion used for solar water splitting. R. Soc. Open Sci. 2018, 5, 171226. [Google Scholar] [CrossRef]
- Takami, S.; Sato, T.; Mousava, T.; Ohara, S.; Umetsu, M.; Adschiri, T. Hydrothermal synthesis of surface-modified iron oxide nanoparticles. Mater. Lett. 2007, 61, 4769–4772. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.X.; Shen, M. Hydrothermal synthesis and functionalization of iron oxide nanoparticles for MR imaging applications. Part. Part. Syst. Charact. 2014, 31, 1223–1237. [Google Scholar] [CrossRef]
- Walker, J.; Tannenbaum, R. Characterization of the sol−gel formation of iron (III) oxide/hydroxide nanonetworks from weak base molecule. Chem. Mater. 2006, 18, 4793–4801. [Google Scholar] [CrossRef]
- Hiralal, P.; Unalan, H.; Wijayantha, K.; Kursumovic, A.; Jefferson, D.; MacManus-Driscoll, J.; Amaratunga, G. Growth and process conditions of aligned and patternable films of iron(III) oxide nanowires by thermal oxidation of iron. Nanotechnology 2008, 19, 455608. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Falker, J.; Yavuz, C.; Colvin, V. Synthesis of monodisperse iron oxide nanocrystals by thermal decomposition of iron carboxylate salts. Chem. Commun. 2004, 2306–2307. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Gao, C.; Liu, Q.; Zhang, L. Preparation and characterization of hematite nanowire arrays. J. Phys. Condens. Matter 2003, 15, 1455. [Google Scholar] [CrossRef]
- Shi, W.; Zheng, Y.; Peng, H.; Wang, N.; Lee, C.S.; Lee, S.T. Laser ablation synthesis and optical characterization of silicon carbide nanowires. J. Am. Ceram. Soc. 2000, 83, 3228–3330. [Google Scholar] [CrossRef]
- Yu, D.; Sun, X.; Lee, C.; Bello, I.; Lee, S.; Gu, H.; Leung, K.; Zhou, G.; Dong, Z.; Zhang, Z. Synthesis of boron nitride nanotubes by means of excimer laser ablation at high temperature. Appl. Phys. Lett. 1998, 72, 1966–1967. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.F.; Tang, Y.H.; Lee, C.S.; Lee, S.T. SiO2-enhanced synthesis of Si nanowires by laser ablation. Appl. Phys. Lett. 1998, 73, 3902–3904. [Google Scholar] [CrossRef]
- Chrisey, D.B. Pulsed Laser Deposition of Thin Films; Wiley-Interscience: New York, NY, USA, 1994; ISBN 978-0471592181. [Google Scholar]
- Eason, R. Pulsed Laser Deposition of Thin Films Applications-Led Growth of Functional Materials; Wiley-Interscience: New York, NY, USA, 2006; ISBN 978-0471447092. [Google Scholar]
- Dijkkamp, D.; Venkatesan, T.; Wu, X.D.; Shaheen, S.A.; Jiswari, N.; Min-lee, Y.H.; McLean, W.L.; Croft, M. Preparation of Y-Ba-Cu oxide superconductor thin films using pulsed laser evaporation from high Tc bulk material. Appl. Phys. Lett. 1987, 51, 619–621. [Google Scholar] [CrossRef]
- de Vero, J.; Lee, D.; Shin, H.; Namuco, S.; Hwang, I.; Sarmago, R.; Song, J.H. Influence of deposition conditions on the growth of micron-thick highly c-axis textured superconducting GdBa2Cu3O7–δ films on SrTiO3 (100). J. Vac. Sci. Technol. 2018, 36, 031506. [Google Scholar] [CrossRef]
- de Vero, J.; Hwang, I.; Santiago, A.; Chang, J.; Kim, J.; Sarmago, R.; Song, J. Growth of Bi2Sr2CaCu2O8+δ thin films with enhanced superconducting properties by incorporating CaIrO3 nanoparticles. Appl. Phys. Lett. 2014, 104, 172603. [Google Scholar] [CrossRef]
- Wu, X.D.; Dye, R.C.; Muenchausen, R.E.; Foltyn, S.R.; Maley, M.; Rollett, A.D.; Garcia, A.R.; Nogar, N.S. Epitaxial CeO2 films as buffer layers for high-temperature superconducting thin films. Appl. Phys. Lett. 1991, 58, 2165. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, D. Pulsed laser deposition and characterization of high-Tc YBa2Cu3O7–x superconducting thin films. Mater. Sci. Eng. Rep. 1998, 22, 113–185. [Google Scholar] [CrossRef]
- Shen, J.; Gai, Z.; Kirschner, J. Growth and magnetism of metallic films and multilayers by pulsed laser deposition. Surf. Sci. Rep. 2004, 52, 163–218. [Google Scholar] [CrossRef]
- Chen, X.; Chien, C. Magnetic properties of epitaxial Mn-doped ZnO thin films. J. Appl. Phys. 2003, 93, 7876–7878. [Google Scholar] [CrossRef] [Green Version]
- Plonczak, P.; Søgaard, A.B.M.; Ryll, T.; Martynczuk, J.; Hendriksen, P.; Gauckler, L. Tailoring of LaxSrxCoyFe1–yO3–δ nanostructure by Pulsed Laser Deposition. Adv. Funct. Mater. 2011, 21, 2764–2775. [Google Scholar] [CrossRef]
- de Vero, J.C.; Develos-Bagarinao, K.; Kishimoto, H.; Ishiyama, T.; Yamaji, K.; Horita, T.; Yokokawa, H. Enhanced stability of solid oxide fuel cells by employing a modified cathode- interlayer interface with a dense La0.6Sr0.4Co0.2Fe0.8O3–δ thin film. J. Power Sources 2018, 377, 128–135. [Google Scholar] [CrossRef]
- de Vero, J.C.; Develos-Bagarinao, K.; Kishimoto, H.; Ishiyama, T.; Yamaji, K.; Horita, T.; Yokokawa, H. Optimization of GDC interlayer against SrZrO3 formation in LSCF/GDC/YSZ triplets. In Proceedings of the 12th European SOFC and SOEC Forum, Lucerne, Switzerland, 5–8 July 2016; Volume 41, p. B1513. [Google Scholar]
- Morales, M.; Pesce, A.; Slodczyk, A.; Torrell, M.; Piccardo, P., II; Montinaro, D.; Tarancón, A.; Morata, A. Enhanced performance of gadolinia-doped ceria diffusion barrier layers fabricated by pulsed laser deposition for large-area solid oxide fuel cells. ACS Appl. Energy Mater. 2018, 1, 1955–1964. [Google Scholar] [CrossRef]
- Yan, J.; Matsumoto, H.; Akbay, T.; Yamada, T.; Ishihara, T. Preparation of LaGaO3-based perovskite oxide film by pulsed-laser ablation method and application as a solid oxide fuel cell electrolyte. J. Power Sources 2006, 157, 714–719. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, Y.; Zhang, X.; Hsu, W.; Gong, Y.; Shin, J.; Wachsman, E.; Dagenais, M.; Takeuchi, I. Fabrication of organic-inorganic perovskite thin films for planar solar cells via pulsed laser deposition. AIP Adv. 2016, 6, 05001. [Google Scholar] [CrossRef]
- Park, J.; Seo, J.; Park, S.; Shin, S.; Kim, Y.; Jeon, N.; Shin, H.; Noh, T.A.J.; Yoon, S.; Hwang, C.; et al. Efficient CH3NH3PbI3 perovskite solar cells employing nanostructured p-Type NiO electrode formed by a pulsed laser deposition. Adv. Mater. 2015, 27, 4013–4019. [Google Scholar] [CrossRef] [PubMed]
- Ghidelli, M.; Mascaretti, L.; Bricchi, B.; Zapelli, A.; Russo, V.; Casari, C.; Bassi, A.L. Engineering plasmonic nanostructured surfaces by pulsed laser deposition. Appl. Surf. Sci. 2018, 433, 1064–1073. [Google Scholar] [CrossRef]
- Bricchi, B.; Ghidelli, M.; Mascaretti, L.; Zapelli, A.; Russo, V.; Casari, C.; Terraneo, G.; Alessandri, I.; Ducati, C.; Bassi, A.L. Intergration of plasmonic Au nanoparticles in TiO2 hierarichal structures in a single-step pulsed laser co-deposition. Mater. Des. 2018, 156, 311–319. [Google Scholar] [CrossRef]
- Develos-Bagarinao, K.; de Vero, J.; Kishimoto, H.; Yamaji, K.; Horita, T.; Yokokawa, H. Multilayered LSC and GDC: An approach for designing cathode materials with superior oxygen exchange properties for solid oxide fuel cells. Nano Energy 2018, 52, 369–380. [Google Scholar] [CrossRef]
- Yang, R. One-Dimensional Nanostructures by Pulsed Laser Ablation. Sci. Adv. Mater. 2012, 4, 401–406. [Google Scholar] [CrossRef]
- Morales, A.; Lieber, C. A laser ablation method for synthesis of crystalline semiconductor nanowires. Science 1998, 279, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Schou, J. Physical aspects of the pulsed laser deposition technique: The stoichiometric transfer of material from target to film. Appl. Surf. Sci. 2009, 10, 5191–5198. [Google Scholar] [CrossRef]
- Arnold, C.B.; Aziz, M.J. Stoichiometry issues in pulsed laser deposition of alloys grown from multicomponent targets. Appl. Phys. A 1999, 69, S23–S27. [Google Scholar] [CrossRef]
- Ichino, Y.; Yoshida, Y.; Yoshimura, T.; Takai, Y.; Yoshizumi, M.; Izumi, T.; Shiohara, Y. Potential of Nd:YAG pulsed laser deposition method for coated conductor production. Phys. C 2010, 470, 1234–1237. [Google Scholar] [CrossRef]
- de Vero, J.; Blanca, G.R.S.; Vitug, J.; Garcia, W.; Sarmago, R. Stoichiometric transfer of material in the infrared pulsed laser deposition of yttrium doped Bi-2212 films. Phys. C 2011, 471, 378–383. [Google Scholar] [CrossRef]
- de Vero, J.; Gabayno, J.F.; Garcia, W.O.; Sarmago, R.V. Growth of Bi2Sr2CaCu2O8+δ thin films deposited by infrared (1064 nm) pulsed laser deposition. Phys. C 2010, 470, 149–154. [Google Scholar] [CrossRef]
- Vitug, J.; de Vero, J.; Blanca, G.R.S.; Sarmago, R.; Garcia, W. Stoichiometric transfer by infrared pulsed laser deposition of y-doped Bi–Sr–Ca–Cu–O investigated using time-resolved optical emission spectroscopy. J. Appl. Spectrosc. 2012, 78, 855–860. [Google Scholar] [CrossRef]
- de Vero, J.; Lopez, R.A.; Garcia, W.O.; Sarmago, R.V. Post deposition heat treatment effects of ceramic superconducting films produced by infrared Nd:YAG pulsed laser deposition. In Heat Treatment; Czerwinski, F., Ed.; InTechOpen: Winchester, UK, 2012; pp. 197–205. ISBN 978-953-51-0768-2. [Google Scholar]
- de Vero, J.C.; Hwang, I.; Shin, H.; Santiago, A.; Lee, D.; Chang, J.; Kim, J.; Sarmago, R.; Song, J.H. Growth and superconducting properties of Bi2Sr2CaCu2O8–δ thin films incorporated with iridate nanoparticles. Phys. Status Solidi A 2014, 211, 1787–1793. [Google Scholar] [CrossRef]
- Nakamura, D.; Tanaka, T.; Ikebuchi, T.; Ueyama, T.; Higashihata, M.; Okada, T. Synthesis of Spherical ZnO Microcrystals by Laser Ablation in Air. Electro Commun. Jpn. 2016, 99, 58–63. [Google Scholar] [CrossRef]
- Santos-Putungan, A.; Singidas, B.; Sarmago, R. Manipulation of low temperature grown ZnO rigid structures via Atomic Force Microscope. HCTL Open Int. J. Technol. Innov. Res. 2014, 11, 1–8. [Google Scholar]
- Santos-Putungan, A.B.; Empizo, M.J.F.; Yamanoi, K.; Vargas, R.M.; Arita, R.; Minami, Y.; Shimizu, T.; Salvador, A.A.; Sarmago, R.V.; Sarukura, N. Intense and fast UV emitting ZnO microrods fabricated by low temperature aqueous chemoical growth method. J. Lum. 2016, 169, 216–219. [Google Scholar]
- Chen, Z.; Cvelbar, U.; Mozetic, M.; He, J.; Sunkara, M. Long-range ordering of oxygen-vacancy planes in Fe2O3 nanowires and nanobelts. Chem. Mater. 2008, 20, 3224–3228. [Google Scholar] [CrossRef]
- Jaeger, R.C. Thermal Oxidation of Silicon. In Introduction to Microelectronic Fabrication; Prentice Hall Inc.: New York, NY, USA, 2001; p. 30. [Google Scholar]
- Arthur, J.R. Reaction between C and O2. Trans. Faraday Soc. 1951, 47, 164–177. [Google Scholar] [CrossRef]
- Jasmin, A.; Rillera, H.; Semblante, O.; Sarmago, R. Surface morphology, microstructure, raman characterization and magnetic ordering of oxidized Fe-sputtered films on silicon substrate. AIP Conf. Proc. 2012, 1482, 572–577. [Google Scholar]
- Maslar, J.; Hurst, W.; Bowers, W.; Hendricks, J.; Aquino, M. In situ raman spectroscopic investigation of aqueous iron corrosion at elevated temperatures and pressures. J. Electrochem. Soc. 2000, 147, 2532–2542. [Google Scholar] [CrossRef]
- Moon, J.; Sahajwalla, V. Kinetic model for the uniform conversion of self-reducing iron oxide carbon briquettes. ISIJ Int. 2003, 43, 1136–1142. [Google Scholar] [CrossRef]
- Crank, J. Mathematics of Diffusion, 2nd ed.; Oxford Science Publications: Oxford, UK, 1975; p. 36. [Google Scholar]
- Chen, M.; Yue, Y.; Jun, Y. Growth of metal and metal oxide nanowires driven by the stress-induced migration. J. Appl. Phys. 2012, 11, 104305. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Cai, R.; Jiang, Q.; Wang, J.; Li, B.; Sharma, A.; Zhou, G. The origin of hematite nanowire growth during thermal oxidation of iron. Mater. Eng. B 2012, 177, 327–336. [Google Scholar] [CrossRef]
- Kim, D.; Gosele, U.; Zacharias, M. Surface-diffusion induced growth of ZnO nanowires. J. Cryst. Growth 2009, 311, 3216–3219. [Google Scholar] [CrossRef]
- Shih, P.-H.; Wu, S. Growth mechanism studies of ZnO nanowires: Experimental observations and short-circuit diffusion analysis. Nanomaterials 2017, 7, 188. [Google Scholar] [CrossRef]
- Cutinho, J.; Chang, B.S.; Oyola-Reynoso, S.; Chen, J.; Akhter, S.S.; Tevis, I.; Bello, N.; Martin, A.; Foster, M.; Thuo, M. Automous thermal-oxidative composition inversion and texture tuning of liquid metal surfaces. ACS Nano 2018, 12, 4744–4753. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vero, J.C.; Jasmin, A.C.; Dasallas, L.L.; Garcia, W.O.; Sarmago, R.V. Synthesis of Iron Oxide Nanostructures via Carbothermal Reaction of Fe Microspheres Generated by Infrared Pulsed Laser Ablation. Coatings 2019, 9, 179. https://doi.org/10.3390/coatings9030179
De Vero JC, Jasmin AC, Dasallas LL, Garcia WO, Sarmago RV. Synthesis of Iron Oxide Nanostructures via Carbothermal Reaction of Fe Microspheres Generated by Infrared Pulsed Laser Ablation. Coatings. 2019; 9(3):179. https://doi.org/10.3390/coatings9030179
Chicago/Turabian StyleDe Vero, Jeffrey C., Alladin C. Jasmin, Lean L. Dasallas, Wilson O. Garcia, and Roland V. Sarmago. 2019. "Synthesis of Iron Oxide Nanostructures via Carbothermal Reaction of Fe Microspheres Generated by Infrared Pulsed Laser Ablation" Coatings 9, no. 3: 179. https://doi.org/10.3390/coatings9030179




