Short- and Long-Term Wettability Evolution and Corrosion Resistance of Uncoated and Polymer-Coated Laser-Textured Steel Surface
Abstract
:1. Introduction
2. Materials and Methods
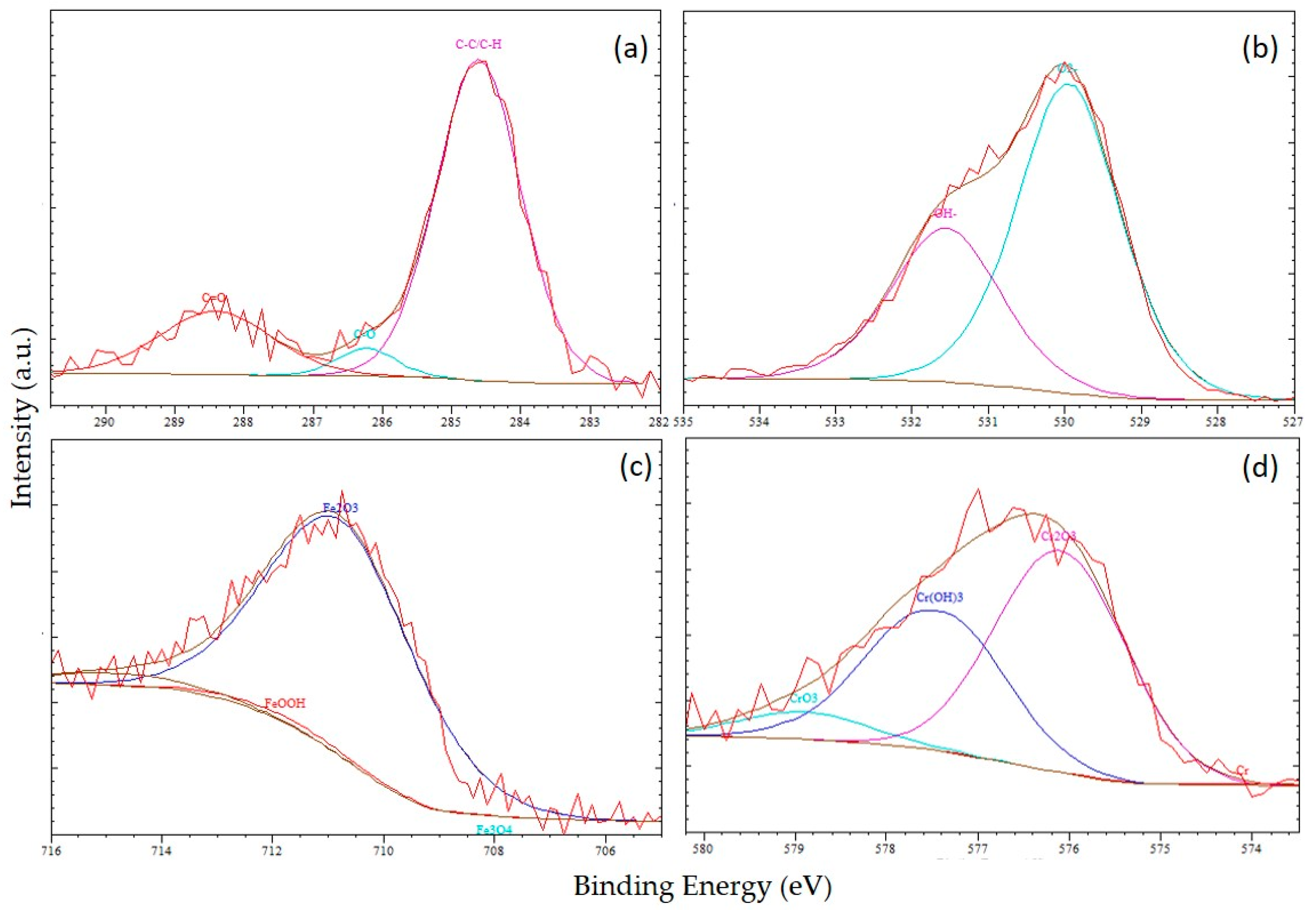
3. Results and Discussion
3.1. Surface Morphology
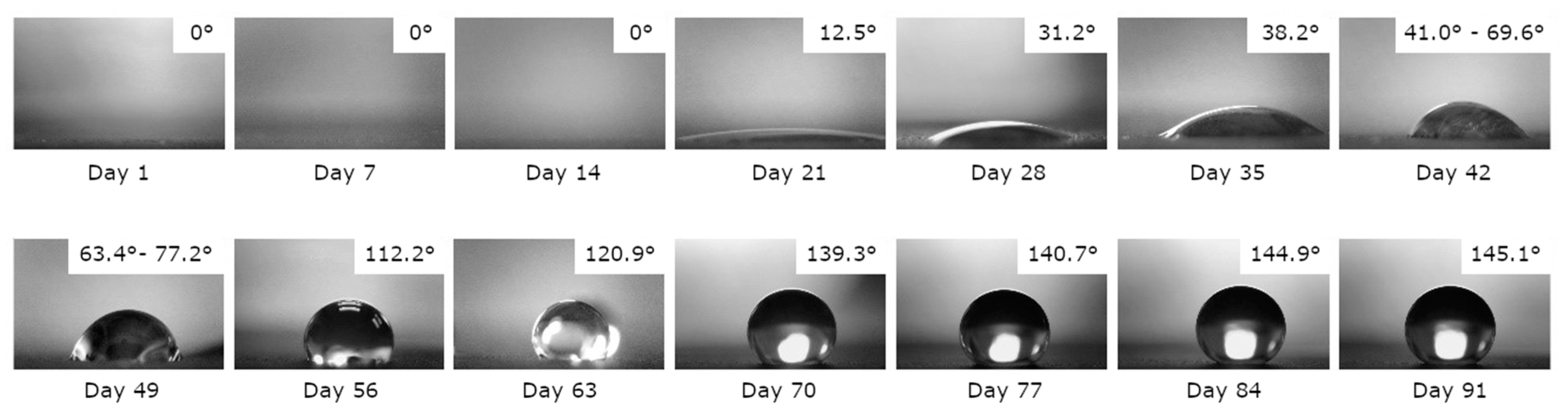
3.2. Surface Wettability and Aging
3.2.1. Uncoated Laser-Textured Surfaces
3.2.2. Coated Laser Textured Surfaces
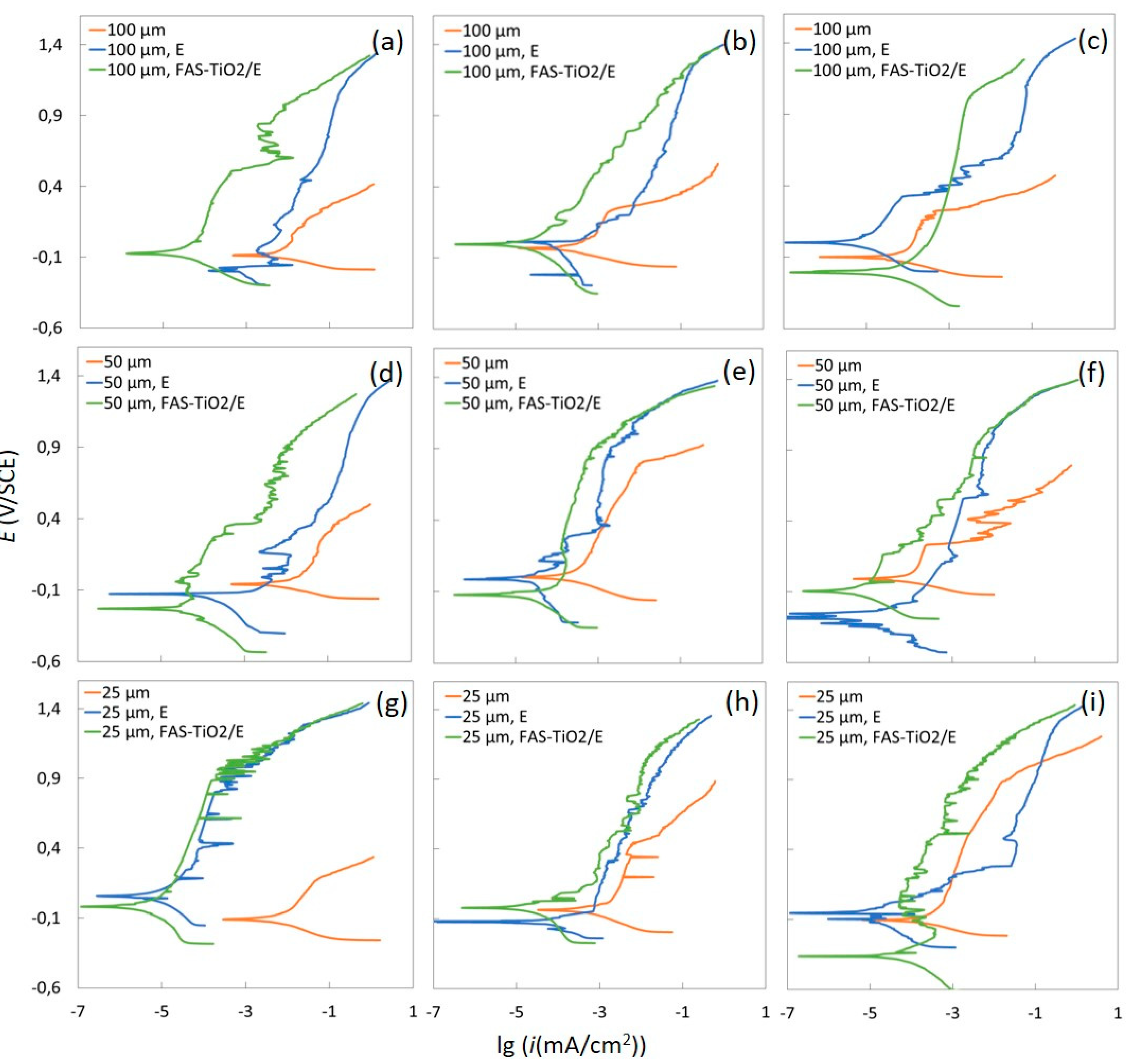
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hryniewicz, T.; Rokicki, R.; Rokosz, K. Corrosion characteristics of medical-grade AISI Type 316L stainless steel surface after electropolishing in a magnetic field. Corrosion 2008, 64, 660–665. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; el Rehim, S.S.A.; Hamza, M.M. Corrosion behavior of some austenitic stainless steels in chloride environments. Mater. Chem. Phys. 2009, 115, 80–85. [Google Scholar] [CrossRef]
- Long, J.Y.; Zhong, M.L.; Zhang, H.J.; Fan, P.X. Superhydrophilicity to superhydrophobicity transition of picosecond laser microstructured aluminum in ambient air. J. Colloid Interface Sci. 2015, 441, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Drzmala, J. Hydrophobicity and collectorless flotation of inorganic materials. Adv. Colloid Interface Sci. 1994, 50, 143–185. [Google Scholar] [CrossRef]
- Long, J.Y.; Zhong, M.L.; Fan, P.X.; Gong, D.W.; Zhang, H.J. Wettability conversion of ultrafast laser structured copper surface. J. Laser Appl. 2015, 27, S29107. [Google Scholar] [CrossRef]
- Kamegawa, T.; Irikawa, K.; Yamashita, H. Multifunctional surface designed by nanocomposite coating of polytetrafluoroethylene and TiO2 photocatalyst: Self-cleaning and superhydrophobicity. Sci. Rep. 2017, 7, 13628. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Karunakaran, R.G.; Guo, J.; Yang, S. Transparent, superhydrophobic surfaces from one-step spin coating of hydrophobic nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 1118–1125. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, N.; Xu, J. Fabrication and application of superhydrophilic surfaces: A review. J. Adhes. Sci. Technol. 2014, 28, 769–790. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, M.; Liu, Z.; Nishimoto, S.; Saito, H.; Murakami, T.; Fujishima, A. Preparation and photocatalytic wettability conversion of TiO2-based superhydrophobic surfaces. Langmuir 2006, 22, 9477–9479. [Google Scholar] [CrossRef]
- Lee, S.; Kim, W.; Yong, K. Overcoming the water vulnerability of electronic devices: A highly water-resistant ZnO nanodevice with multifunctionality. Adv. Mater. 2011, 23, 4398. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Sun, Y.; Zheng, Y.; Wang, X.; Shang, Y.; Wang, L.; Liu, C. Facile fabrication of superhydrophobic surfaces with corrosion resistance by nanocomposite coating of TiO2 and polydimethylsiloxane. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 471–477. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Synergistic effect of superhydrophobicity and oxidized layers on corrosion resistance of aluminum alloy surface textured by nanosecond laser treatment. ACS Appl. Mater. Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef] [PubMed]
- Ta, V.D.; Dunn, A.; Wasley, T.J.; Li, J.; Kay, R.W.; Stringer, J.; Smith, P.J.; Esenturk, E.; Connaughton, C.; Shephard, J.D. Laser textured superhydrophobic surfaces and their applications for homogeneous spot deposition. Appl. Surf. Sci. 2016, 365, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Gregorcic, P.; Setina-Batic, B.; Hocevar, M. Controlling the stainless steel surface wettability by nanosecond direct laser texturing at high fluences. Appl. Phys. A Mater. Sci. Process. 2017, 123, 766. [Google Scholar] [CrossRef] [Green Version]
- Ta, V.D.; Dunn, A.; Wasley, T.J.; Li, J.; Kay, R.W.; Stringer, J.; Smith, P.J.; Esenturk, E.; Connaughton, C.; Shephard, J.D. Laser textured surface gradients. Appl. Surf. Sci. 2016, 371, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Ta, D.V.; Dunn, A.; Wasley, T.J.; Kay, R.W.; Stringer, J.; Smith, P.J.; Connaughton, C.; Shephard, J.D. Nanosecond laser textured superhydrophobic metallic surfaces and their chemical sensing applications. Appl. Surf. Sci. 2015, 357, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Kietzig, A.M.; Hatzikiriakos, S.G.; Englezos, P. Patterned Superhydrophobic Metallic Surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef]
- Gregorčič, P.; Conradi, M.; Hribar, L.; Hočevar, M.; Gregorcic, P.; Conradi, M.; Hribar, L.; Hocevar, M. Long-term influence of laser-processing parameters on (super)hydrophobicity development and stability of stainless-steel surfaces. Materials 2018, 11, 2240. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Gnedenkov, S.V.; Alpysbaeva, D.A.; Egorkin, V.S.; Emelyanenko, A.M.; Sinebryukhov, S.L.; Zaretskaya, A.K. Corrosion resistance of composite coatings on low-carbon steel containing hydrophobic and superhydrophobic layers in combination with oxide sublayers. Corros. Sci. 2012, 55, 238–245. [Google Scholar] [CrossRef]
- Trdan, U.; Hocevar, M.; Gregorcic, P. Transition from superhydrophilic to superhydrophobic state of laser textured stainless steel surface and its effect on corrosion resistance. Corros. Sci. 2017, 123, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Emelyanenko, A.M.; Shagieva, F.M.; Domantovsky, A.G.; Boinovich, L.B. Nanosecond laser micro- and nanotexturing for the design of a superhydrophobic coating robust against long-term contact with water, cavitation, and abrasion. Appl. Surf. Sci. 2015, 332, 513–517. [Google Scholar] [CrossRef]
- Fairley, N. Casa XPS VAMAS Processing Software; Casa Software Ltd.: Teignmouth, UK, 2018. [Google Scholar]
- Conradi, M.; Drnovšek, A.; Gregorčič, P. Wettability and friction control of a stainless steel surface by combining nanosecond laser texturing and adsorption of superhydrophobic nanosilica particles. Sci. Rep. 2018, 8, 7457. [Google Scholar] [CrossRef] [PubMed]
- McHale, G.; Shirtcliffe, N.J.; Newton, M.I. Super-hydrophobic and super-wetting surfaces: Analytical potential? Analyst 2004, 129, 284–287. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zheng, H.Y.; Lim, C.P.; Lam, Y.C. Polymer hydrophilicity and hydrophobicity induced by femtosecond laser direct irradiation. Appl. Phys. Lett. 2009, 95, 111110. [Google Scholar] [CrossRef]
- Lai, J.N.; Sunderland, B.; Xue, J.M.; Yan, S.; Zhao, W.J.; Folkard, M.; Michael, B.D.; Wang, Y.G. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef]




| Scan Line Separation ∆x = ∆y [μm] | Sa [μm] | AR/A0 |
|---|---|---|
| 100 μm | 10.8 ± 0.1 | 2.0 ± 0.3 |
| 100 μm, E | 10.9 ± 0.1 | 1.7 ± 0.4 |
| 100 μm, FAS-TiO2/E | 11.2 ± 0.1 | 1.8 ± 0.2 |
| 50 μm | 10.7 ± 0.1 | 4.3 ± 0.1 |
| 50 μm, E | 10.4 ± 0.2 | 4.3 ± 0.2 |
| 50 μm, FAS-TiO2/E | 10.7 ± 0.2 | 4.1 ± 0.2 |
| 25 μm | 5.2 ± 0.1 | 1.5 ± 0.1 |
| 25 μm, E | 5.3 ± 0.2 | 1.4 ± 0.2 |
| 25 μm, FAS-TiO2/E | 5.6 ± 0.2 | 1.6 ± 0.2 |
| Scan Line Separations [μm] | Complete Wetting Regime | Transition Period | Time Constant, τ |
|---|---|---|---|
| 100, ambient | 4 days | 16 days | 10.6 days |
| 100, chamber | 15 days | 31 days | 20.8 days |
| 50, ambient | 8 days | 13 days | 6.5 days |
| 50, chamber | 21 days | 59 days | 29.4 days |
| 25, ambient | 21 days | 14 days | 4.1 days |
| 25, chamber | 35 days | 122 days | 74.1 days |
| Scan Line Separation [μm] | Uncoated Contact angle [°] | E Contact Angle [°] | FAS-TiO2/E Contact Angle [°] |
|---|---|---|---|
| Immediately after preparation | |||
| 100, ambient | 0 | 97.5 ± 1.4 | 143.4 ± 3.1 |
| 50, ambient | 0 | 112.1 ± 1.9 | 151.1 ± 2.1 |
| 25, ambient | 0 | 108.3 ± 2.3 | 151.9 ± 0.7 |
| 3 months after preparation | |||
| 100, ambient | 130.5 ± 0.6 | 104.3 ± 2.6 | 140.5 ± 2.2 |
| 100, chamber | 138.6 ± 3.2 | 100.2 ± 1.7 | 147.3 ± 1.2 |
| 50, ambient | 146.3 ± 1.6 | 115.0 ± 2.2 | 151.1 ± 0.9 |
| 50, chamber | 145.1 ± 1.6 | 108.8 ± 1.5 | 151.5 ± 1.0 |
| 25, ambient | 150.7 ± 1.1 | 115.0 ± 1.2 | 153.7 ± 1.3 |
| 25, chamber | 56.5 ± 7.8 | 107.3 ± 2.2 | 152.0 ± 1.1 |
| 1 year after preparation | |||
| 100, ambient | 154.5 ± 1.5 | 105.8 ± 2.2 | 149.8 ± 1.9 |
| 100, chamber | 155.3 ± 1.8 | 103.0 ± 2.1 | 154.3 ± 0.9 |
| 50, ambient | 155.1 ± 2.1 | 117.3 ± 1.6 | 154.2 ± 1.6 |
| 50, chamber | 154.2 ± 2.1 | 110.8 ± 2.2 | 155.1 ± 1.2 |
| 25, ambient | 155.8 ± 1.8 | 118.4 ± 1.9 | 156.3 ± 1.8 |
| 25, chamber | 156.3 ± 1.6 | 112.5 ± 1.8 | 156.1 ± 1.5 |
| Scan Line Separation [μm] | Ecorr [mV] | icorr [µA/cm2] | vcorr [µm/year] | Contact Angle [o] |
|---|---|---|---|---|
| Immediately after preparation | ||||
| 100 µm | −86.6 ± 0.5 | 4.345 ± 0.008 | 16.25 ± 0.07 | 0 |
| 100 µm, E | −172.9 ± 0.6 | 2.500 ± 0.006 | 3.45 ± 0.05 | 97.5 ± 1.4 |
| 100 µm, FAS-TiO2/E | −70.7 ± 0.4 | 0.010 ± 0.001 | 0.25 ± 0.04 | 143.4 ± 3.1 |
| 50 µm | −59.7 ± 0.3 | 2.402 ± 0.005 | 20.51 ± 0.08 | 0 |
| 50 µm, E | −127.7 ± 0.5 | 0.209 ± 0.004 | 0.58 ± 0.03 | 112.1 ± 1.9 |
| 50 µm, FAS-TiO2/E | −230.4 ± 0.9 | 0.007 ± 0.001 | 0.07 ± 0.01 | 151.1 ± 2.1 |
| 25 µm | −108.8 ± 0.5 | 5.540 ± 0.009 | 39.73 ± 0.07 | 0 |
| 25 µm, E | 55.9 ± 0.2 | 0.013 ± 0.001 | 0.13 ± 0.01 | 108.3 ± 2.3 |
| 25 µm, FAS-TiO2/E | −17.6 ± 0.1 | 0.002 ± 0.001 | 0.07 ± 0.01 | 151.9 ± 0.7 |
| 3 months after preparation | ||||
| 100 µm | −30.6 ± 0.2 | 0.130 ± 0.007 | 0.70 ± 0.03 | 130.5 ± 0.6 |
| 100 µm, E | 0.6 ± 0.1 | 0.155 ± 0.008 | 1.20 ± 0.04 | 104.3 ± 2.6 |
| 100 µm, FAS-TiO2/E | −4.6 ± 0.1 | 0.005 ± 0.001 | 0.15 ± 0.01 | 140.5 ± 2.2 |
| 50 µm | 1.6 ± 0.1 | 0.040 ± 0.004 | 0.42 ± 0.02 | 146.3 ± 1.6 |
| 50 µm, E | −11.1 ± 0.1 | 0.005 ± 0.001 | 0.12 ± 0.01 | 115.0 ± 2.2 |
| 50 µm, FAS-TiO2/E | −121.4 ± 0.9 | 0.005 ± 0.001 | 0.05 ± 0.01 | 151.1 ± 0.9 |
| 25 µm | −35.7 ± 0.2 | 0.367 ± 0.006 | 2.60 ± 0.05 | 150.7 ± 1.1 |
| 25 µm, E | −120.3 ± 0.7 | 0.067 ± 0.003 | 0.40 ± 0.02 | 115.0 ± 1.2 |
| 25 µm, FAS-TiO2/E | −20.4 ± 0.1 | 0.013 ± 0.002 | 0.27 ± 0.03 | 153.7 ± 1.3 |
| 1 year after preparation | ||||
| 100 µm | −105.0 ± 0.9 | 0.015 ± 0.002 | 0.15 ± 0.01 | 155.3 ± 1.8 |
| 100 µm, E | −4.9 ± 0.8 | 0.005 ± 0.001 | 0.05 ± 0.01 | 103.0 ± 2.1 |
| 100 µm, FAS-TiO2/E | −210.7 ± 0.9 | 0.035 ± 0.004 | 0.15 ± 0.01 | 154.3 ± 0.9 |
| 50 µm | −8.4 ± 0.1 | 0.009 ± 0.001 | 0.09 ± 0.01 | 154.2 ± 2.1 |
| 50 µm, E | −298.8 ± 0.3 | 0.009 ± 0.001 | 0.12 ± 0.01 | 110.8 ± 2.2 |
| 50 µm, FAS-TiO2/E | −96.6 ± 0.5 | 0.002 ± 0.001 | 0.02 ± 0.01 | 155.1 ± 1.2 |
| 25 µm | −122.5 ± 0.6 | 0.127 ± 0.009 | 1.47 ± 0.02 | 156.3 ± 1.6 |
| 25 µm, E | −50.8 ± 0.9 | 0.007 ± 0.001 | 0.07 ± 0.01 | 112.5 ± 1.8 |
| 25 µm, FAS-TiO2/E | −366.4 ± 0.3 | 0.060 ± 0.005 | 0.53 ± 0.05 | 156.1 ± 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conradi, M.; Sever, T.; Gregorčič, P.; Kocijan, A. Short- and Long-Term Wettability Evolution and Corrosion Resistance of Uncoated and Polymer-Coated Laser-Textured Steel Surface. Coatings 2019, 9, 592. https://doi.org/10.3390/coatings9090592
Conradi M, Sever T, Gregorčič P, Kocijan A. Short- and Long-Term Wettability Evolution and Corrosion Resistance of Uncoated and Polymer-Coated Laser-Textured Steel Surface. Coatings. 2019; 9(9):592. https://doi.org/10.3390/coatings9090592
Chicago/Turabian StyleConradi, Marjetka, Tina Sever, Peter Gregorčič, and Aleksandra Kocijan. 2019. "Short- and Long-Term Wettability Evolution and Corrosion Resistance of Uncoated and Polymer-Coated Laser-Textured Steel Surface" Coatings 9, no. 9: 592. https://doi.org/10.3390/coatings9090592




