Synthetic Strategies for the Fabrication of Cationic Surface-Modified Cellulose Nanocrystals
Abstract
:1. Introduction
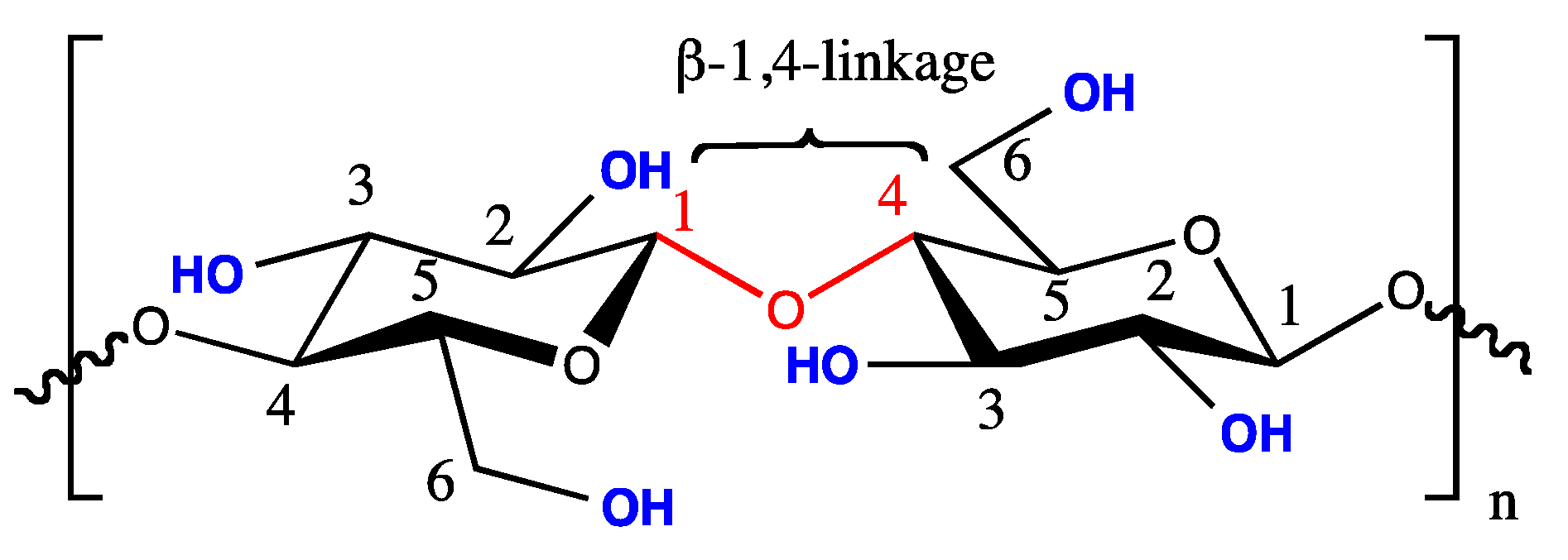
1.1. Cellulose and Cellulose Nanocrystals
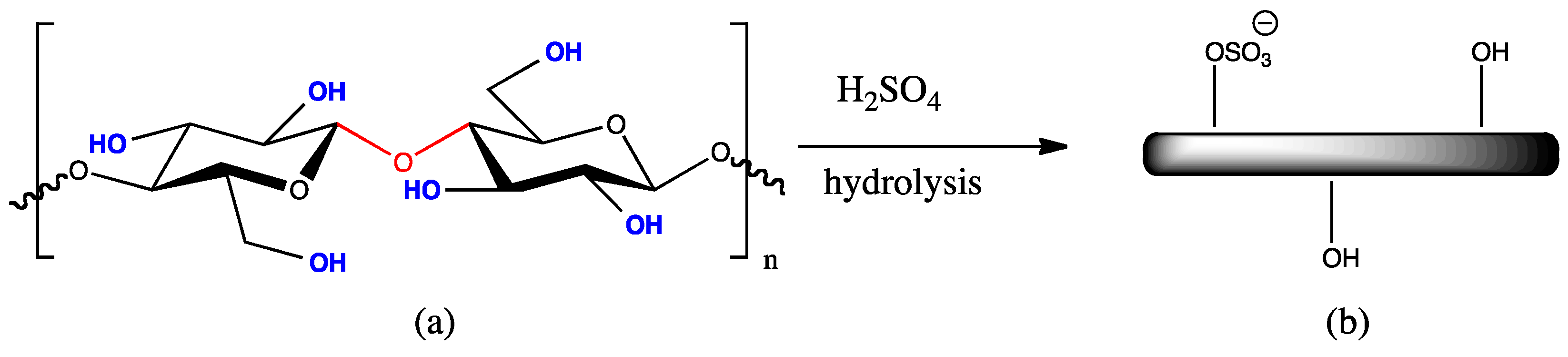
1.2. Preparation of CNCs via Acid Hydrolysis of Native Cellulose
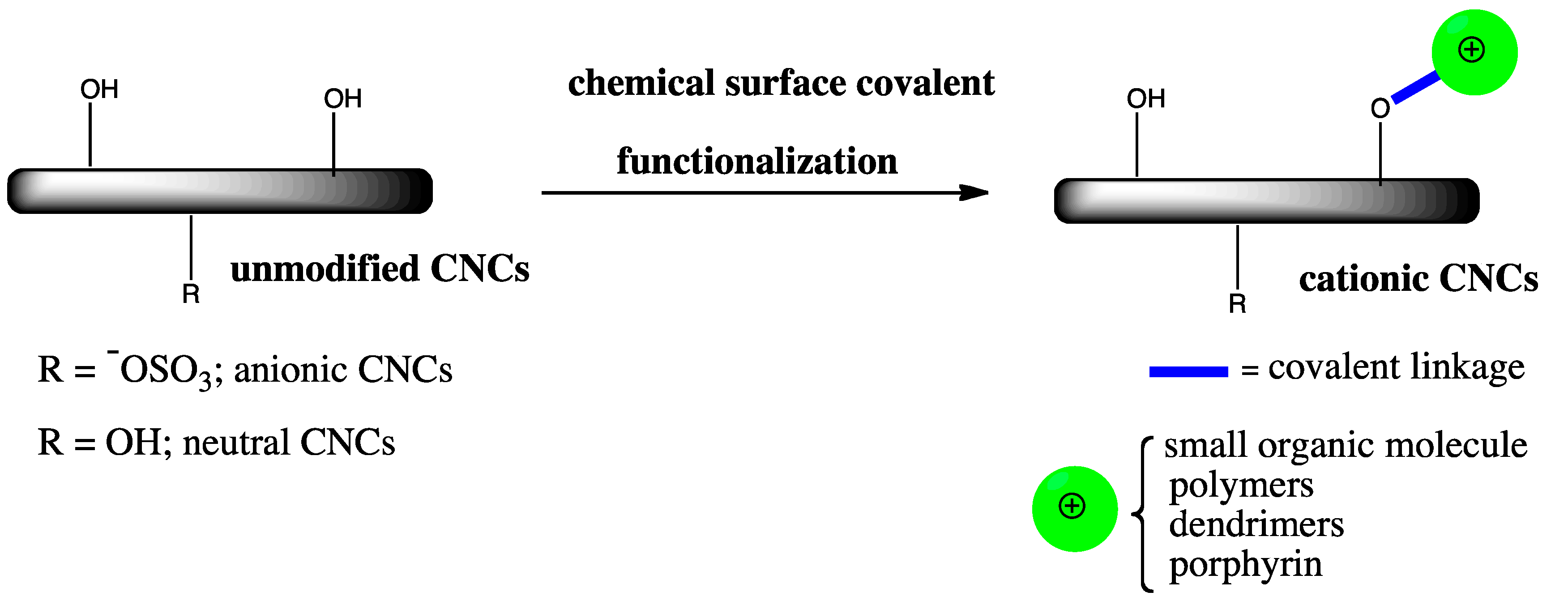
1.3. Surface Covalent Functionalization of CNCs
2. Surface Covalent Functionalization Methods for the Fabrication of Cationic CNCs
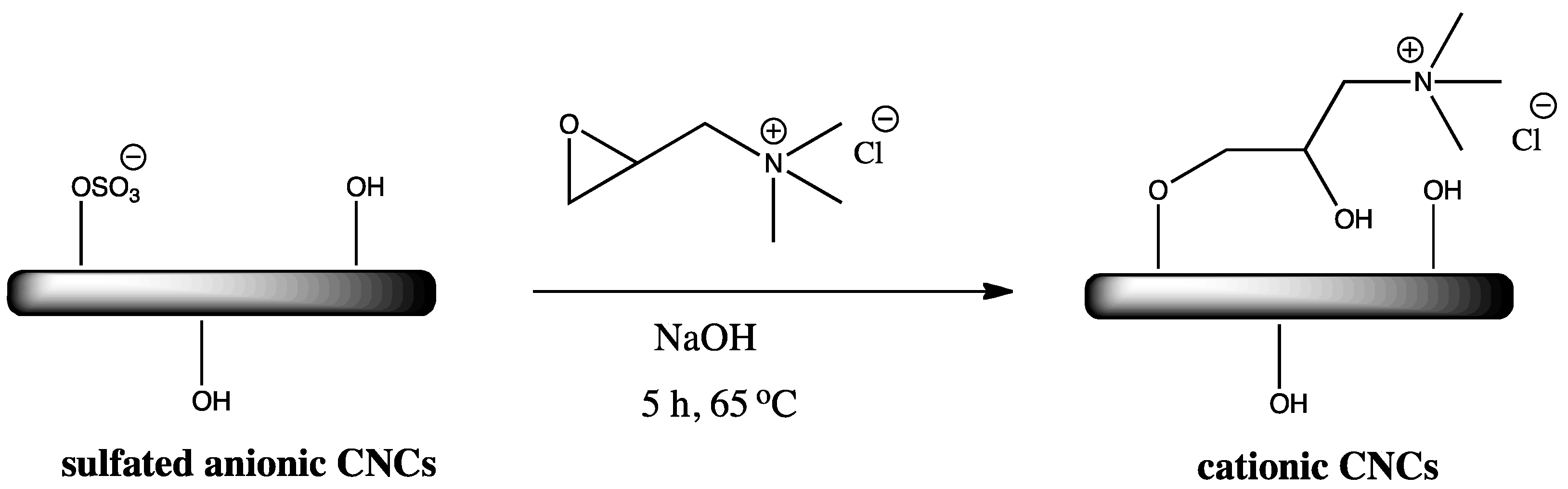
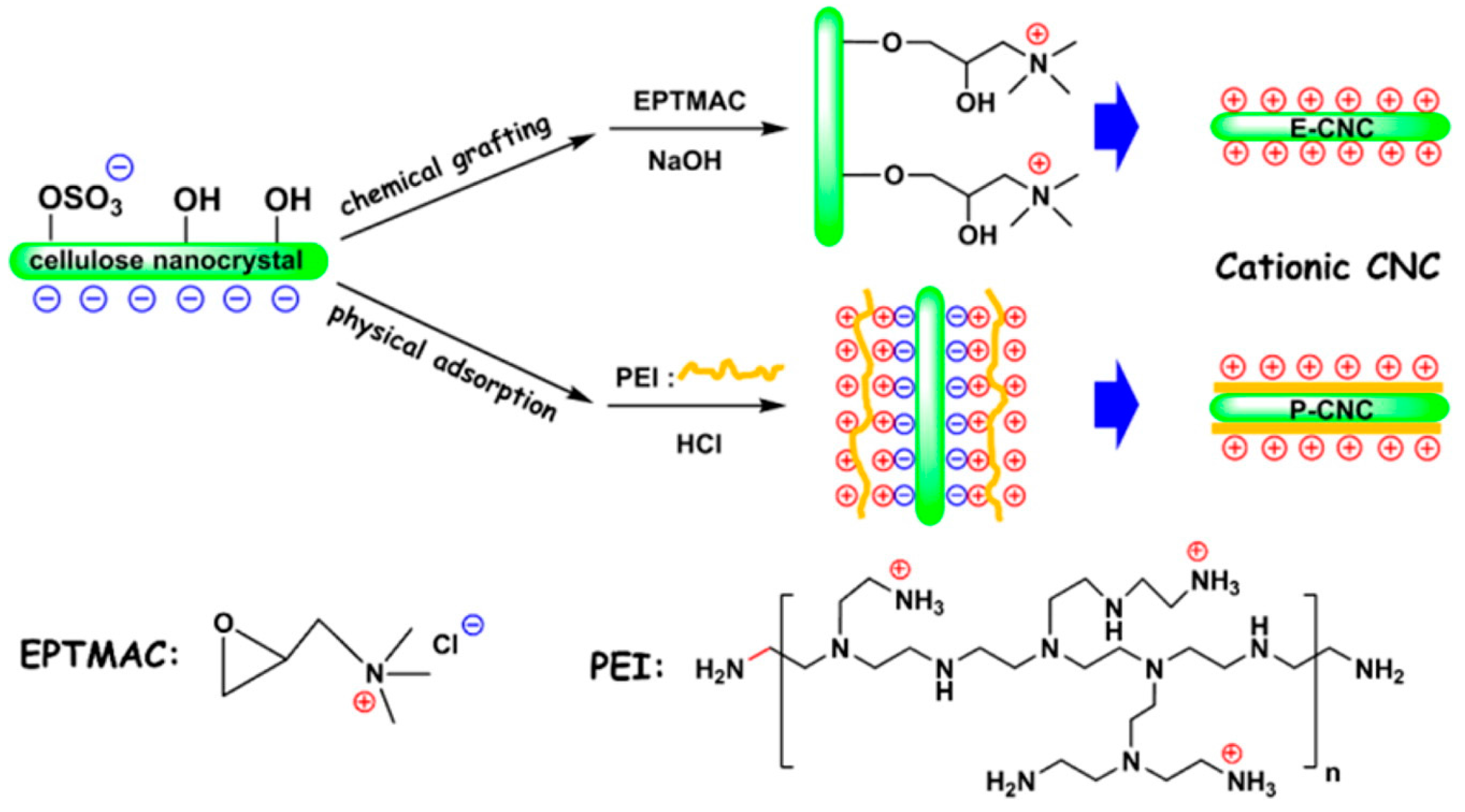
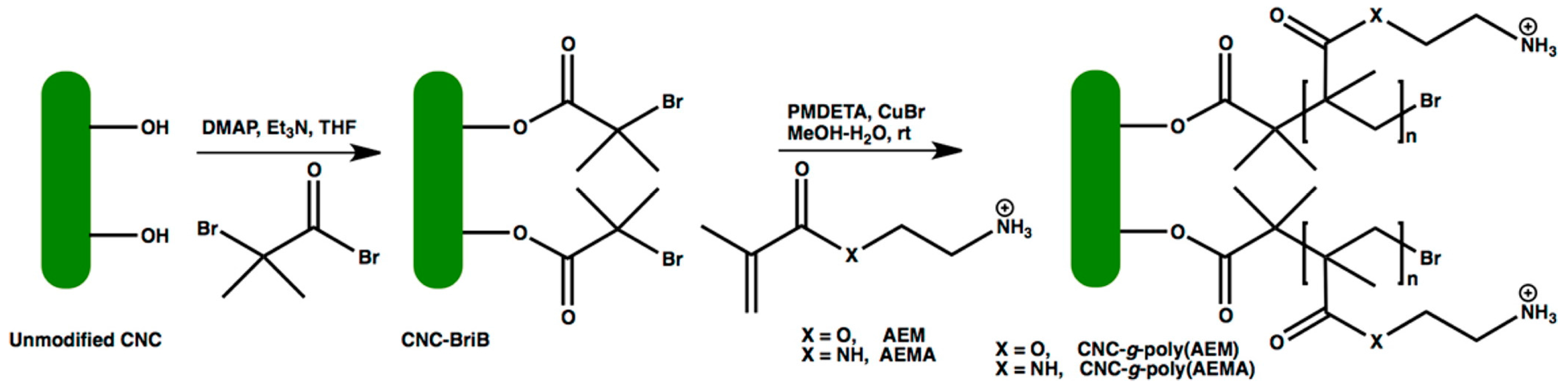
2.1. Surface Cationization of CNCs via Etherification Reaction
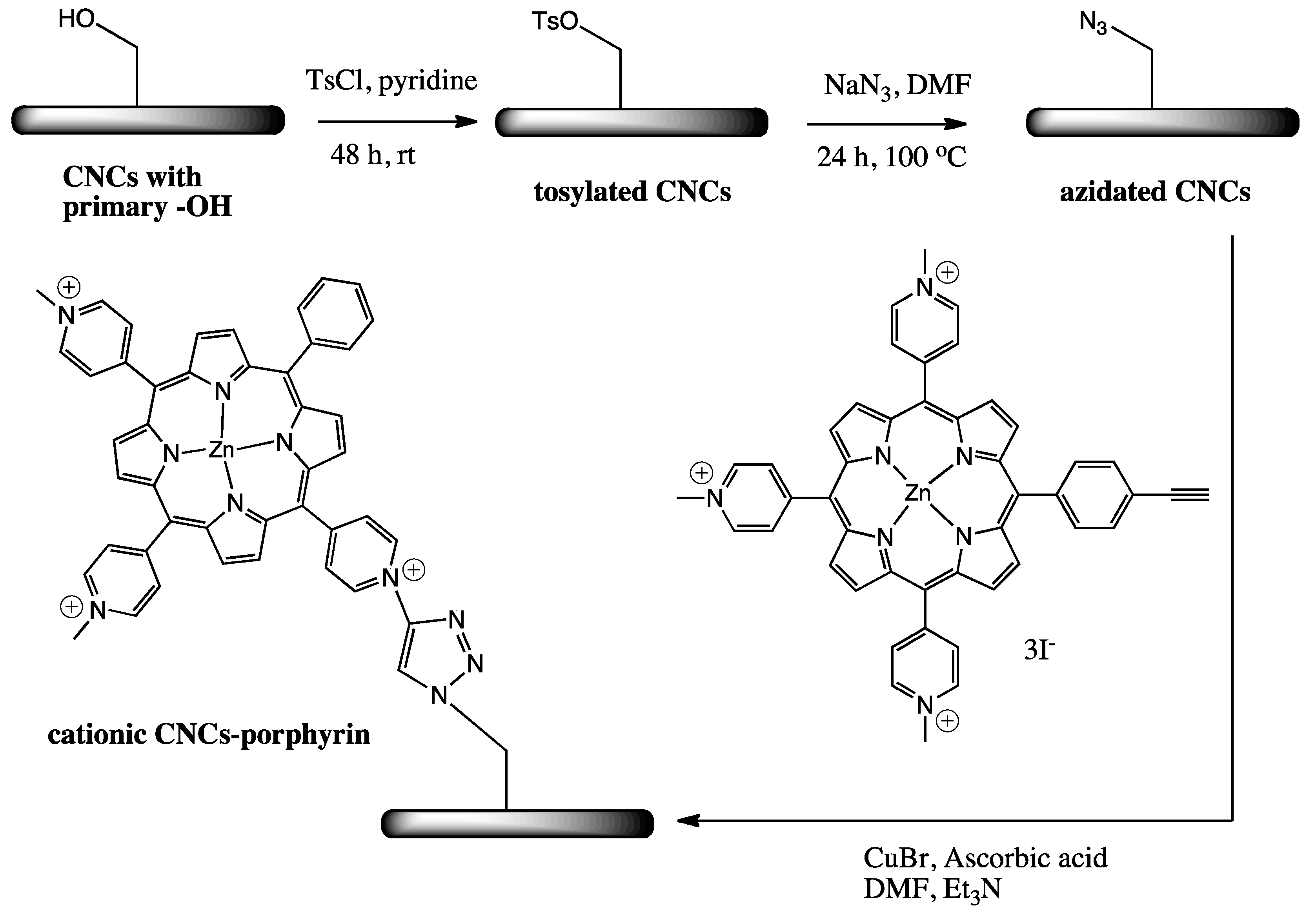
2.2. Cationic Porphyrin-Based CNCs with Photobactericidal Activity
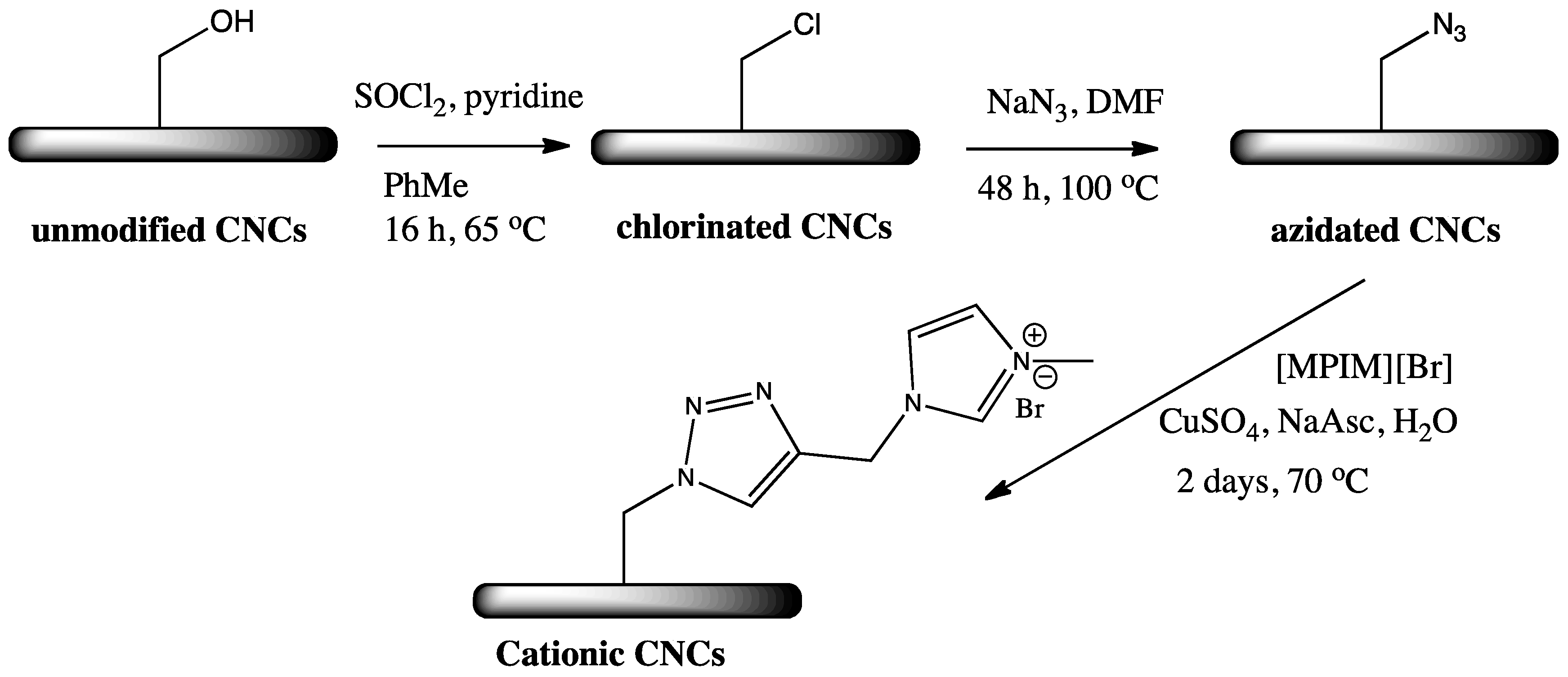
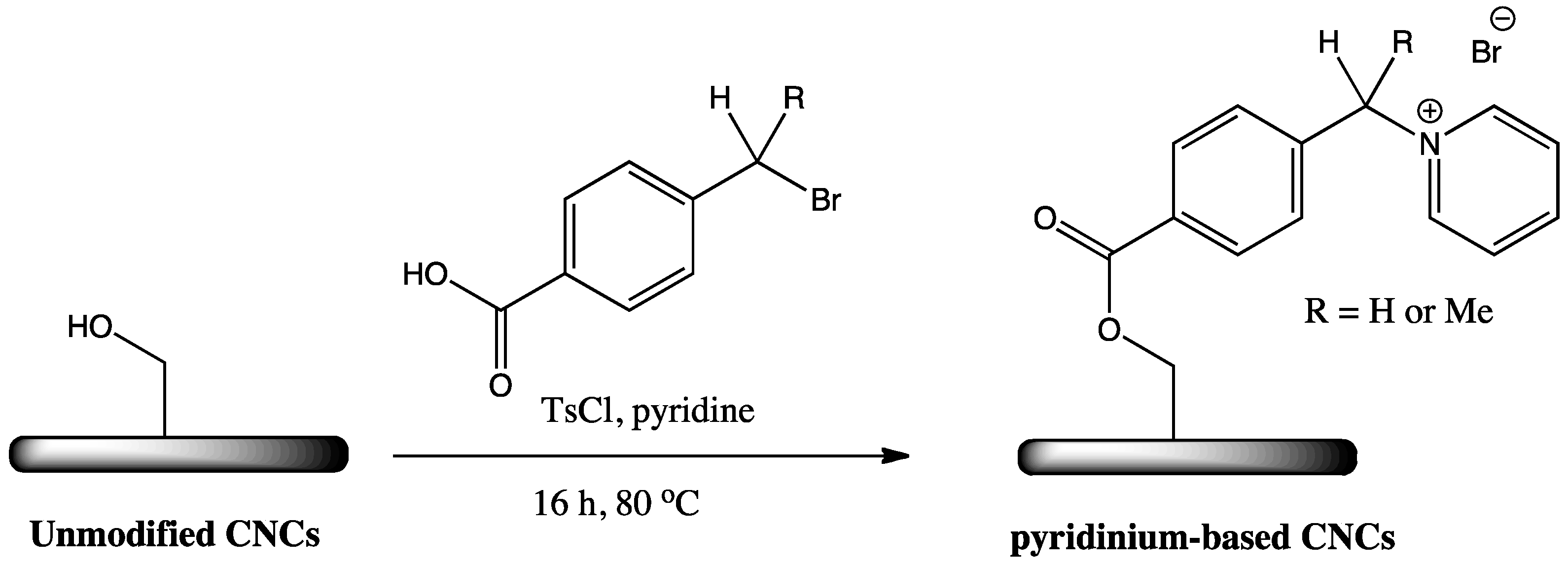
2.3. Cationic Imidazolium and Pyridinium Grafted CNCs
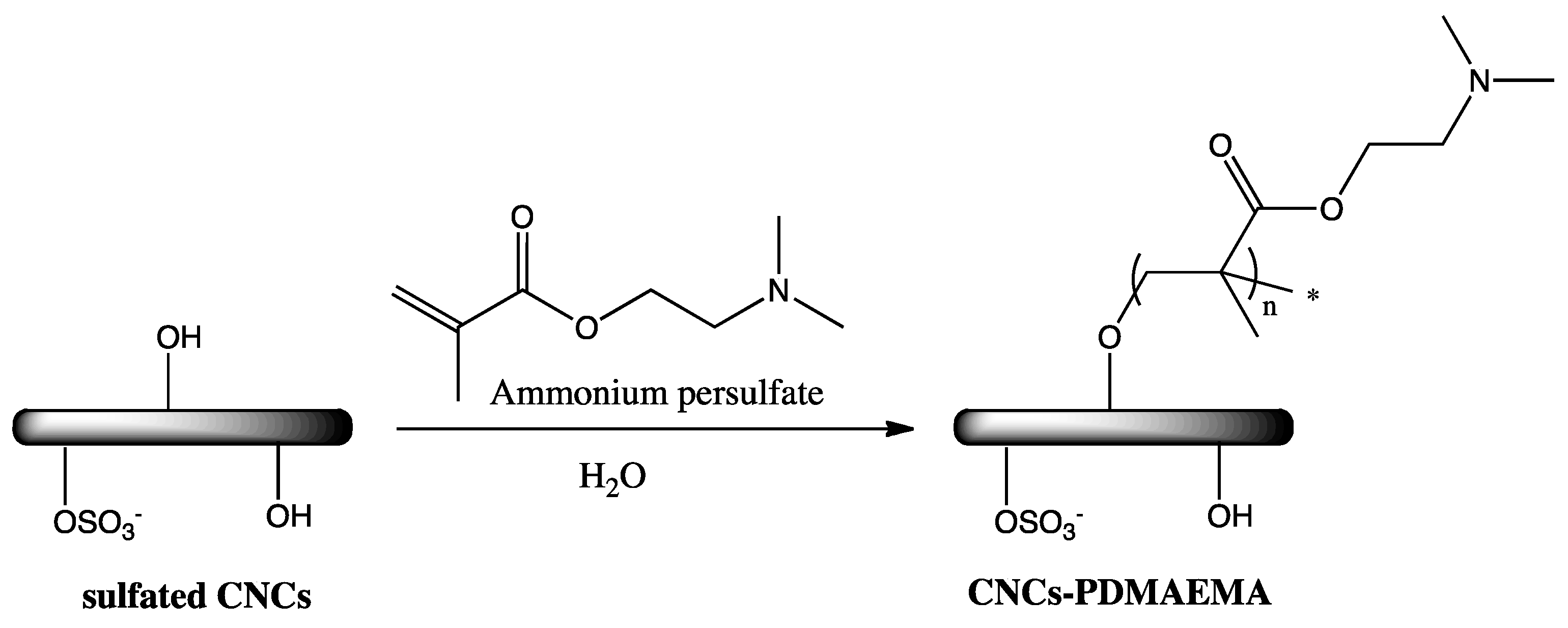
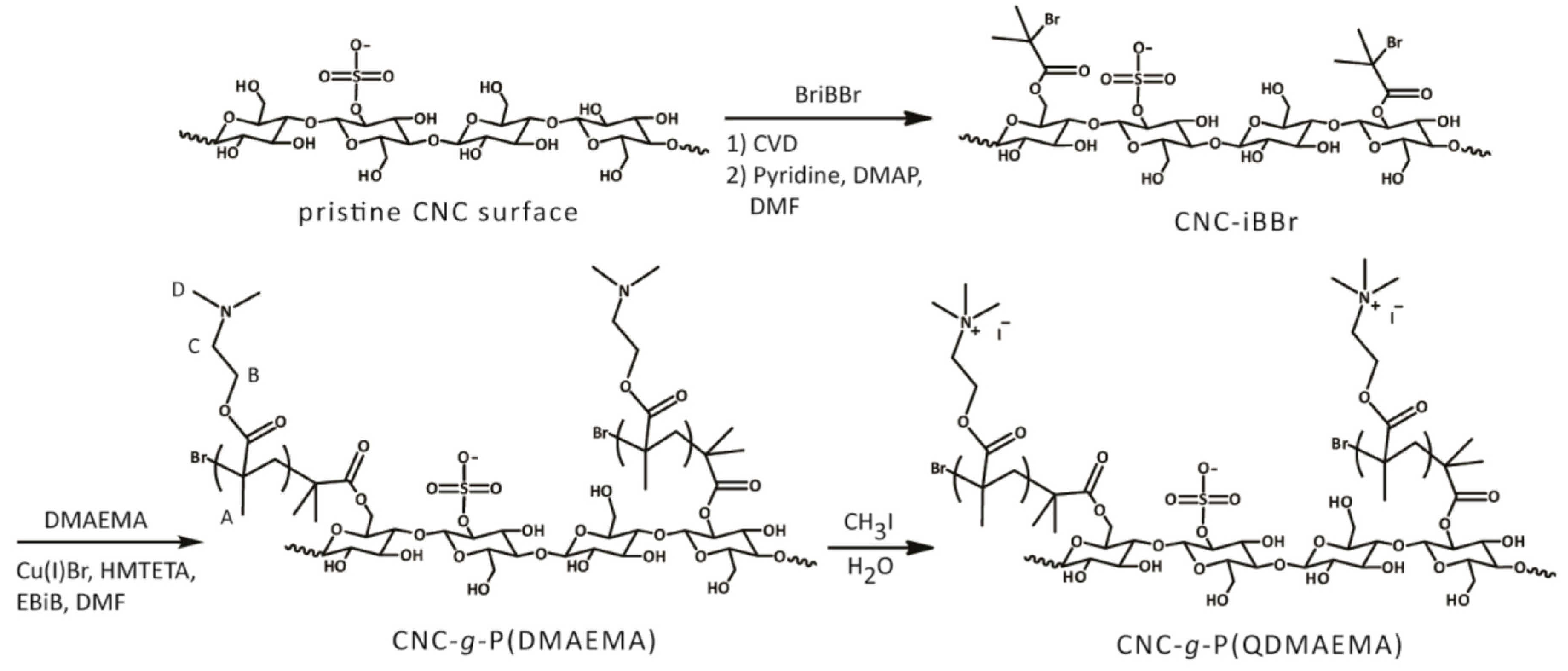
2.4. Cationic CNCs via Polymerization Techniques
2.5. Other Covalent Cationization Synthetic Methods
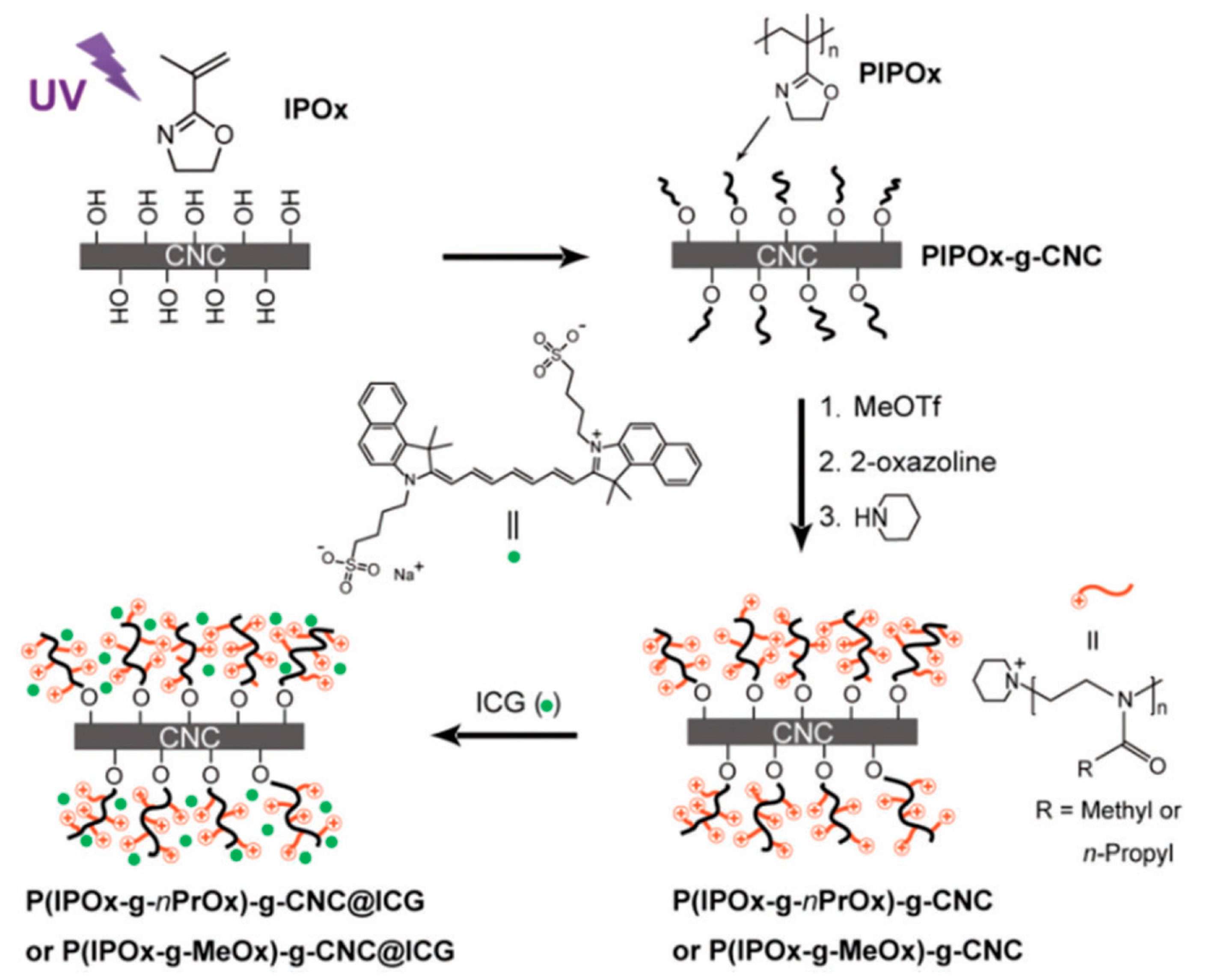
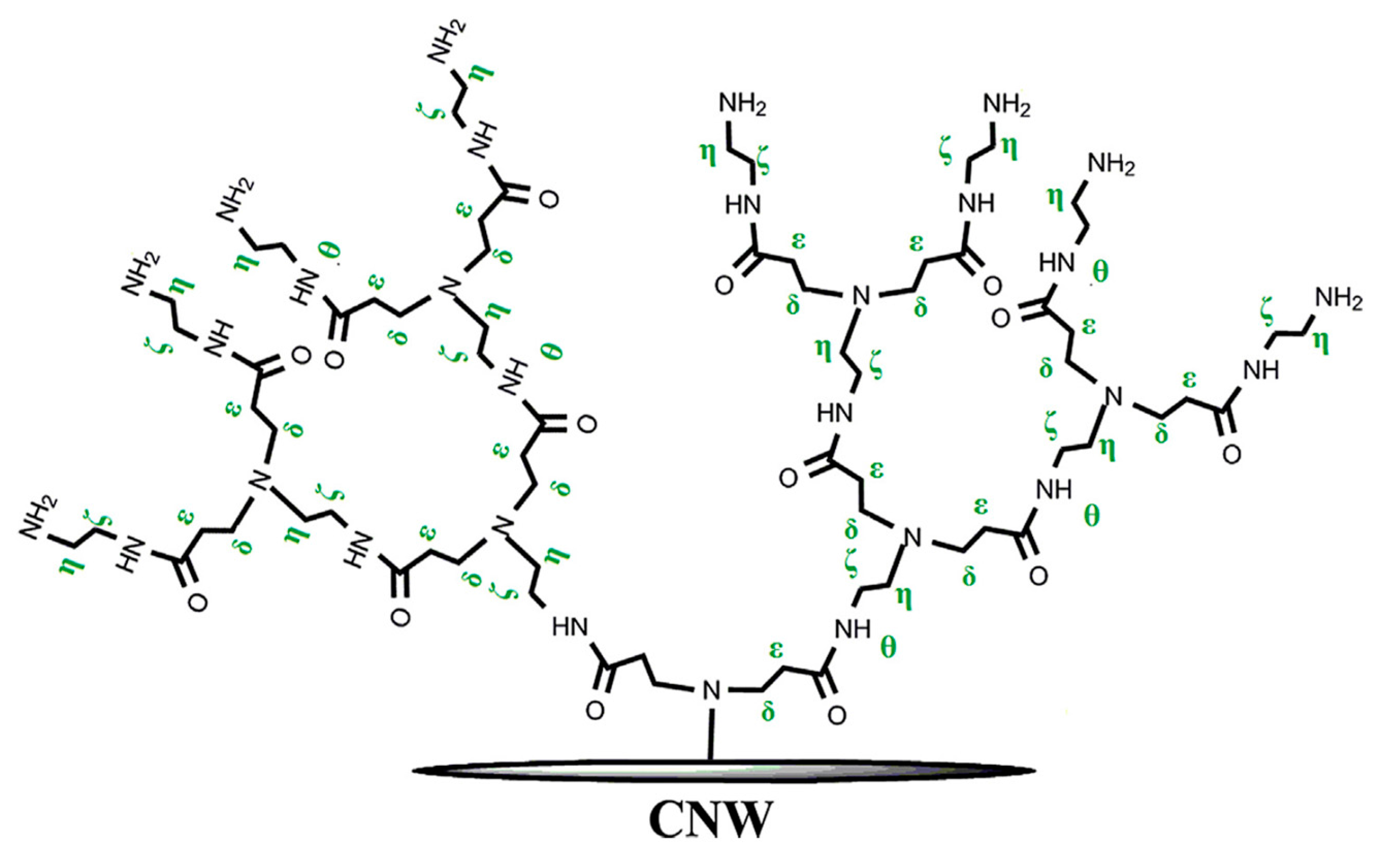
2.5.1. Cationization of CNCs via Grafting of Cationic Hyperbranched Dendrimers
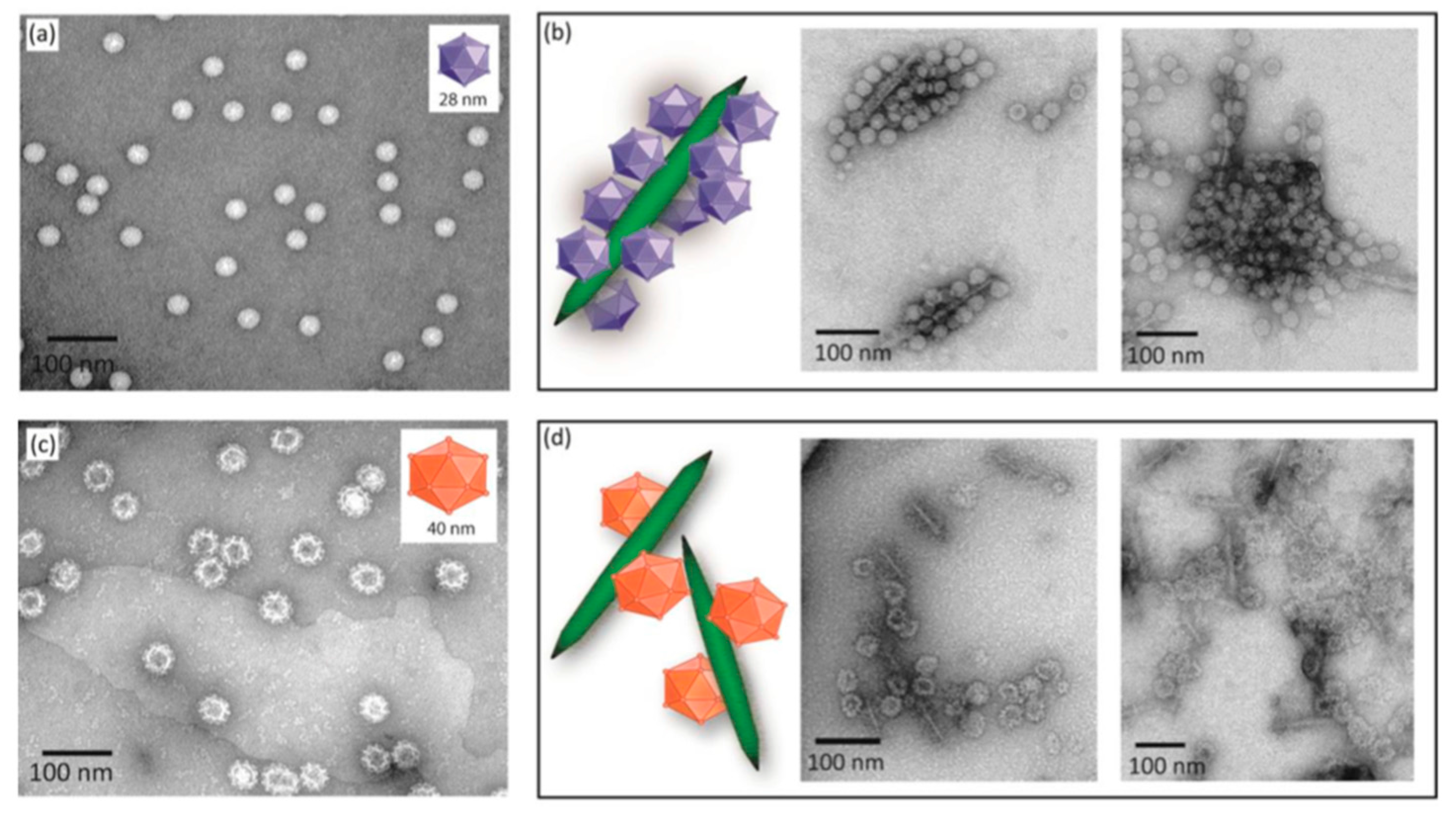
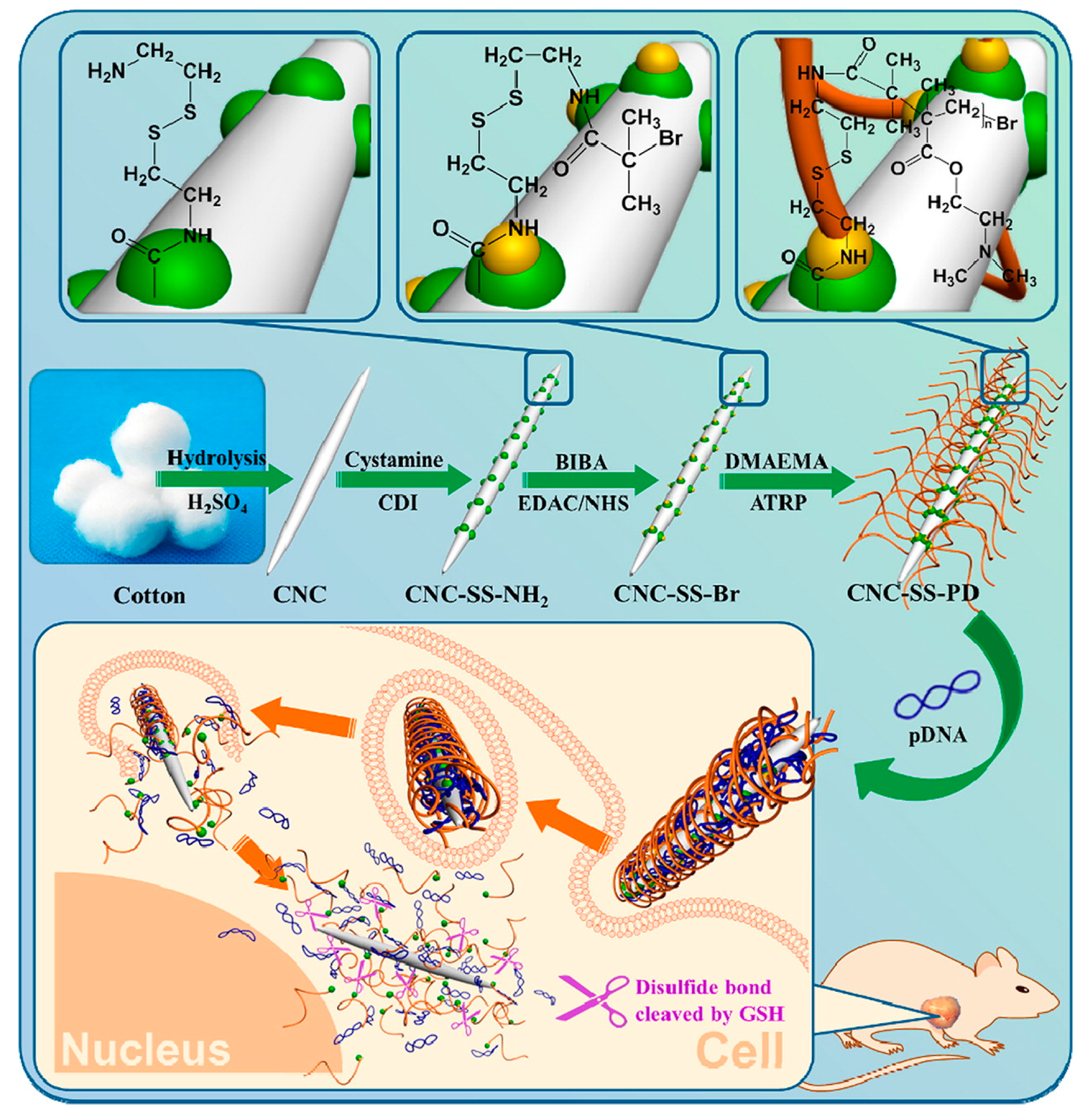
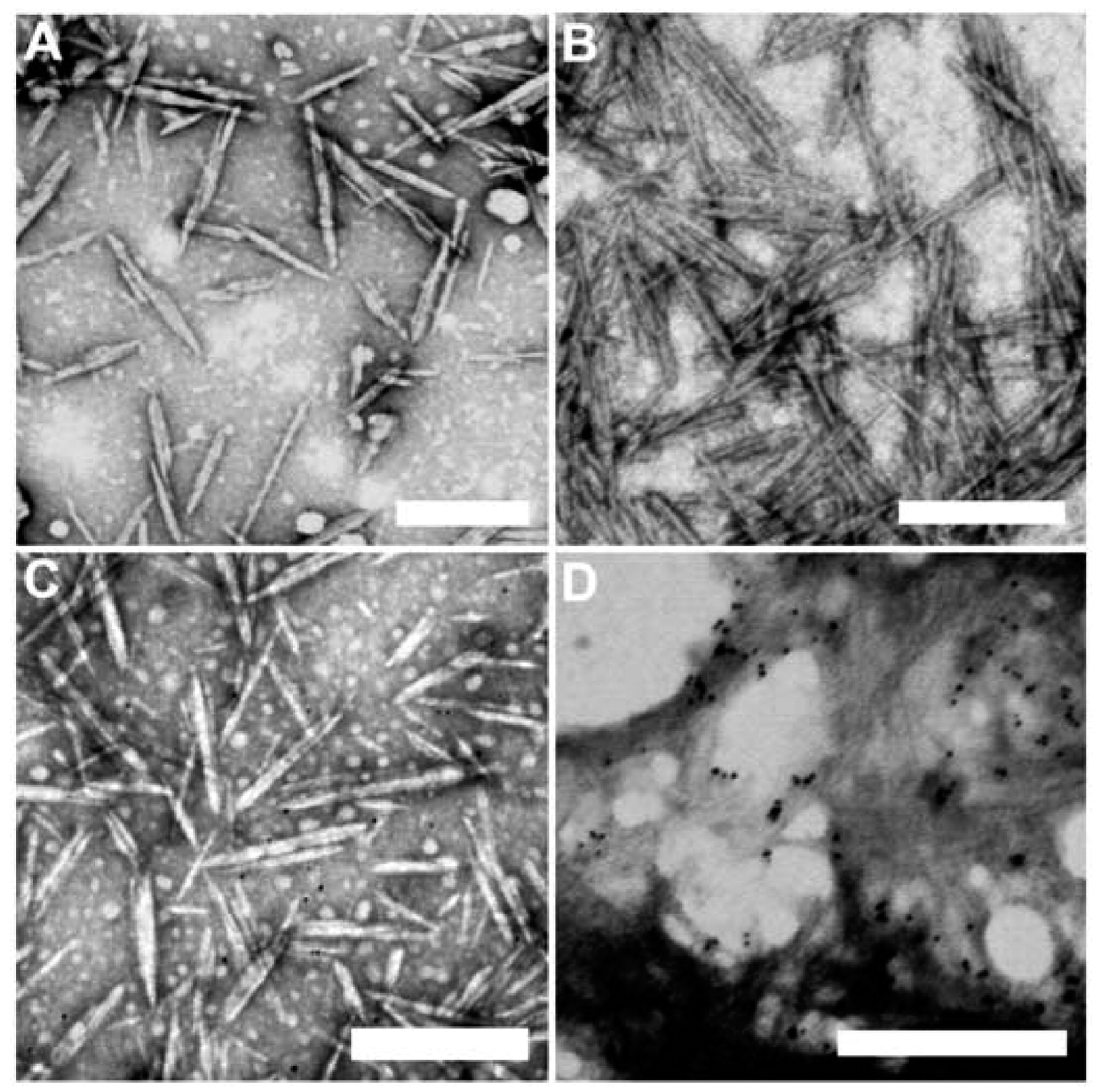
2.5.2. Cationic CNCs as siRNA Delivery Agents
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Ranby, B.G. Aqueous colloidal solutions of cellulose micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef]
- Ranby, B.G.; Ribi, E. Uber Den Feinbau Zellulose. Experientia 1950, 6, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaillé, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Favier, V.; Canova, G.R.; Cavaillé, J.Y.; Chanzy, H.; Dufresne, A.; Gauthier, C. Nanocomposites materials from latex and cellulose whiskers. Polym. Adv. Technol. 1995, 6, 351–355. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose nanocrystals: A versatile nanoplatform for emerging biomedical applications. Expert Opin. Drug Deliv. 2016, 13, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Future Markets Inc. The Global Market for Nanocellulose to 2020; Future Markets Inc.: Edinburgh, UK, 2013. [Google Scholar]
- Microfibrillar cellulose project from borregaard gets EU funding. BioPlastics Magazine. 27 April 2016. Available online: http://www.bioplasticsmagazine.com/en/news/meldungen/20160427-Microfibrillar-cellulose-project-from-Borregaard-gets-EU-funding--.php (accessed on 1 February 2018).
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking cellulose nanocrystals: From the laboratory to industrial production. Langmuir 2017, 33, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Hamad, W.Y.; Hu, T.Q. Structure-process-yield interrelations in nanocrystalline cellulose extraction. Can. J. Chem. Eng. 2010, 88, 392–402. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Hirth, K.; Baez, C.; Agarwal, U.P.; Zhu, J.Y. Tailoring the yield and characteristics of wood cellulose nanocrystals (CNC) using concentrated acid hydrolysis. Cellulose 2015, 22, 1753–1762. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.-F.; Gray, D.G. Effect of microscystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Bortner, M.J.; Roman, M. Analysis of the sulfuric acid hydrolysis of wood pulp for cellulose nanocrystal production: A central composite design study. Ind. Crops Prod. 2016, 93, 76–87. [Google Scholar] [CrossRef]
- Filpponen, I.; Argyropoulos, D.S. Regular linking of cellulose nanocrystals via click chemistry: Synthesis and formation of cellulose nanoplatelet gels. Biomacromolecules 2010, 11, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Filpponen, I.; Clarke, S.P.; Broughman, F.; Argyropoulos, D.S. Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J. Mater. Sci. 2011, 46, 7344–7355. [Google Scholar] [CrossRef]
- Lemke, C.H.; Dong, R.Y.; Michal, C.A.; Hamad, W.Y. New insights into nano-crystalline cellulose structure and morphology based on solid-state NMR. Cellulose 2012, 19, 1619–1629. [Google Scholar] [CrossRef]
- Espinosa, S.C.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of Sulfate Groups from Sulfuric Acid Hydrolysis on the Thermal Degradation Behavior of Bacterial Cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Méthot, M.; Bouchard, J. General procedure for determining cellulose nanocrystal sulfate half-ester content by conductometric titration. Cellulose 2015, 22, 101–116. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed]
- Hemraz, U.D.; Sunasee, R. Functionalization of Nanocrystalline Cellulose Surfaces. In Dekker Encyclopedia of Nanoscience and Nanotechnology, 3rd ed.; Lyshevski, S.E., Ed.; CRC Press: Boca Raton, FL, USA, 2014; Volume II. [Google Scholar]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2016, 6, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. Tempo-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.; Liu, Z.; Ni, Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R. Single-Step Method for the Isolation and Surface Functionalization of Cellulosic Nanowhiskers. Biomacromolecules 2009, 10, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Avila Ramirez, J.A.; Fortunati, E.; Kenny, J.M.; Torre, L.; Foresti, M.L. Simple citric acid-catalyzed surface esterification of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Wada, M.; Kuga, S. Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Azzam, F.; Heux, L.; Putaux, J.-L.; Jean, B. Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromolecules 2010, 11, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Dufresne, A. Highly filled bionanocomposites from functionalized polysaccharide nanocrystals. Biomacromolecules 2008, 9, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Peresin, M.S.; Habibi, Y.; Venditti, R.A.; Rojas, O.J. Reinforcing poly(epsilon-caprolactone) nanofibers with cellulose nanocrystals. ACS Appl. Mater. Interfaces 2009, 1, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.P.; Zaman, M.; Mohammed, N.; Brinatti, C.; Batmaz, R.; Berry, R.; Loh, W.; Tam, K.C. Synthesis of amine functionalized cellulose nanocrystals: Optimization and characterization. Carbohydr. Res. 2015, 409, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hemraz, U.D.; Boluk, Y.; Sunasee, R. Amine-decorated nanocrystalline cellulose surfaces: Synthesis, characterization and surface properties. Can. J. Chem. 2013, 91, 974–981. [Google Scholar] [CrossRef]
- Morandi, G.; Heath, L.; Thielemans, W. Cellulose nanocrystals grafted with polystyrene chains through surface-initiated atom transfer radical polymerization (ATRP). Langmuir 2009, 25, 8280–8286. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Habibi, Y.; Rojas, O.J.; Venditti, R.A.; Johansson, L.S.; Efimenko, K.; Osterberg, M.; Laine, J. Poly(N-isopropylacrylamide) brushes grafted from cellulose nanocrystals via surface-initiated single-electron transfer living radical polymerization. Biomacromolecules 2010, 11, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Hemraz, U.D.; Lu, A.; Sunasee, R.; Boluk, Y. Structure of poly(N-isopropylacrylamide) brushes and steric stability of their grafted cellulose nanocrystal dispersions. J. Colloid Interface Sci. 2014, 430, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Hemraz, U.D.; Campbell, K.A.; Burdick, J.S.; Ckless, K.; Boluk, Y.; Sunasee, R. Cationic poly(2-aminoethylmethacrylate) and poly(N-(2-aminoethylmethacrylamide) modified cellulose nanocrystals: Synthesis, characterization, and cytotoxicity. Biomacromolecules 2015, 16, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Rosilo, H.; McKee, J.R.; Kontturi, E.; Koho, T.; Hytönen, V.P.; Ikkala, O.; Kostiainen, M.A. Cationic Polymer Brush-Modified Cellulose Nanocrystals for High-Affinity Virus Binding. Nanoscale 2014, 6, 11871–11881. [Google Scholar] [CrossRef] [PubMed]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rena, J.L.; Peng, F.; Sun, R.C.; Kennedy, J.F. Influence of hemicellulosic derivatives on the sulfate kraft pulp strength. Carbohydr. Polym. 2009, 75, 338–342. [Google Scholar] [CrossRef]
- Lin, N.; Gèze, A.; Wouessidjewe, D.; Huang, J.; Dufresne, A. Biocompatible double-membrane hydrogels from cationic cellulose nanocrystals and anionic alginate as complexing drugs codelivery. ACS Appl. Mater. Interfaces 2016, 8, 6880–6889. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved Mechanical Properties and Sustained Release Behavior of Cationic Cellulose Nanocrystals Reinforeced Cationic Cellulose Injectable Hydrogels. Biomacromolecules 2016, 17, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Kedzior, S.A.; Marway, H.S.; Cranston, E.D. Tailoring cellulose nanocrystal and surfactant behavior in miniemulsion polymerization. Macromolecules 2017, 50, 2645–2655. [Google Scholar] [CrossRef]
- Li, M.-C.; Mei, C.; Xu, X.; Lee, S.; Wu, Q. Cationic surface modification of cellulose nanocrystals: Toward tailoring dispersion and interface in carboxymethyl cellulose films. Polymers 2016, 107, 200–210. [Google Scholar] [CrossRef]
- Feese, E.; Sadeghifar, H.; Gracz, H.S.; Argyropoulos, D.S.; Ghiladi, R.A. Photobactericidal porphyrin-cellulose nanocrystals: Synthesis, characterization, and antimicrobial Properties. Biomacromolecules 2011, 12, 3528–3539. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Agut, W.; Agnaou, R.; Lecommandoux, S.; Taton, D. Synthesis of block copolypeptides by click chemistry. Macromol. Rapid Commun. 2008, 29, 1147–1155. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Imidazolium grafted cellulose nanocrystals for ion exchange applications. Chem. Commun. 2011, 47, 4177–4179. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.L.; Dawsey, T.R.; Newman, J.K. Competitive formation of cellulose p-toluenesulfonate and chlorodeoxycellulose during homogeneous reaction of p-toluenesulfonyl chloride with cellulose in N,N-dimethylacetamide-lithium chloride. Carbohydr. Res. 1990, 208, 183–191. [Google Scholar] [CrossRef]
- Liebert, T.; Hänsch, C.; Heinze, T. Click chemistry with polysaccharides. Macromol. Rapid Commun. 2006, 27, 208–213. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, H.; Piekarski, C.; Shreeve, J.M. Azolium salts functionalized with cyanomethyl, vinyl, or propargyl substituents and dicyanamide, dinitramide, perchlorate and nitrate anions. Eur. J. Inorg. Chem. 2007, 2007, 4965–4972. [Google Scholar] [CrossRef]
- Jasmani, L.; Eyley, S.; Wallbridge, R.; Thielemans, W. A facile one-pot route to cationic cellulose nanocrystals. Nanoscale 2013, 5, 10207–10211. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Eyley, S.; den Mooter, G.V.; Muylaert, K.; Thielemans, W. Highly charged cellulose-based nanocrystals as flocculants for harvesting Chlorella vulgaris. Bioresour. Technol. 2015, 194, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasmani, L.; Eyley, S.; Schütz, C.; Gorp, H.-V.; De Feyter, S.; Thielemans, W. One-pot functionalization of cellulose nanocrystals with various cationic groups. Cellulose 2016, 23, 3569–3576. [Google Scholar] [CrossRef]
- Tizzotti, M.; Charlot, A.; Fleury, E.; Stenzel, M.; Bernard, J. Modification of polysaccharides through controlled/living radical polymerization grafting-Towards the generation of high performance hybrids. Macromol. Rapid Commun. 2010, 31, 1751–1772. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Narain, R. Glycopolymer syntheses. In Glycopolymers: Synthesis and Applications, 1st ed.; Narain, R., Ed.; Smithers Rapra: Shawbury, UK, 2014; pp. 1–44. [Google Scholar]
- Habibi, Y. Key advances in the chemical modification of nanacelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Kan, K.H.M.; Li, J.; Wijesekera, K.; Cranston, E.D. Polymer-grafted cellulose nanocrystals as pH-responsive reversible flocculants. Biomacromolecules 2013, 14, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Morandi, G.; Thielemans, W. Synthesis of cellulose nanocrystals bearing photocleavable grafts by ATRP. Polym. Chem. 2012, 3, 1402–1407. [Google Scholar] [CrossRef]
- Roeder, R.D.; Garcia-Valdez, O.; Whitney, R.A.; Champagne, P.; Cunningham, M.F. Graft modification of cellulose nanocrystals via nitroxide-mediated polymerization. Polym. Chem. 2016, 7, 6383–6390. [Google Scholar] [CrossRef]
- Saigal, T.; Dong, H.; Matyjaszewski, K.; Tilton, R.R.D. Pickering emulsions stabilized by nanoparticles with thermally responsive grafted polymer brushes. Langmuir 2010, 26, 15200–15209. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.L.; Tam, K.C. Synthesis and self-assembly of stimuli-responsive poly(2-(dimethylamino) ethyl methacrylate)-block-fullerene (PDMAEMA-b-C60) and the demicellization induced by free PDMAEMA chains. Langmuir 2011, 27, 6668–6673. [Google Scholar] [CrossRef] [PubMed]
- Rinkenauer, A.C.; Schallon, A.; Günther, U.; Wagner, M.; Betthausen, E.; Schubert, U.S.; Schacher, F.H. A Paradigm Change: Efficient Transfection of Human Leukemia Cells by Stimuli-Responsive Multicompartment Micelles. ACS Nano 2013, 7, 9621–9631. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive pickering emulsion stabilized by poly[2-(dimethylamino)ethyl methacrylate] grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xiu, K.M.; Xu, S.L.; Yang, W.T.; Xu, F.J. Functionalized layered double hydroxide nanoparticles conjugated with disulfide-linked polycation brushes for advanced gene delivery. Bioconjug. Chem. 2013, 24, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Yang, W.T. Polymer vectors via controlled/living radical polymerization for gene delivery. Prog. Polym. Sci. 2011, 36, 1099–1131. [Google Scholar] [CrossRef]
- Hu, H.; Yuan, W.; Liu, F.-S.; Cheng, G.; Xu, F.-J.; Ma, J. Redox-responsive polycation-functionalized cotton cellulose nanocrystals for effective cancer treatment. ACS Appl. Mater. Interfaces 2015, 7, 8942–8951. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, A.; Nikolaou, V.; Nurumbetov, G.; Wilson, P.; Kempe, K.; Quinn, J.F.; Davis, T.P.; Whittaker, M.R.; Haddleton, D.M. Cu(0)-Mediated Living Radical Polymerization: A Versatile Tool for Materials Synthesis. Chem. Rev. 2016, 116, 835–877. [Google Scholar] [CrossRef] [PubMed]
- Lligadas, G.; Grama, S.; Percec, V. Recent developments in the synthesis of biomacromolecules and their conjugates by single electron transfer–living radical polymerization. Biomacromolecules 2017, 18, 1039–1063. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Araoye, E.; Pyram, D.; Hemraz, U.D.; Boluk, Y.; Ckless, K. Cellulose nanocrystal cationic derivative induces NLRP3 inflammasome-dependent IL-1β secretion associated with mitochondrial ROS production. Biochem. Biophys. Rep. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Hou, L.; Fang, J.; Wang, W.; Xie, Z.; Dong, D.; Zhang, N. Indocyanine green-functionalized bottle brushes of poly(2-oxazoline) on cellulose nanocrystals for photothermal cancer therapy. J. Mater. Chem. B 2017, 5, 3348–3354. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Frechet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Satija, J.; Gupta, U.; Jain, N.K. Pharmaceutical and biomedical potential of surface engineered dendrimers. Crit. Rev. Ther. Drug Carrier Syst. 2007, 24, 257–306. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, A.D.; Rabe, J.P. Dendronized polymers: Synthesis, characterization, assembly at Interfaces, and manipulation. Angew. Chem. Int. Ed. 2000, 39, 864–883. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, Y.; Xu, B.; Mai, K.; Deng, Y.; Zhang, L.-M. Dendronized chitosan derivative as a biocompatible gene delivery carrier. Biomacromolecule 2011, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, A.D.; Basiryan, A. Dendronization of cellulose nanowhisker with cationic hyperbranched dendritic polyamidoamine. Carbohydr. Polym. 2015, 120, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Pecot, C.V.; Calin, G.A.; Coleman, R.L.; Lopez-Berestein, G.; Sood, A.K. RNA interference in the clinic: Challenges and future directions. Nat. Rev. Cancer 2011, 11, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-P.; Nah, J.-W. Target gene delivery from targeting ligand conjugated chitosan-PEI copolymer for cancer therapy. Carbohydr. Polym. 2016, 135, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cai, J.; Borjihan, W.; Ganbold, T.; Rana, T.M.; Baigude, H. Preparation of novel curdlan nanoparticles for intracellular siRNA delivery. Carbohydr. Polym. 2015, 117, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, W.; Wang, J.S.; Wang, J.J.; Tong, X.; Hu, Q.; Qi, L. Highly efficient Gab2 siRNA delivery to ovarian cancer cells mediated by chitosan-polyethyleneimine nanoparticles. J. Mater. Chem. B 2016, 4, 273–281. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; De Simone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, N.; Yan, P.; Hu, H.; Xu, F.J. The shape and size effects of polycation functionalized silica nanoparticles on gene transfection. Acta Biomater. 2015, 11, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Ndong Ntoutoume, G.M.; Grassot, V.; Brégier, F.; Chabanais, J.; Petit, J.M.; Granet, R.; Sol, V. PEI-cellulose nanocrystal hybrids as efficient siRNA delivery agents-Synthesis, physicochemical characterization and in vitro evaluation. Carbohydr. Polym. 2017, 164, 258–267. [Google Scholar] [CrossRef] [PubMed]
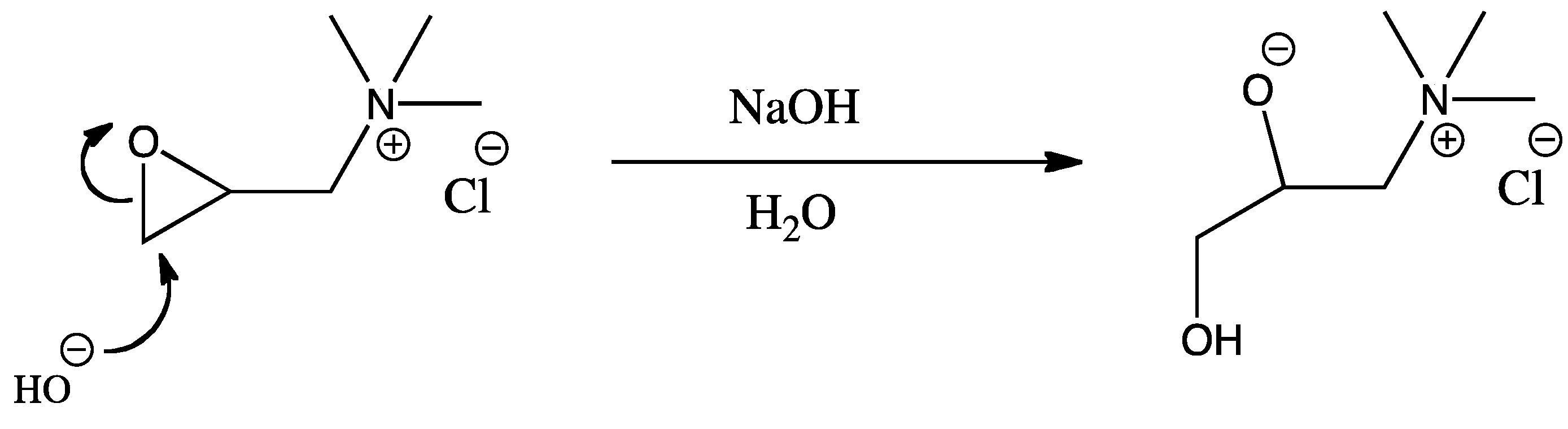


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunasee, R.; Hemraz, U.D. Synthetic Strategies for the Fabrication of Cationic Surface-Modified Cellulose Nanocrystals. Fibers 2018, 6, 15. https://doi.org/10.3390/fib6010015
Sunasee R, Hemraz UD. Synthetic Strategies for the Fabrication of Cationic Surface-Modified Cellulose Nanocrystals. Fibers. 2018; 6(1):15. https://doi.org/10.3390/fib6010015
Chicago/Turabian StyleSunasee, Rajesh, and Usha D. Hemraz. 2018. "Synthetic Strategies for the Fabrication of Cationic Surface-Modified Cellulose Nanocrystals" Fibers 6, no. 1: 15. https://doi.org/10.3390/fib6010015




