Integrated Proteomics and Metabolomics Analysis of Perirenal Adipose Tissue in Obese Rabbits Treated with a Restricted Diet
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histomorphological Observation
2.3. TMT Labeling
2.4. Separation of Fractions
2.5. LC-MS/MS Analysis
2.6. Proteomics Data Processing and Analysis
2.7. UHPLC-MS/MS Analysis
2.8. Metabolomics Data Processing and Analysis
2.9. Integrated Analysis of Proteomics and Metabolomics
2.10. Statistical Analysis
3. Results
3.1. Histomorphological Changes in the Perirenal Adipose Tissue of Dieting Rabbits
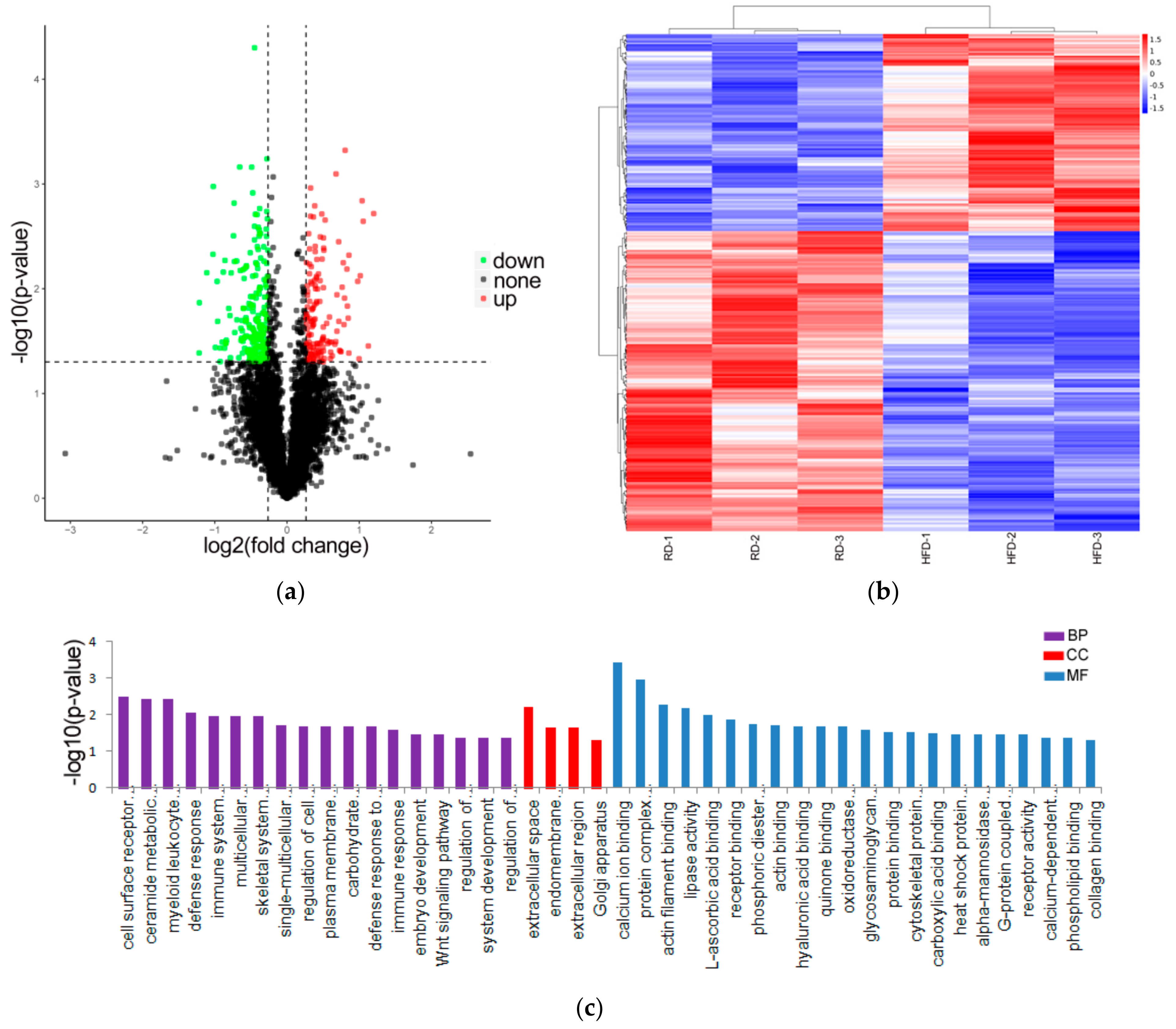
3.2. Identification and Analysis of DE Proteins
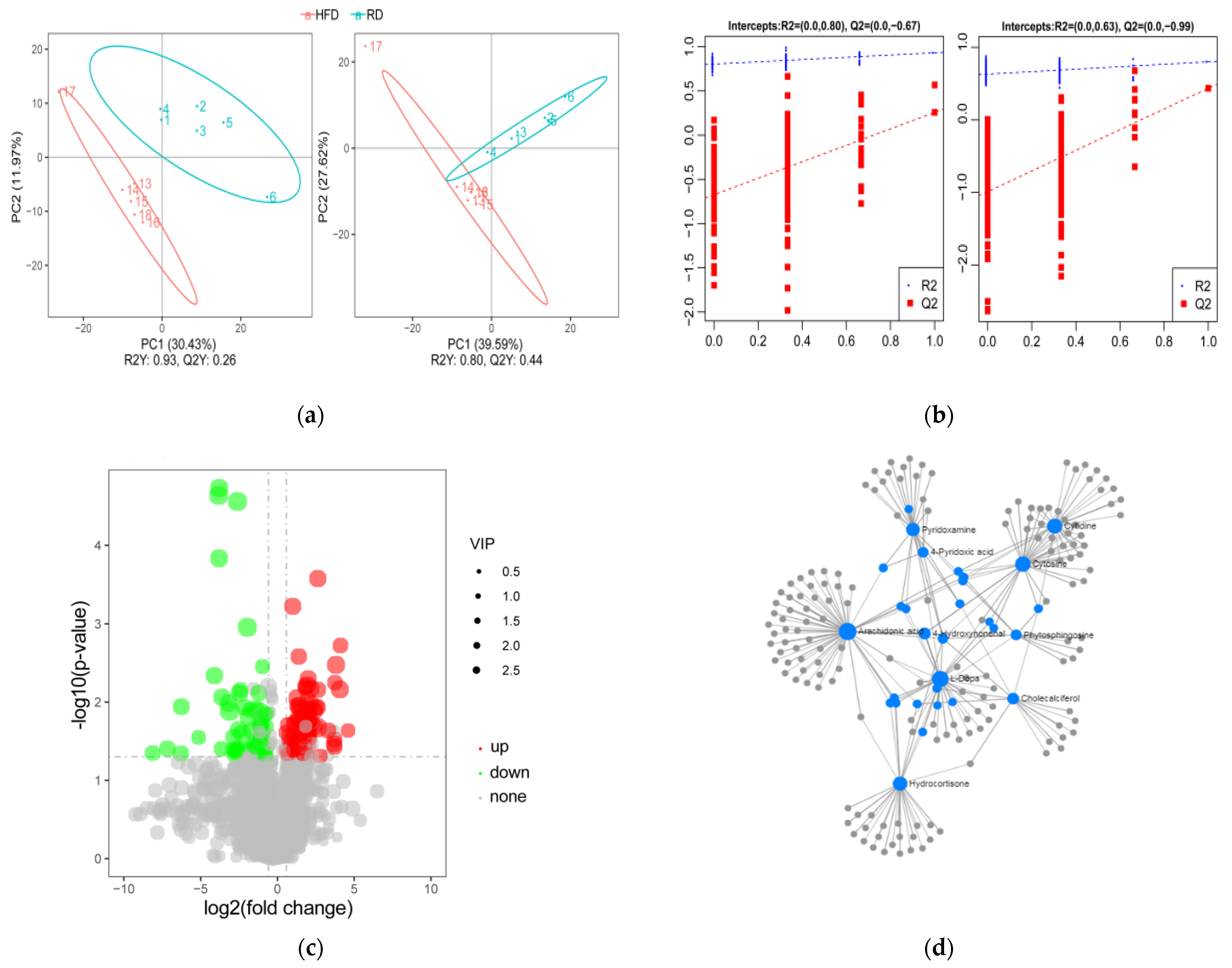
3.3. Identification and Analysis of DE Metabolites
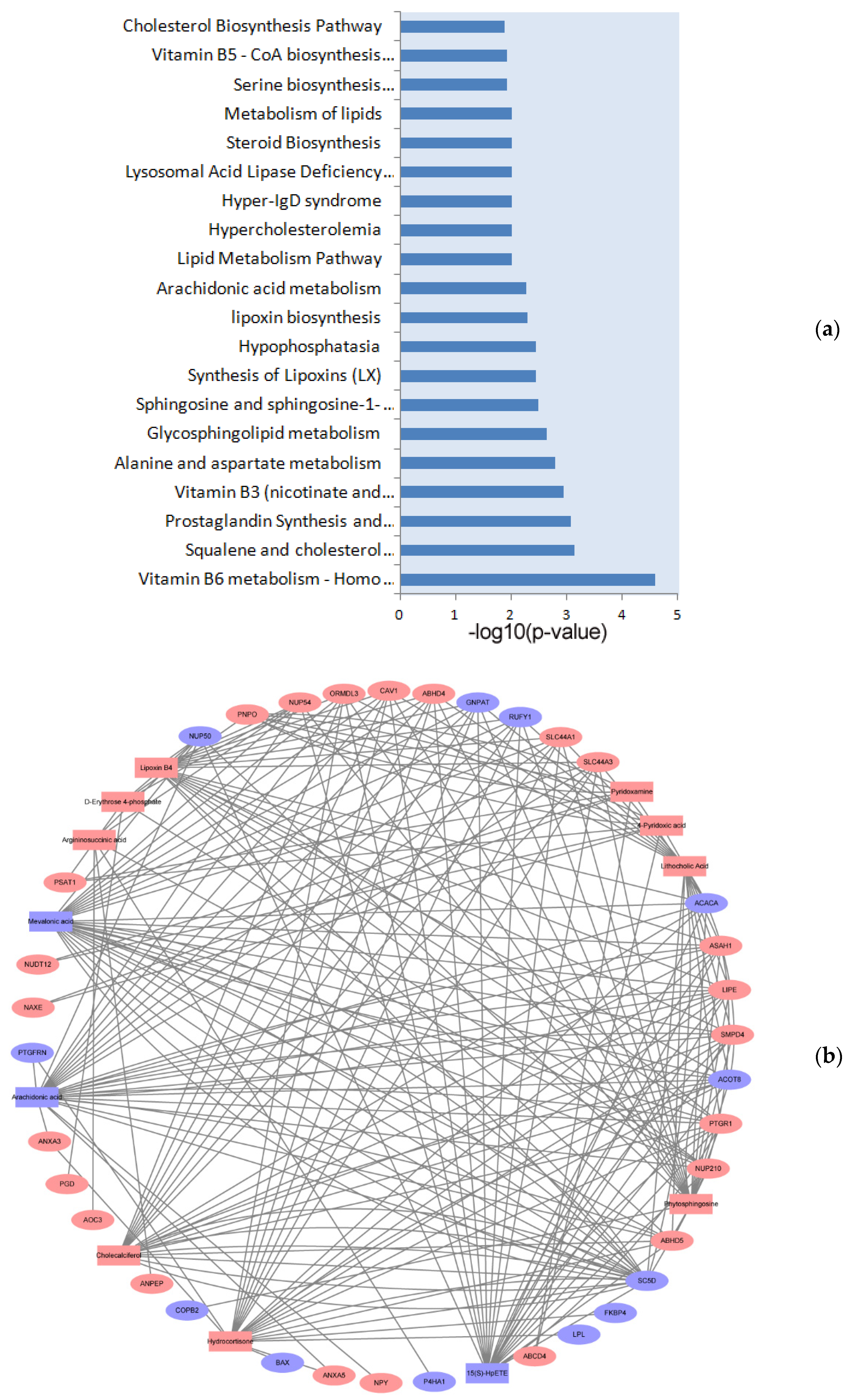
3.4. Integrated Analysis of Proteomics and Metabolomics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popkin, B.M.; Doak, C.M. The Obesity Epidemic Is a Worldwide Phenomenon. Nutr. Rev. 2010, 56, 106–114. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Llewellyn, C.; Wardle, J. Behavioral susceptibility to obesity: Gene–Environment interplay in the development of weight. Physiol. Behav. 2015, 152, 494–501. [Google Scholar] [CrossRef]
- Swinburn, B.; Sacks, G.; Hall, K. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–818. [Google Scholar] [CrossRef]
- Branco, J.C.; Motta, J.; Wiener, C.; Oses, J.P.; Pedrotti Moreira, F.; Spessato, B.; Dias, L.; Da Silva, R. Association between obesity and suicide in woman, but not in man: A population-based study of young adults. Psychol. Health Med. 2016, 22, 275–281. [Google Scholar] [CrossRef]
- Brugman, E.; Meulmeester, J.F.; Spee-Van, D.W.A.; Beuker, R.J.; Zaadstra, B.M.; Radder, J.J.; Verloove-Vanhorick, P.S. Dieting, weight and health in adolescents in The Netherlands. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1997, 21, 54–60. [Google Scholar] [CrossRef] [Green Version]
- French, S.A.; Jeffery, R.W. Consequences of dieting to lose weight: Effects on physical and mental health. Health Psychol. 1994, 13, 195–212. [Google Scholar] [CrossRef]
- Kemps, E.; Tiggemann, M.; Marshall, K. Relationship between dieting to lose weight and the functioning of the central executive. Appetite. 2005, 45, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Cogan, J.C.; Ernsberger, P. Dieting, Weight, and Health: Reconceptualizing Research and Policy. J. Soc. Issues. 2010, 55, 187–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Pu, J.; Yang, L.; Xie, P. Integrated Metabolomics and Proteomics Analysis of Hippocampus in a Rat Model of Depression. Neuroence. 2017, 371, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, J.; Li, Y.; Elzo, M.A.; Tang, T.; Lai, T.; Ma, Y.; Gan, M.; Wang, L.; Jia, X.; et al. Growth, behavioural, serum biochemical and morphological changes in female rabbits fed high-fat diet %J Journal of Animal Physiology & Animal Nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 105, 345–353. [Google Scholar]
- Tang, H.; Zhang, X.; Gong, B.; Yan, Y.; Shi, Q. Proteomics and metabolomics analysis of tomato fruit at different maturity stages and under salt treatment. Food Chem. 2020, 311, 126009.126001–126009.126010. [Google Scholar] [CrossRef]
- James, P.T.; Neville, R.; Rachel, L. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur. J. Cardiovasc. Prev. 2019, 11, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Molarius, A.; Lindén Ostrm, M.; Karlsson, J. Desire to lose weight and need of weight loss support in the adult population Results from a cross: Ectional study in Sweden. Obes. Ence Pract. 2020, 6, 373–381. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Large, V.; Peroni, O.; Letexier, D.; Ray, H.; Beylot, M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004, 30, 294–309. [Google Scholar] [CrossRef]
- Mezghanni, N.; Chaabouni, K.; Chtourou, H.; Masmoudi, L.; Mejdoub, H. Effect of exercise training intensity on body composition, lipid profile, and insulin resistance in young obese women. Afr. J. Microbiol. Res. 2012, 6, 2481–2488. [Google Scholar]
- Eckel, R.H. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N. Engl. J. Med. 1989, 320, 1060. [Google Scholar] [PubMed]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.-R.; Nikkilä, E.A. Basal and postprandial lipoprotein lipase activity in adipose tissue during caloric restriction and refeeding. Metabolism 1987, 36, 625–630. [Google Scholar] [CrossRef]
- Guenter, H.; Robert, Z.; Marianne, H.; Christian, T.; Georg, W.; Elke, W.; Wolfgang, S.; Thomas, M.M.; Erwin, F.W.; Rudolf, Z. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002, 277, 4806–4815. [Google Scholar]
- Mao, J.; Marcos, S.; Davis, S.K.; Burzlaff, J.; Seyfert, H.M. Genomic distribution of three promoters of the bovine gene encoding acetyl-CoA carboxylase alpha and evidence that the nutritionally regulated promoter I contains a repressive element different from that in rat. Biochem. J. 2001, 358, 127–135. [Google Scholar] [CrossRef]
- Alexaki, A.; Clarke, B.A.; Gavrilova, O.; Ma, Y.; Zhu, H.; Ma, X.; Xu, L.; Tuymetova, G.; Larman, B.C.; Allende, M.L. De Novo Sphingolipid Biosynthesis Is Required for Adipocyte Survival and Metabolic Homeostasis. J. Biol. Chem. 2017, 292, 3929–3939. [Google Scholar] [CrossRef] [Green Version]
- Min, Z.; Xiaomo, L.I.; Hong, Q.U. EDdb: A web resource for eating disorder and its application to identify an extended adipocytokine signaling pathway related to eating disorder. Sci. China. Life Sci. 2013, 56, 1086–1096. [Google Scholar]
- Wu, C.-W.; Zhao, X.-L.; Wu, X.-J.; Wen, C.; Li, H.; Chen, X.-H.; Peng, X.-X. Exogenous glycine and serine promote growth and antifungal activity of Penicillium citrinum W1 from the south-west Indian Ocean. FEMS Microbiol. Lett. 2015, 362, fnv040. [Google Scholar]
- Zhao, X.; Liu, Z.; Liu, Z.; Meng, R.; Shi, C.; Chen, X.; Bu, X.; Guo, N. Phenotype and RNA-seq-Based transcriptome profiling of Staphylococcus aureus biofilms in response to tea tree oil. Microb. Pathog. 2018, 123, 304–313. [Google Scholar]
- Salter-Cid, L.M. Anti-Inflammatory Effects of Inhibiting the Amine Oxidase Activity of Semicarbazide-Sensitive Amine Oxidase. J. Pharmacol. Exp. Ther. 2005, 315, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, E.J. Effects of H2O2 on insulin signaling the glucose transport system in mammalian skeletal muscle. Methods Enzymol. 2013, 528, 269–278. [Google Scholar] [PubMed]
- Shimizu, M.; Furuya, S.; Shinoda, Y.; Mitoma, J.; Okamura, T.; Miyoshi, I.; Kasai, N.; Hirabayashi, Y.; Suzuki, Y. Functional analysis of mouse 3-phosphoglycerate dehydrogenase (Phgdh) gene promoter in developing brain. J. Neurosci. Res. 2004, 76, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiao, F.; Guo, Y.; Deng, J.; Liu, B.; Zhang, Q.; Li, K.; Wang, C.; Chen, S.; Guo, F. Hepatic Phosphoserine Aminotransferase 1 Regulates Insulin Sensitivity in Mice via Tribbles Homolog 3. Diabetes. 2015, 64, 1591–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Ge, A.; Xu, S.; You, Z.; Ning, S.; Zhao, Y.; Pang, D. PSAT1 is regulated byATF4 and enhances cell proliferation via the GSK3β/β-catenin/cyclin D1 signaling pathway in ER-negative breast cancer. J. Exp. Clin. Cancer Res. 2017, 36, 179. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-P.; Chan, Y.-C.; Yu, S.; Lin, Y.-F. Overexpression of PSAT1 Gene is a Favorable Prognostic Marker in Lower-Grade Gliomas and Predicts a Favorable Outcome in Patients with IDH1 Mutations and Chromosome 1p19q Codeletion. Cancers 2019, 12, 13. [Google Scholar] [CrossRef] [Green Version]
- Pietilainen, K.H.; Róg, T.; Seppanen-Laakso, T.; Virtue, S.; Gopalacharyulu, P.; Tang, J.; Rodriguez-Cuenca, S.; Maciejewski, A.; Naukkarinen, J. Association of Lipidome Remodeling in the Adipocyte Membrane with Acquired Obesity in Humans. PLoS Biol. 2011, 9, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Niemel, P.S.; Ollila, S.; Hyv Nen, M.T.; Karttunen, M.; Vattulainen, I. Assessing the Nature of Lipid Raft Membranes. Plos Comput. Biol. 2007, 3, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mysore, R.; Liebisch, G.; Zhou, Y.; Olkkonen, V.M. Angiopoietin-like 8 (Angptl8) controls adipocyte lipolysis and phospholipid composition. Chem. Phys. Lipids 2017, 207, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsukov, L.I.; Bergelson, L.D.; Spiess, M.; Hauser, H.; Semenza, G. Phospholipid topology and flip-flop in intestinal brush-border membrane. Biochim. Biophys. Acta 2019, 862, 87–99. [Google Scholar] [CrossRef]
- Larijani, B.; Dufourc, E.J. Polyunsaturated phosphatidylinositol and diacylglycerol substantially modify the fluidity and polymorphism of biomembranes: A solid-state deuterium NMR study. Lipids 2006, 41, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, F.; Smith, T.K. The Kennedy pathway—Synthesis of phosphatidylethanolamine and phosphatidylcholine. Iubmb Life. 2010, 62, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Brose, N.; Betz, A.; Wegmeyer, H. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr. Opin. Neurobiol. 2004, 14, 328–340. [Google Scholar] [CrossRef]
- Yang, L.; Andrews, D.A.; Low, P.S. Lysophosphatidic acid opens a Ca++ channel in human erythrocytes. Blood 2000, 95, 2420–2425. [Google Scholar] [CrossRef]
- Grewal, T.; Enrich, C.; Rentero, C.; Buechler, C. Annexins in Adipose Tissue: Novel Players in Obesity. Int. J. Mol. Sci. 2019, 20, 3449. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Ito, Y.; Sato, A.; Hosono, T.; Niimi, S. Annexin A3 as a negative regulator of adipocyte differentiation. J. Biochem. 2012, 152, 355–363. [Google Scholar] [CrossRef]
- Nobusue, H.; Onishi, N.; Shimizu, T.; Sugihara, E.; Oki, Y.; Sumikawa, Y.; Chiyoda, T.; Akashi, K.; Saya, H.; Kano, K. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat. Commun. 2014, 5, 3368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, N. ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating 1 integrin function. J. Cell Sci. 2003, 116, 3893–3904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; You, W.; Cheng, H.; Zhang, Q.; Song, E. Effect of mevalonic acid on cholesterol synthesis in bovine intramuscular and subcutaneous adipocytes. J. Appl. Genet. 2016, 57, 113–118. [Google Scholar] [CrossRef]
- Goto, T.; Nagai, H.; Egawa, K.; Kim, Y.I.; Kato, S.; Taimatsu, A.; Sakamoto, T.; Ebisu, S.; Hohsaka, T.; Miyagawa, H.J.B.J. Farnesyl pyrophosphate regulates adipocyte functions as an endogenous PPARγ agonist. Biochem. J. 2011, 438, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Nishi, S.; Nishino, H.; Ishibashi, T. cDNA cloning of the mammalian sterol C5-desaturase and the expression in yeast mutant. Biochim. Biophys. Acta Gene Struct. Expr. 2000, 1492, 211. [Google Scholar] [CrossRef]
- Mori, M.; Li, G.; Abe, I.; Nakayama, J.; Guo, Z.; Sawashita, J.; Ugawa, T.; Nishizono, S.; Serikawa, T.; Higuchi, K. Lanosterol synthase mutations cause cholesterol deficiency–associated cataracts in the Shumiya cataract rat. J. Clin. Investig. 2006, 116, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Soumya, S.J.; Binu, S.; Helen, A.; Kumar, K.A.; Reddanna, P.; Sudhakaran, P.R. Effect of 15-lipoxygenase metabolites on angiogenesis: 15(S)-HPETE is angiostatic and 15(S)-HETE is angiogenic. Inflamm. Res. 2012, 61, 707–718. [Google Scholar] [CrossRef]
- Soumya, S.J.; Binu, S.; Helen, A.; Reddanna, P.; Sudhakaran, P.R. 15-LOX metabolites and angiogenesis: Angiostatic effect of 15(S)-HPETE involves induction of apoptosis in adipose endothelial cells. PeerJ 2014, 2, e635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavel, G.; Valvason, C.; Yamaoka, K.; Lemeiter, D.; Bessis, N. Relationship between angiogenesis and inflammation in experimental arthritis. Eur. Cytokine Netw. 2006, 17, 202–210. [Google Scholar] [PubMed]
- Naldini, A.; Carraro, F. Role of inflammatory mediators in angiogenesis. Curr. Drug Targets Inflamm Allergy 2005, 4, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, P.J.; Jorge, C.; Ferreira, C.; Borges, M.; Aires, I.; Amaral, T.; Gil, C.; Cortez, J.; Ferreira, A. Cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin. J. Am. Soc. Nephrol. 2010, 5, 905. [Google Scholar] [CrossRef] [PubMed]
- Schleimer, R.P. Potential regulation of inflammation in the lung by local metabolism of hydrocortisone. Am. J. Respir. Cell Mol. Biol. 2012, 4, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Karra, L.; Haworth, O.; Priluck, R.; Levy, B.D.; Levi-Schaffer, F. Lipoxin BLipoxin B-4 promotes the resolution of allergic inflammation in the upper and lower airways of mice. Mucosal Immunol. 2015, 8, 852–862. [Google Scholar] [CrossRef] [Green Version]
- Moretti, A.; Li, Q.; Chmielowski, R.; Joseph, L.; Moghe, P.; Uhrich, K. Nanotherapeutics Containing Lithocholic Acid-Based Amphiphilic Scorpion-Like Macromolecules Reduce In Vitro Inflammation in Macrophages: Implications for Atherosclerosis. Nanomaterials 2018, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.; Ertunc, M.E.; Konduri, S.; Zhang, J. Discovery of FAHFA-containing Triacylglycerols and Their Metabolic Regulation. J. Am. Chem. Soc. 2019, 141, 8798–8806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, S.; Syed, I.; St Hlman, M.; Kolar, M.J.; Homan, E.A.; Chu, Q.; Smith, U.; Borén, J.; Kahn, B.B. A LC-MS-based workflow for measurement of branched fatty acid esters of hydroxy fatty acids. Nat. Protoc. 2016, 11, 747. [Google Scholar] [CrossRef] [Green Version]
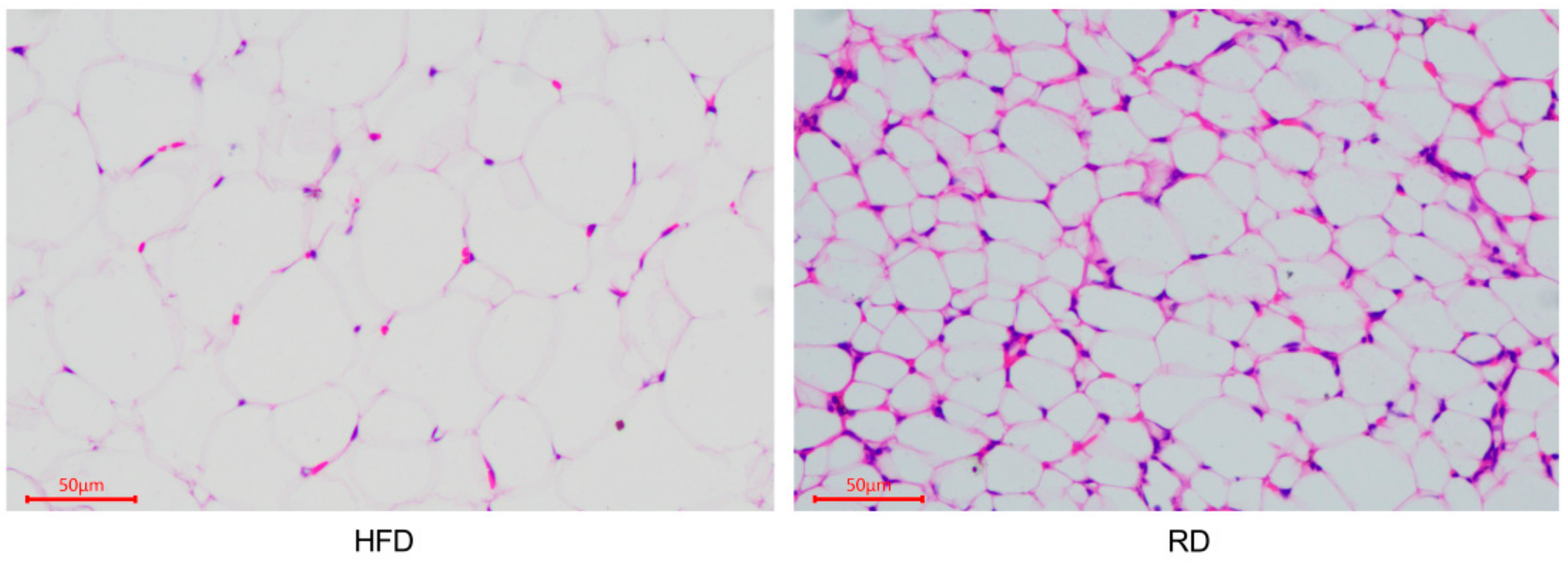
| Parameters | HFD | RD |
|---|---|---|
| Adipocytes area (μm2) | 1098 ± 92.13 | 793.6 ± 57.55 * |
| Adipocytes diameter (μm) | 39.81 ± 1.485 | 34.44 ± 1.038 ** |
| Adipocytes number | 37.22 ± 4.651 | 54.89 ± 3.3448 ** |
| Adipocytes density (number/mm2) | 417.8 ± 52.32 | 619.4 ± 37.73 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Pan, T.; Wang, J.; Tang, T.; Li, Y.; Jia, X.; Lai, S. Integrated Proteomics and Metabolomics Analysis of Perirenal Adipose Tissue in Obese Rabbits Treated with a Restricted Diet. Biology 2021, 10, 321. https://doi.org/10.3390/biology10040321
Shao J, Pan T, Wang J, Tang T, Li Y, Jia X, Lai S. Integrated Proteomics and Metabolomics Analysis of Perirenal Adipose Tissue in Obese Rabbits Treated with a Restricted Diet. Biology. 2021; 10(4):321. https://doi.org/10.3390/biology10040321
Chicago/Turabian StyleShao, Jiahao, Ting Pan, Jie Wang, Tao Tang, Yanhong Li, Xianbo Jia, and Songjia Lai. 2021. "Integrated Proteomics and Metabolomics Analysis of Perirenal Adipose Tissue in Obese Rabbits Treated with a Restricted Diet" Biology 10, no. 4: 321. https://doi.org/10.3390/biology10040321






