Possible Synergistic Antidiabetic Effects of Quantified Artemisia judaica Extract and Glyburide in Streptozotocin-Induced Diabetic Rats via Restoration of PPAR-α mRNA Expression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Experimental Animals
2.3. Induction of Experimental Diabetes
2.4. Experimental Design
2.5. Estimation of Biochemical Parameters
2.5.1. Effect on Blood Glucose and Insulin Levels
2.5.2. Effect on Total Hemoglobin and Glycosylated Hemoglobin Levels
2.5.3. Effect on Blood Lipid Profile
2.6. Tissue Collection
2.7. Effect on Oxidative Stress and Lipid Peroxidation (LPO) Markers
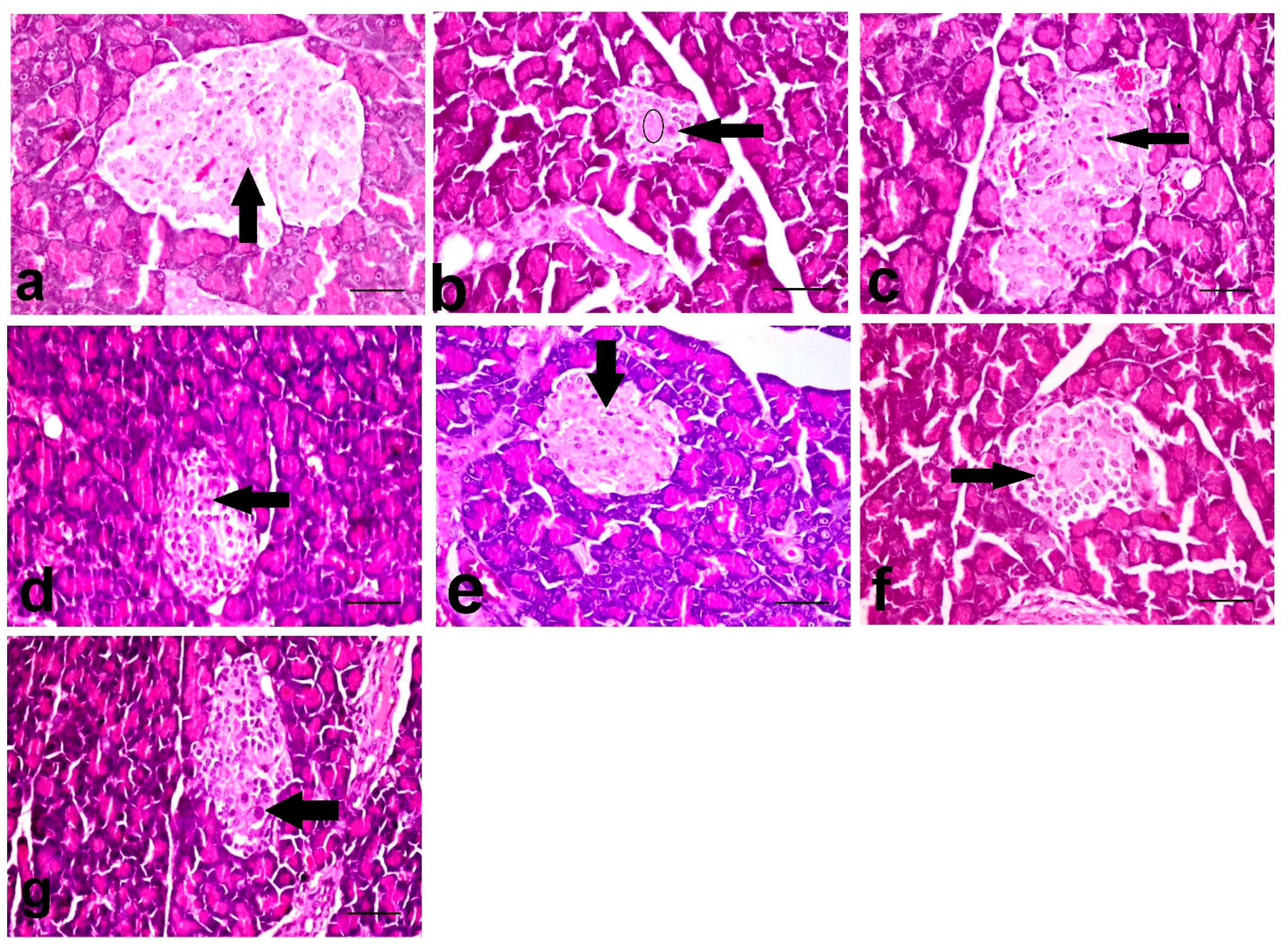
2.8. Histopathological Investigation of Pancreas
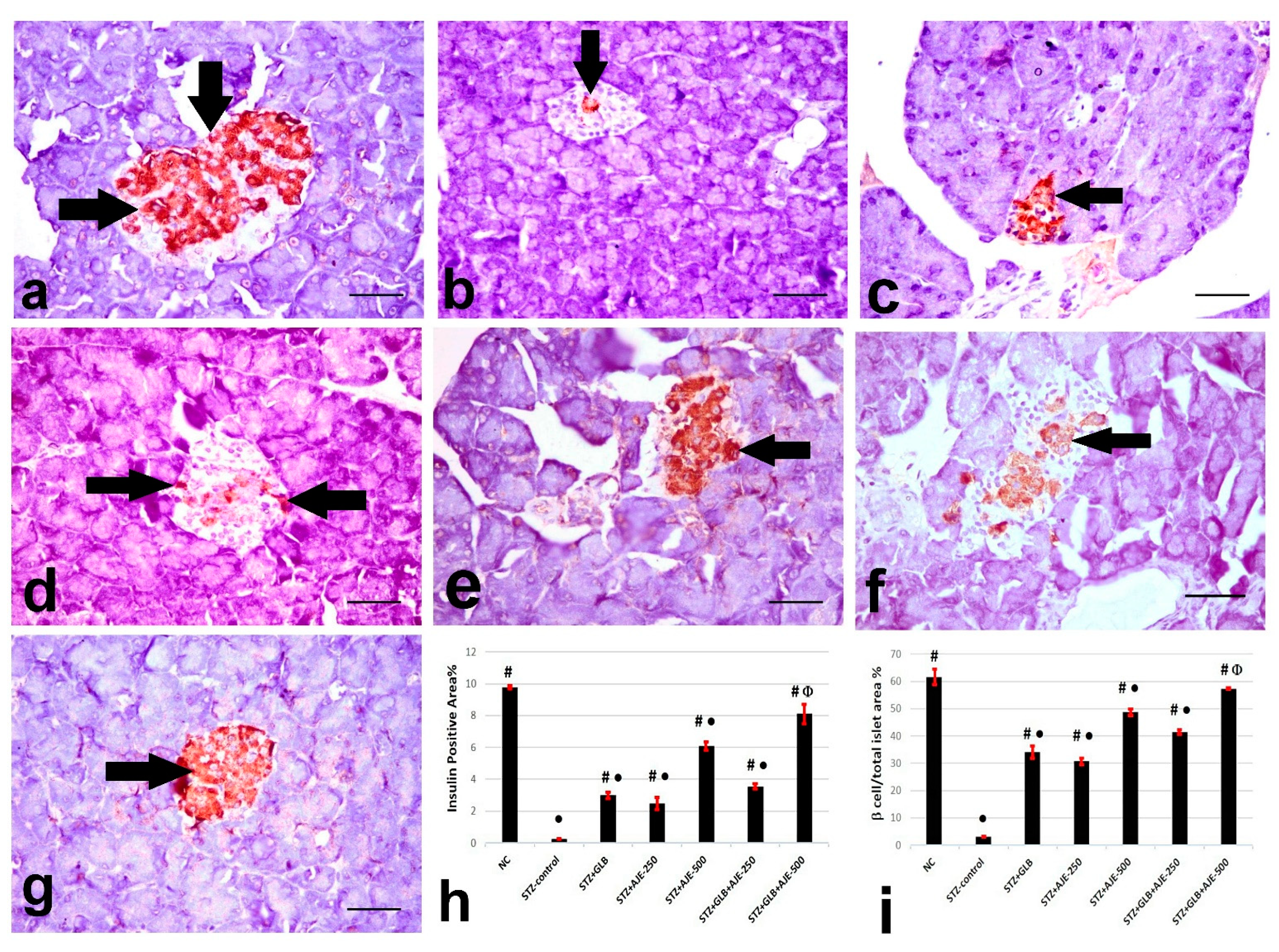
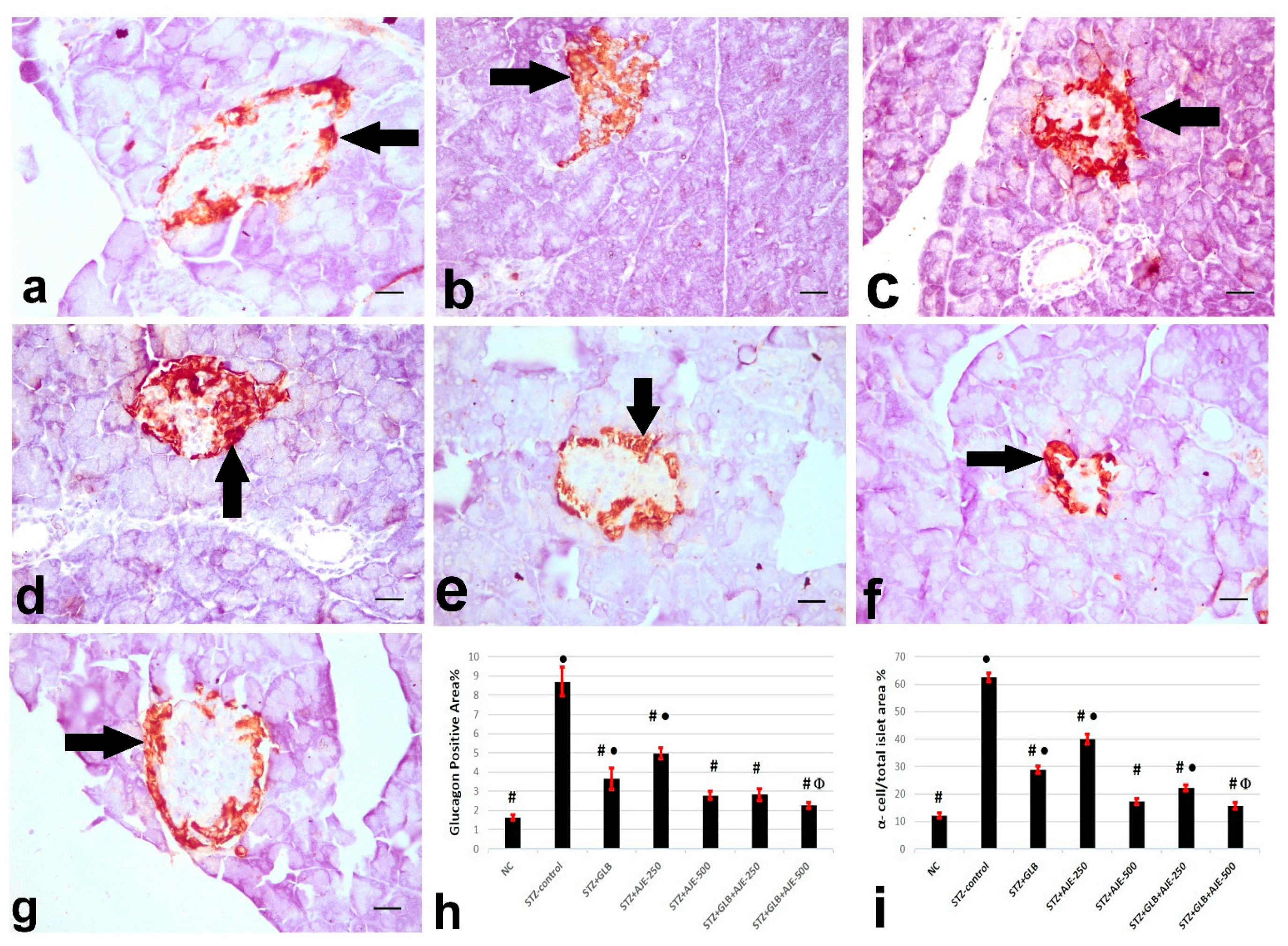
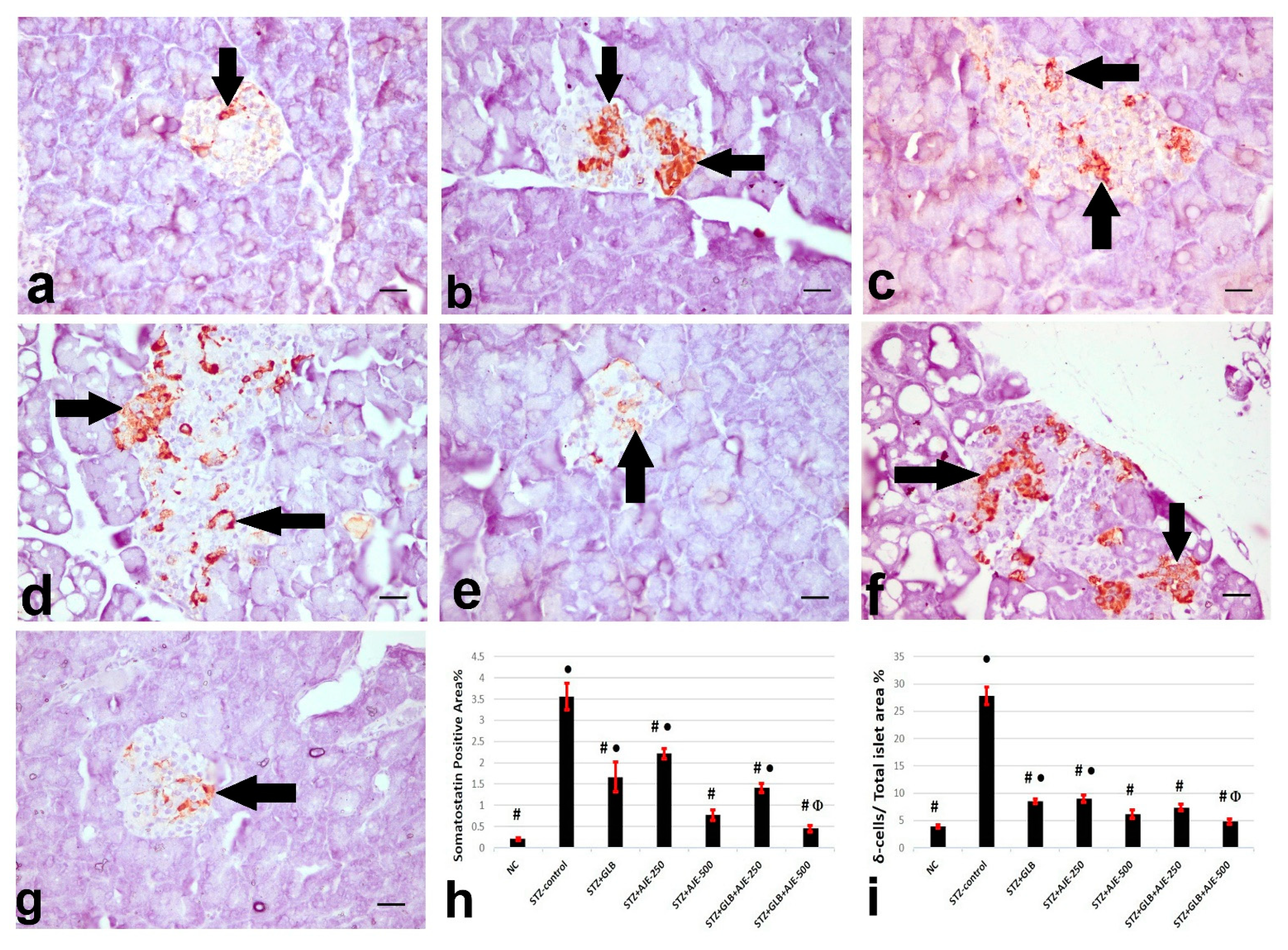
2.9. Immunohistochemical Analysis
2.10. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect on Body Weight
3.2. Effect on Blood Glucose Levels
3.3. Effect on Blood Insulin Levels
3.4. Effect on Total Hemoglobin and Glycosylated Hemoglobin Levels
3.5. Effect on Serum Lipid Profile
3.6. Effect on Oxidative Stress and Lipid Peroxidation Markers
3.7. Histopathological Investigation of Pancreas
3.8. Immunohistochemical Analysis of Insulin, Glucagon, and Somatostatin
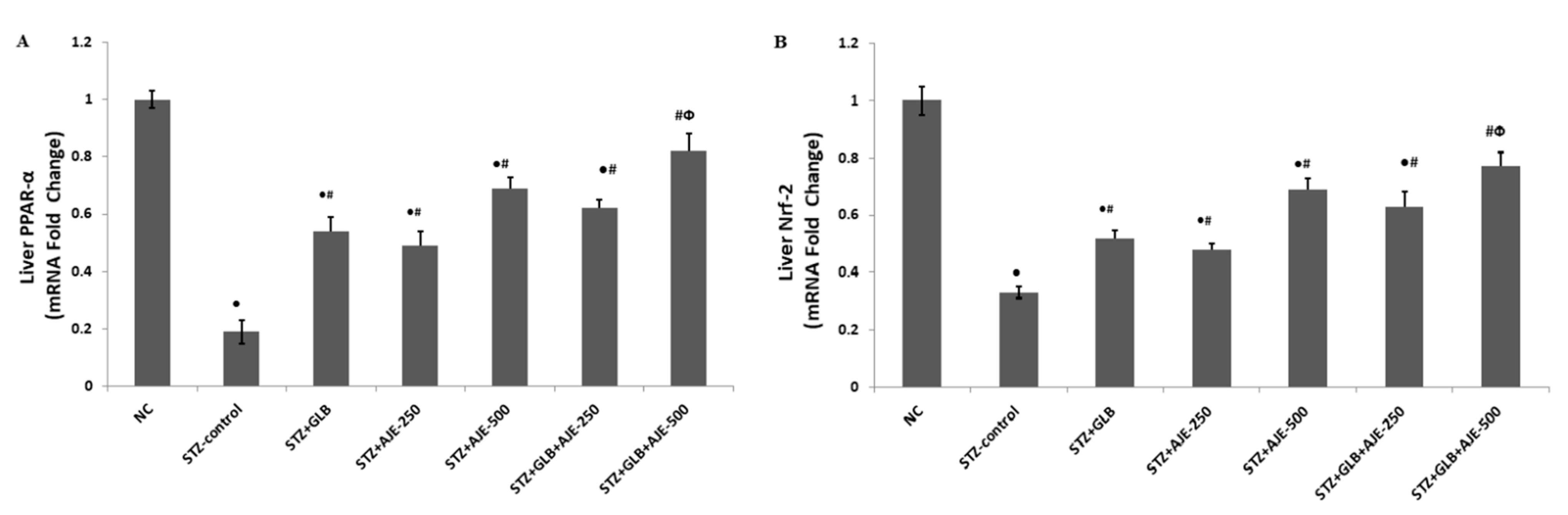
3.9. Real Time-PCR for Hepatic Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bahmanzadeh, M.; Vahidinia, A.; Mehdinejadiani, S.; Shokri, S.; Alizadeh, Z. Dietary supplementation with astaxanthin may ameliorate sperm parameters and DNA integrity in streptozotocin-induced diabetic rats. Clin. Exp. Reprod. Med. 2016, 43, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfar, K.; Ganai, B.A.; Akbar, S.; Mubashir, K.; Dar, S.A.; Dar, M.Y.; Tantry, M.A. Antidiabetic activity of Artemisia amygdalina decne in streptozotocin induced diabetic rats. Biomed Res. Int. 2014, 2014, 185676. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.Y.; Wallis, M.; Tiralongo, E. Use of complementary and alternative medicine among people living with diabetes: Literature review. J. Adv. Nurs. 2007, 58, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Liu, E.H.; Chu, C.; Peng, Y.B.; Cai, H.X.; Li, P. Anti-diabetic agents from natural products—An update from 2004 to 2009. Curr. Top. Med. Chem. 2010, 10, 434–457. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Clinical and experimental studies on polyherbal formulations for diabetes: Current status and future prospective. J. Integr. Med. 2014, 12, 336–345. [Google Scholar] [CrossRef]
- Komaki, E.; Yamaguchi, S.; Kinoshita, M.; Kakehi, K.; Ohta, Y.; Tsukada, Y. Identification of anti-α-amylase components from olive leaf extracts. Food Sci. Technol. Res. 2003, 9, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Virdi, J.; Sivakami, S.; Shahani, S.; Suthar, A.; Banavalikar, M.; Biyani, M. Antihyperglycemic effects of three extracts from Momordica charantia. J. Ethnopharmacol. 2003, 88, 107–111. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive Phenolics of the Genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS Profile of the Siberian Species and Their Inhibitory Potential Against α-Amylase and α -Glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef]
- Wyk, V.B.E.; Wink, M. Medicinal Plants of the World; Briza Publications: Pretoria, South Africa, 2004; pp. 54–56. [Google Scholar]
- Choi, J.Y.; Shin, S.K.; Jeon, S.M.; Baek, N.I.; Chung, H.G.; Jeong, T.S.; Lee, K.T.; Lee, M.K.; Choi, M.S. Dose-response study of sajabalssuk ethanol extract from Artemisia princeps Pampanini on blood glucose in subjects with impaired fasting glucose or mild type 2 diabetes. J. Med. Food. 2011, 14, 101–107. [Google Scholar] [CrossRef]
- Kirk-Ballard, H.; Wang, Z.Q.; Acharya, P.; Zhang, X.H.; Yu, Y.; Kilroy, G.; Ribnicky, D.; Cefalu, W.T.; Floyd, Z.E. An extract of Artemisia dracunculus L. inhibits ubiquitin-proteasome activity and preserves skeletal muscle mass in a murine model of diabetes. PLoS ONE 2013, 8, e57112. [Google Scholar] [CrossRef] [Green Version]
- Eshetu, N.; Afework, M.; Makonnen, E.; Debella, A.; Ergete, W.; Tolesssa, T. Evaluation of the acute and sub-chronic toxic effects of aqueous leaf extracts of Artemisia afra on liver, kidney and some blood parameters in Wistar rats. J. Biomed. Eng. Sci. 2016, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mesa, L.E.; Lutgen, P.; Velez, I.D.; Segura, A.M.; Robledo, S.M. Artemisia annua L., potential source of molecules with pharmacological activity in human diseases. Am. J. Phytomed. Clin. Ther. 2015, 3, 436–450. [Google Scholar]
- Tackholm, V. Students’ Flora of Egypt; Cooperative Printing Co.: Beirut, Lebanon, 1974. [Google Scholar]
- Nofal, S.M.; Mahmoud, S.S.; Ramadan, A.; Soliman, G.; Fawzy, R. Antidiabetic effect of Artemisia judaica extracts. Res. J. Med. Med. Sci. 2009, 4, 42–48. [Google Scholar]
- Ahmed, E.S.; Mabrouk, D.M.; Hassanane, M.M.; Khalil, W.K. Protective effect of Artemisia judaica against doxorubicin-induced toxicity in mice. Annu. Res. Rev. Biol. 2017, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.; Fahad Ullah, M.; Abu-Duhier, F. Bioactive extract of Artemisia judaica causes in vitro inhibition of dipeptidyl peptidase IV and pancreatic/intestinal enzymes of the carbohydrate absorption cascade: Implication for anti-diabetic new molecular entities (NMEs). Orient. Pharm. Exp. Med. 2019, 19, 71–80. [Google Scholar] [CrossRef]
- Alzweiri, M.; Sarhan, A.A.; Mansi, K.; Hudaib, M.; Aburjai, T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 2011, 137, 27–35. [Google Scholar] [CrossRef]
- Rodriguez de Vera, C.; Peran Mesam, S.; de Diego, A.M.; Villar del Fresno, A. Preliminary study on the effects produced in the lipid fraction of various rat organs after administration of vulgarin (a new oral hypoglycemic agent of natural origin). Bollettino Chimico Farmaceutico 1976, 115, 445–456. [Google Scholar]
- Xu, H.; Williams, K.M.; Liauw, W.S.; Murray, M.; Day, R.O.; McLachlan, A.J. Effects of St John’s wort and CYP2C9 genotype on the pharmacokinetics and pharmacodynamics of gliclazide. Br. J. Pharmacol. 2008, 153, 1579–1586. [Google Scholar] [CrossRef] [Green Version]
- Han, D.G.; Cho, S.S.; Kwak, J.H.; Yoon, I.S. Medicinal plants and phytochemicals for diabetes mellitus: Pharmacokinetic characteristics and herb-drug interactions. J. Pharm. Investig. 2019, 49, 603–612. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alam, P.; Abdel-Kader, M.S. High-Performance Thin-Layer Chromatography Method for the Simultaneous Determination of Vulgarin and Epivulgarin Biomarkers in Artemisia judaica L. J. Planar Chromatogr. Mod. TLC. 2018, 31, 202–206. [Google Scholar] [CrossRef]
- Saeedan, A.S.; Soliman, G.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A.; Foudah, A.I.; Abdel-Kader, M.S. Artemisia judaica L. diminishes diabetes-induced reproductive dysfunction in male rats via activation of Nrf2/HO-1-mediated antioxidant responses. Saudi J. Biol. Sci. 2021, 28, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.F.; Soliman, G.A.; Saeedan, A.S.; Ogaly, H.A.; Abd-Elsalam, R.M.; Alqasoumi, S.I.; Abd-Elsalam, R.M. Molecular and biochemical monitoring of the possible herb-drug interaction between Momordica charantia extract and glibenclamide in diabetic rats. Saudi Pharma. J. 2019, 27, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Alqasoumi, S.I.; Ogaly, H.A.; Abd-Elsalam, R.M.; El-Banna, H.A.; Soliman, G.A. Propolis ameliorates cerebral injury in focal cerebral ischemia/reperfusion (I/R) rat model via upregulation of TGF-β1. Saudi Pharma. J. 2020, 28, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.F.; Ezzat, S.M.; Ogaly, H.A.; Abd-Elsalam, R.M.; Hessin, A.F.; Fekry, M.I.; Mansour, D.F.; Mohamed, S.O. Ficus deltoidea extract down-regulates protein tyrosine phosphatase 1B expression in a rat model of type 2 diabetes mellitus: A new insight into its antidiabetic mechanism. J. Nutr. Sci. 2020, 9 e2, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Khamis, G.; Hassan, M.; Morsy, M.; Ibrahim, M.A.; Abd-Elsalam, R.M.; El Badawy, S.A.; Azouz, A.A.; Galal, M. Innovative application of helium-neon laser: Enhancing the germination of Adansonia digitata and evaluating the hepatoprotective activities in mice. Environ. Sci. Pollut. Res. 2020, 27, 26520–26531. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Abd-Elsalam, R.M.; Marzouk, M.S. Molecular and immunohistochemical diagnosis of Photobacterium damselae subspecies piscicida during naturally occurring disease in Egypt. J. World Aquac. Soc. 2015, 46, 583–595. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Soliman, G.A.; Abdel-Rahman, R.F.; Saeedan, A.S.; Abd-Elsalam, R.M.; Ogaly, H.A. Effect of olive leaves extract on the antidiabetic effect of glyburide for possible herb-drug interaction. Saudi Pharma. J. 2019, 27, 1182–1195. [Google Scholar] [CrossRef]
- Chen, V.; Ianuzzo, C. Dosage effect of Streptozotocin on rat tissue enzyme activities and glycogen concentration. Can. J. Physiol. Pharmacol. 1982, 60, 1251–1256. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, M.; Sirohi, P.; Varsha. Effects of alcoholic extract of Momordica charantia (Linn.) whole fruit powder on the pancreatic islets of alloxan diabetic albino rats. J. Environ. Biol. 2008, 29, 101–106. [Google Scholar]
- Ramkumar, K.M.; Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Sujatha, J. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 3390–3394. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.J.; Sunmonu, T.O. Artemisia afra Jacq. Ameliorates oxidative stress in the pancreas of streptozotocin-induced diabetic Wistar rats. Biosci. Biotechnol. Biochem. 2011, 75, 2083–2086. [Google Scholar] [CrossRef] [PubMed]
- Awad, N.E.; Seida, A.A.; Shaffie, Z.E.; El-Aziz, A.M.; Awad, N.E. Hypoglycemic activity of Artemisia herba-alba (Asso) used in Egyptian traditional medicine as Hypoglycemic remedy. J. Appl. Pharm. Sci. 2012, 2, 30–39. [Google Scholar]
- Daradka, H.M.; Abas, M.M. Antidiabetic effect of Artemisia absinthium extracts on alloxan-induced diabetic rats. Comp. Clin. Pathol. 2014, 23, 1733–1742. [Google Scholar] [CrossRef]
- Al-Shamaony, L.; Al-Khazraji, S.M.; Twaij, H.A. Hypoglycaemic effect of Artemisia herba alba. II Effect of a valuable extract on some blood parameters in diabetic animals. J. Ethnopharmacol. 1994, 43, 167–171. [Google Scholar] [CrossRef]
- Subramoniam, A.; Pushpangadan, P.; Rajasekharan, S.; Evans, D.; Latha, P.; Valsaraj, R. Effects of Artemisia pallens wall. On blood glucose levels in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 1996, 50, 13–17. [Google Scholar] [CrossRef]
- Albasher, G.; Alwahaibi, M.; Abdel-Daim, M.; Alkahtani, S.; Almeer, R. Protective effects of Artemisia judaica extract compared to metformin against hepatorenal injury in high-fat diet/streptozotocine-induced diabetic rats. Environ. Sci. Pollut. Res. 2020, 27, 40525–40536. [Google Scholar] [CrossRef]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Shailendra, G.; Ribnicky, D.M.; Burk, D.; Karki, N.; Qingxia Wang, M.S. An extract of Artemisia dracunculus L. stimulates insulin secretion from β-cells, activates AMPK and suppresses inflammation. J. Ethnopharmacol. 2015, 170, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Ribnicky, D.; Zhang, X.H.; Zuberi, A.; Raskin, I.; Yu, Y.; Cefalu, W.T. An extract of Artemisia dracunculus L. enhances insulin receptor signaling and modulates gene expression in skeletal muscle in KK-Ay mice. J. Nutr. Biochem. 2011, 22, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Al-Wahaibi, L.H.N.; Mahmood, A.; Khan, M.; Alkhathlan, H.Z. Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arab. J. Chem. 2020, 13, 2053–2065. [Google Scholar] [CrossRef]
- Kondeti, V.K.; Badri, K.R.; Maddirala, D.R.; Thur, S.K.; Fatima, S.S.; Kasetti, R.B.; Rao, C.A. Effect of Pterocarpus santalinus bark, on blood glucose, serum lipids, plasma insulin and hepatic carbohydrate metabolic enzymes in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2010, 48, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, B.; Siddiquee, T.; Mujumder, A.; Afsana, F.; Ahmed, T.; Mdala, I.; Moreira, N.; Khan, A.; Hussain, A.; Holmboe-Ottesen, G.; et al. Serum Lipid Profile and Its Association with Diabetes and Prediabetes in a Rural Bangladeshi Population. Int. J. Environ. Res. Public Health 2018, 15, 1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juarez-Rojop, I.E.; Diaz-Zagoya, J.C.; Ble-Castillo, J.L.; Miranda-Osorio, P.H.; Castell-Rodriguez, A.E.; Tovilla-Zarate, C.A.; Rodríguez-Hernández, A.; Aguilar-Mariscal, H.; Ramón-Frías, T.; Bermúdez-Ocaña, D.Y. Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2012, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, S.; Pari, L. Antihyperlipidemic effect of diosmin: A citrus flavonoid on lipid metabolism in experimental diabetic rats. J. Funct. Foods 2013, 5, 484–492. [Google Scholar] [CrossRef]
- Pandhare, R.; Sangameswaran, B.; Mohite, P.; Khanage, S. Anti-hyperglycaemic and lipid lowering potential of Adenanthera pavonina Linn. in streptozotocin induced diabetic rats. Orient. Pharm. Exp. Med. 2012, 12, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, X.T.; Zhang, H.; Yao, H.; Zhang, H. Effect of Vacinium bracteatum Thunb. leaves extract on blood glucose and plasma lipid levels in streptozotocin-induced diabetic mice. J. Ethnopharmacol. 2010, 130, 465–469. [Google Scholar] [CrossRef]
- Ahmad, W.; Khan, I.; Khan, M.A.; Ahmad, M.; Subhan, F.; Karim, N. Evaluation of antidiabetic and antihyperlipidemic activity of Artemisia indica Linn (aeriel parts) in Streptozotocin induced diabetic rats. J. Ethnopharmacol. 2014, 151, 618–623. [Google Scholar] [CrossRef]
- Sefi, M.; Fetoui, H.; Makni, M.; Zeghal, N. Mitigating effects of antioxidant properties of Artemisia campestris leaf extract on hyperlipidemia, advanced glycation end products and oxidative stress in alloxan-induced diabetic rats. Food Chem. Toxicol. 2010, 48, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Rajasekaran, A.; Manisenthilkumar, K.T. Investigation of hypoglycemic, hypolipidemic and antioxidant activities of aqueous extract of Terminalia paniculata bark in diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress activated signaling pathways mediators of insulin resistance and beta cell dysfunction. Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef]
- Yusufoglu, H.S.; Soliman, G.A.; Abdel-Rahman, R.F.; Abdel-Kader, M.S.; Ganaie, M.A.; Bedir, E.; Baykan, S.; Ozturk, B. Antihyperglycemic and antihyperlipidemic effects of Ferula duranii in experimental type 2 diabetic rats. Int. J. Pharmacol. 2015, 11, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.T.; Chin, S.; Ya, W.C.; Ching, Y.Y.; Chin, C.W.; Dong, Z.H.; Chen, C.H.; Cheng, P.W.; Liu, S.H.; Huang, C.F. Arsenic induces pancreatic cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol. Lett. 2011, 20, 15–26. [Google Scholar] [CrossRef]
- Manna, P.; Das, J.; Ghosh, J.; Sil, P.C. Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IkappaBalpha/NFkappaB, MAPKs, and mitochondria-dependent pathways: Prophylactic role of arjunolic acid. Free Radic. Biol. Med. 2010, 48, 1465–1484. [Google Scholar] [CrossRef]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Kaul, U.; Parmar, D.; Manjunath, K.; Shah, M.; Parmar, K.; Patil, K.P.; Jaiswal, A. New dual peroxisome proliferator activated receptor agonist—Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: Integrated analysis of the real world evidence. Cardiovasc. Diabetol. 2019, 18, 80. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Jian, L.; Cheung, C.W.; Zhang, L.; Xia, Z. Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovasc. Diabetol. 2021, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Roul, D.; Recchia, F.A. Metabolic alterations induce oxidative stress in diabetic and failing hearts: Different pathways, same outcome. Antioxid. Redox Signal. 2015, 22, 1502–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, J.M.; Aleksunes, L.M.; Dieter, M.Z.; Tanaka, Y.; Peters, J.M.; Manautou, J.E.; Klaassen, C.D. Nrf2- and PPARα-Mediated Regulation of Hepatic Mrp Transporters after Exposure to Perfluorooctanoic Acid and Perfluorodecanoic Acid. Toxicol. Sci. 2008, 106, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, S.A.; Ogaly, H.A.; Abd-Elsalam, R.M.; Azouz, A.A. Benzyl isothiocyanates modulate inflammation, oxidative stress, and apoptosis via Nrf2/HO-1 and NF-κB signaling pathways on indomethacin-induced gastric injury in rats. Food Funct. 2021, 12, 6001–6013. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- ALTamimi, J.Z.; AlFaris, N.A.; Alshammari, G.M.; Alagal, R.I.; Aljabryn, D.H.; Aldera, H.; Alrfaei, B.M.; Alkhateeb, M.A.; Yahya, M.A. Ellagic acid protects against diabetic nephropathy in rats by regulating the transcription and activity of Nrf2. J. Funct. Foods 2021, 79, 104397. [Google Scholar] [CrossRef]
- Gupta, A.; Behl, T.; Sehgal, A.; Bhatia, S.; Jaglan, D.; Bungau, S. Therapeutic potential of Nrf-2 pathway in the treatment of diabetic neuropathy and nephropathy. Mol. Biol. Rep. 2021, 22, 1–4. [Google Scholar]
- Ebrahimpour, S.; Shahidi, S.B.; Abbasi, M.; Tavakoli, Z.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) increases Nrf2 expression via miR-27a mediation to prevent memory dysfunction in diabetic rats. Sci. Rep. 2020, 10, 15957. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence (5′-3′) | Accession no. | |
|---|---|---|---|
| PPAR-α | Forward | TTCGGAAACTGCAGACCT | NM_013196.1 |
| Reverse | TTAGGAACTCTCGGGTGAT | ||
| Nrf2 | Forward | CACATCCAGACAGACACCAGT | XM_006234398.3 |
| Reverse | CTACAAATGGGAATGTCTCTGC | ||
| β actin | Forward | ATGGTGGGTATGGGTCAG | NM_031144.3 |
| Reverse | CAATGCCGTGTTCAATGG | ||
| Treatment Groups | % of Body Weight Gain (g) | ||
|---|---|---|---|
| 2 Weeks | 4 Weeks | 8 Weeks | |
| NC | 13.65 ± 0.88 | 33.96 ± 1.82 | 49.84 ± 2.93 |
| STZ-control | −2.90 ± 0.18 ● | −7.15 ± 0.31 ● | −12.02 ± 0.47 ● |
| STZ + GLB | 7.16 ± 0.41 ●# | 14.71 ± 0.96 ●# | 28.34 ± 2.11 ●# |
| STZ + AJE-250 | 7.35 ± 0.43 ●# | 15.72 ± 0.97 ●# | 29.12 ± 1.98 ●# |
| STZ + AJE-500 | 8.52 ± 0.75 ●# | 18.17 ± 1.74 ●# | 34.25 ± 1.75 ●# |
| STZ + GLB + AJE-250 | 7.86 ± 0.55 ●# | 17.51 ± 1.47 ●# | 32.73 ± 1.95 ●# |
| STZ + GLB + AJE-500 | 9.45 ± 0.76 ●#Փ | 29.14 ± 1.73 #Փ | 44.64 ± 2.82 #Փ |
| Treatment Groups | FBG (mg/dL) | ||||||
|---|---|---|---|---|---|---|---|
| 0-Time | 2 Weeks | 4 Weeks | 8 Weeks | ||||
| M ± SEM | M ± SEM | % | M ± SEM | % | M ± SEM | % | |
| NC | 96.3 ± 5.43 | 94.5 ± 4.67 | −1.87 | 95.7 ± 6.97 | −0.62 | 94.4 ± 5.70 | −1.97 |
| STZ-control | 349.2 ± 7.57 ● | 352.2 ± 9.58 ● | 0.86 | 347.8 ± 16.47 ● | −0.40 | 340.5 ± 16.52 ● | −2.49 |
| STZ + GLB | 347.1 ± 5.08 ● | 186.2 ± 6.53 ●# | −46.36 | 169.2 ± 8.62 ●# | −51.25 | 135.3 ± 7.43 ●# | −61.02 |
| STZ + AJE-250 | 339.4 ± 9.87 ● | 194.7 ± 9.20 ●# | −42.63 | 175.3 ± 8.50 ●# | −48.35 | 143.2 ± 8.11 ●# | −57.81 |
| STZ + AJE-500 | 347.8 ± 8.97 ● | 176.5 ± 8.18 ●# | −49.25 | 152.1 ± 7.1 7●# | −56.27 | 118.5 ± 7.92 ●# | −65.93 |
| STZ + GLB + AJE-250 | 345.9 ± 7.66 ● | 179.6 ± 8.22 ●# | −48.08 | 161.3 ± 8.50 ●# | −53.37 | 126.7 ± 6.37 ●# | −63.37 |
| STZ + GLB + AJE-500 | 358.5 ± 6.98 ● | 157.5 ± 6.49 ●#Փ | −56.07 | 112.6 ± 8.62 #Փ | −68.59 | 96.62 ± 6.52 #Փ | −73.05 |
| Treatment Groups | Insulin (U/L) | ||||||
|---|---|---|---|---|---|---|---|
| 0-Time | 2 Weeks | 4 Weeks | 8 Weeks | ||||
| M ± SEM | M ± SEM | % | M ± SEM | % | M ± SEM | % | |
| NC | 7.5 ± 0.34 | 7.3 ± 0.48 | −2.67 | 7.4 ± 0.57 | −1.33 | 7.2 ± 0.48 | −4.00 |
| STZ-control | 3.8 ± 0.26 ● | 3.4 ± 0.10 ● | −10.53 | 3.6 ± 0.28 ● | −5.26 | 3.5 ± 0.27 ● | −7.89 |
| STZ + GLB | 3.5 ± 0.18 ● | 3.9 ± 0.11 ●# | 11.43 | 4.7 ± 0.32 ●# | 34.29 | 5.2 ± 0.36 ●# | 48.57 |
| STZ + AJE-250 | 3.5 ± 0.25 ● | 3.8 ± 0.10 ●# | 8.57 | 4.5 ± 0.25 ●# | 28.57 | 5.0 ± 0.30 ●# | 42.86 |
| STZ + AJE-500 | 3.4 ± 0.20 ● | 4.2 ± 0.28 ●# | 23.53 | 5.3 ± 0.32 ●# | 55.88 | 5.9 ± 0.33 ●# | 73.53 |
| STZ + GLB + AJE-250 | 3.6 ± 0.17 ● | 4.0 ± 0.23 ●# | 21.21 | 4.8 ± 0.31 ●# | 45.45 | 5.3 ± 0.39 ●# | 60.61 |
| STZ + GLB + AJE-500 | 3.5 ± 0.21 ● | 4.6 ± 0.25 ●#Փ | 31.43 | 5.9 ± 0.29 #Փ | 68.57 | 6.5 ± 0.41 #Փ | 85.71 |
| Treatment Groups | Total Hemoglobin (mg/dL) | HbA1c (%) |
|---|---|---|
| NC | 14.4 ± 0.64 | 3.9 ± 0.21 |
| STZ-control | 9.7 ± 0.36 ● | 8.5 ± 0.36 ● |
| STZ + GLB | 11.1 ± 0.48 ●# | 6.3 ± 0.48 ●# |
| STZ + AJE-250 | 11.4 ± 0.57 ●# | 6.7 ± 0.30 ●# |
| STZ + AJE-500 | 12.3 ± 0.62 ●# | 5.0 ± 0.42 ●# |
| STZ + GLB + AJE-250 | 12.0 ± 0.60 ●# | 5.5 ± 0.29 ●# |
| STZ + GLB + AJE-500 | 13.6 ± 0.65 #Փ | 4.5 ± 0.32 #Փ |
| Treatment Groups | TGs (mg/dL) | TC (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | VLDL (mg/dL) |
|---|---|---|---|---|---|
| NC | 27.7 ± 0.97 | 44.5 ± 2.27 | 24.6 ± 0.74 | 14.4 ± 1.15 | 5.5 ± 0.28 |
| STZ-control | 49.3 ± 2.55 ● | 66.2 ± 1.32 ● | 14.5 ± 0.75 ● | 41.8 ± 1.26 ● | 9.9 ± 0.45 ● |
| STZ + GLB | 40.2 ± 1.37 ●# | 59.2 ± 1.72 ●# | 17.1 ± 0.63 ●# | 34.1 ± 2.97 ●# | 8.0 ± 0.41 ●# |
| STZ + AJE-250 | 42.4 ± 1.42 ●# | 60.6 ± 2.14 ●# | 16.9 ± 0.48 ●# | 35.2 ± 1.58 ●# | 8.5 ± 0.42 ●# |
| STZ + AJE-500 | 36.4 ± 1.55 ●# | 52.6 ± 2.61 ●# | 19.2 ± 0.75 ●# | 26.1 ± 2.50 ●# | 7.3 ± 0.36 ●# |
| STZ + GLB + AJE-250 | 37.5 ± 1.17 ●# | 54.1 ± 2.27 ●# | 18.9 ± 0.76 ●# | 27.7 ± 1.96 ●# | 7.5 ± 0.39 ●# |
| STZ + GLB + AJE-500 | 33.5 ± 2.58 #Փ | 49.5 ± 1.65 #Փ | 23.7 ± 0.65 #Փ | 19.1 ± 1.89 #Փ | 6.7 ± 0.30 #Փ |
| Treatment Groups | SOD (U/mg protein) | GPx (U/mg protein) | CAT (U/mg protein) | GSH (µmol/g tissue) | MDA (nmol/g tissue) |
|---|---|---|---|---|---|
| NC | 57.6 ± 3.20 | 6.8 ± 0.55 | 11.4 ± 0.15 | 9.7 ± 0.52 | 26.5 ± 1.76 |
| STZ-control | 21.5 ± 1.63 ● | 2.2 ± 0.14 ● | 5.1 ± 0.31 ● | 3.8 ± 0.20 ● | 47.4 ± 3.17 ● |
| STZ + GLB | 30.6 ± 2.87 ●# | 3.7 ± 0.30 ●# | 7.0 ± 0.52 ●# | 5.7 ± 0.47 ●# | 38.2 ± 2.55 ●# |
| STZ + AJE-250 | 33.5 ± 2.23 ●# | 3.8 ± 0.25 ●# | 7.3 ± 0.41 ●# | 6.3 ± 0.49 ●# | 37.3 ± 2.27 ●# |
| STZ + AJE-500 | 40.6 ± 3.71 ●# | 4.9 ± 0.47 ●# | 8.3 ± 0.42 ●# | 7.4 ± 0.66 ●# | 32.4 ± 1.57 ●# |
| STZ + GLB + AJE-250 | 36.2 ± 2.73 ●# | 4.7 ± 0.38 ●# | 7.9 ± 0.55 ●# | 7.0 ± 0.52 ●# | 35.2 ± 2.61 ●# |
| STZ + GLB + AJE-500 | 49.5 ± 3.24 #Փ | 5.5 ± 0.36 #Փ | 10.2 ± 0.72 #Փ | 8.5 ± 0.57 #Փ | 30.7 ± 2.13 #Փ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeedan, A.S.; Soliman, G.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A.; Alharthy, K.M.; Abdel-Kader, M.S. Possible Synergistic Antidiabetic Effects of Quantified Artemisia judaica Extract and Glyburide in Streptozotocin-Induced Diabetic Rats via Restoration of PPAR-α mRNA Expression. Biology 2021, 10, 796. https://doi.org/10.3390/biology10080796
Saeedan AS, Soliman GA, Abdel-Rahman RF, Abd-Elsalam RM, Ogaly HA, Alharthy KM, Abdel-Kader MS. Possible Synergistic Antidiabetic Effects of Quantified Artemisia judaica Extract and Glyburide in Streptozotocin-Induced Diabetic Rats via Restoration of PPAR-α mRNA Expression. Biology. 2021; 10(8):796. https://doi.org/10.3390/biology10080796
Chicago/Turabian StyleSaeedan, Abdulaziz S., Gamal A. Soliman, Rehab F. Abdel-Rahman, Reham M. Abd-Elsalam, Hanan A. Ogaly, Khalid M. Alharthy, and Maged S. Abdel-Kader. 2021. "Possible Synergistic Antidiabetic Effects of Quantified Artemisia judaica Extract and Glyburide in Streptozotocin-Induced Diabetic Rats via Restoration of PPAR-α mRNA Expression" Biology 10, no. 8: 796. https://doi.org/10.3390/biology10080796





