The Influences of Rainfall Intensity and Timing on the Assemblage of Dung Beetles and the Rate of Dung Removal in an Alpine Meadow
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Sampling
2.3. Data Analysis
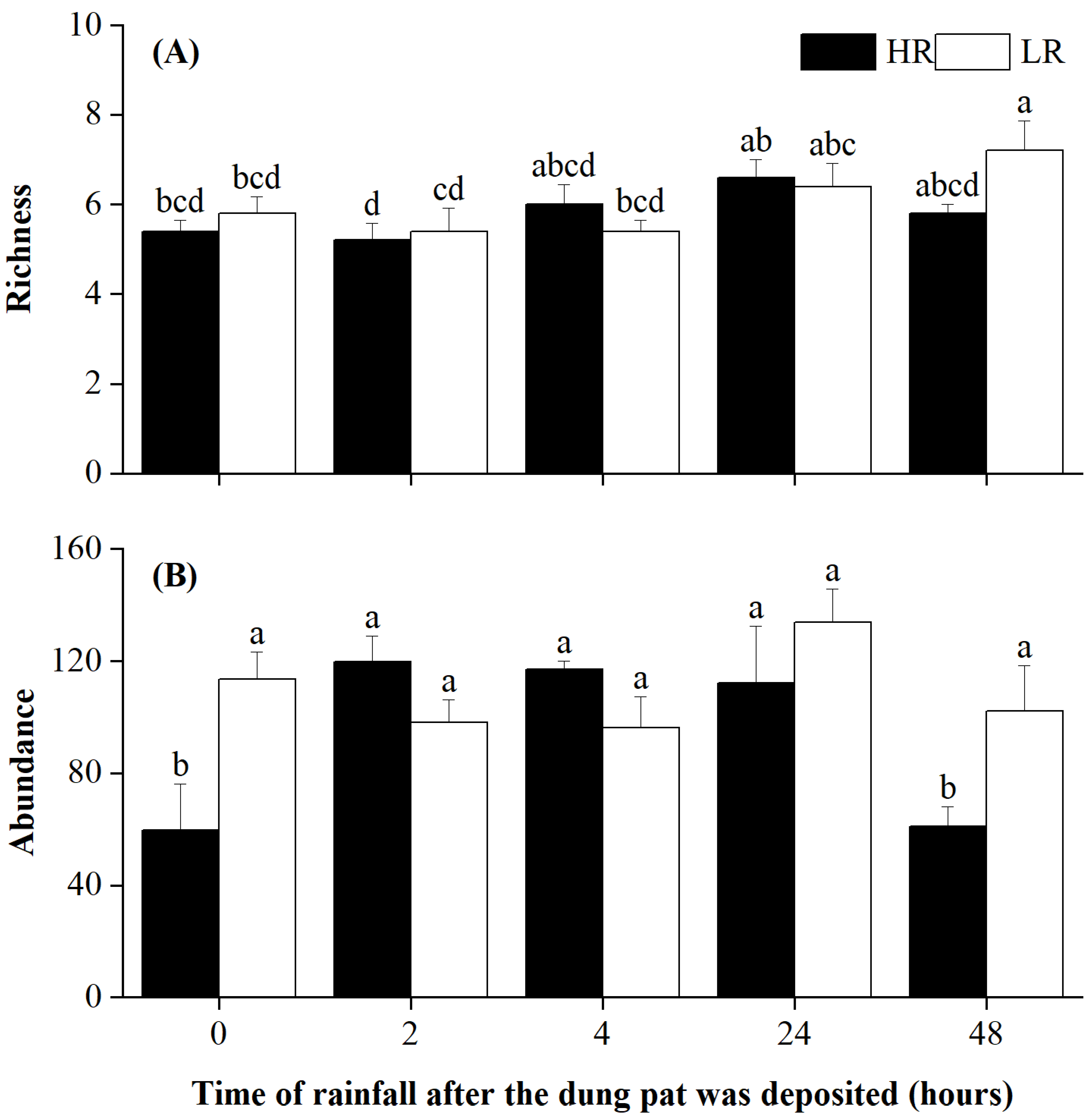
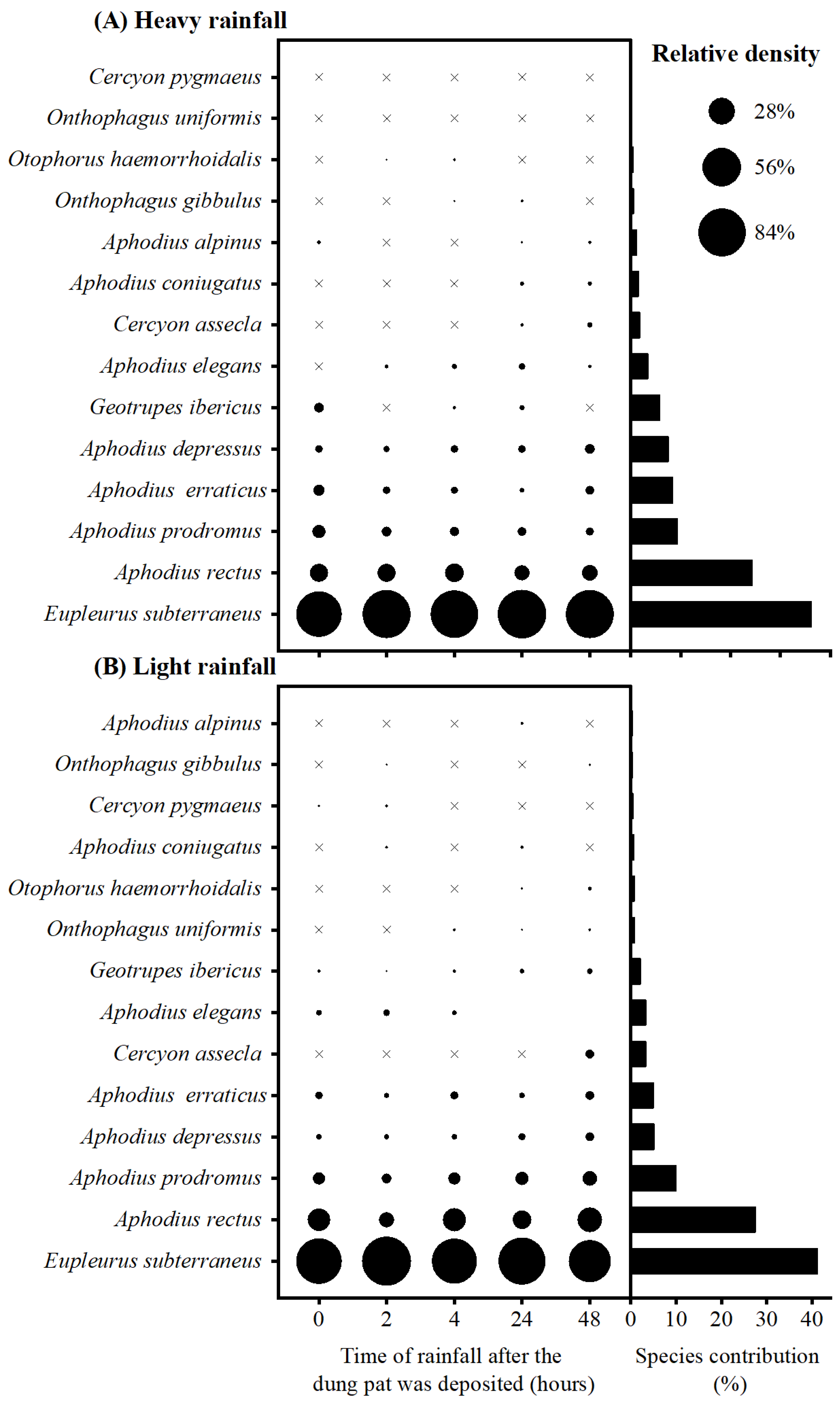
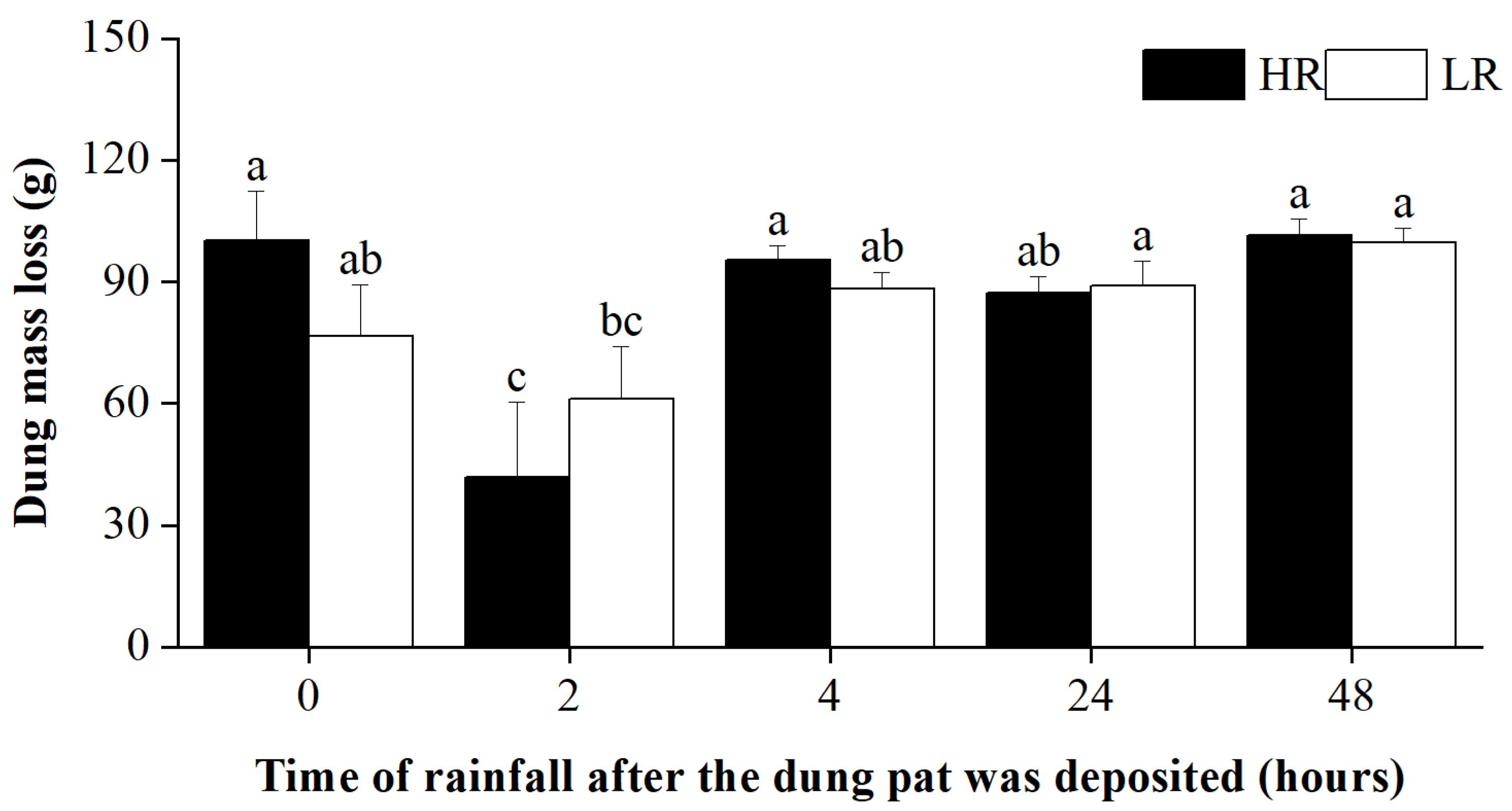
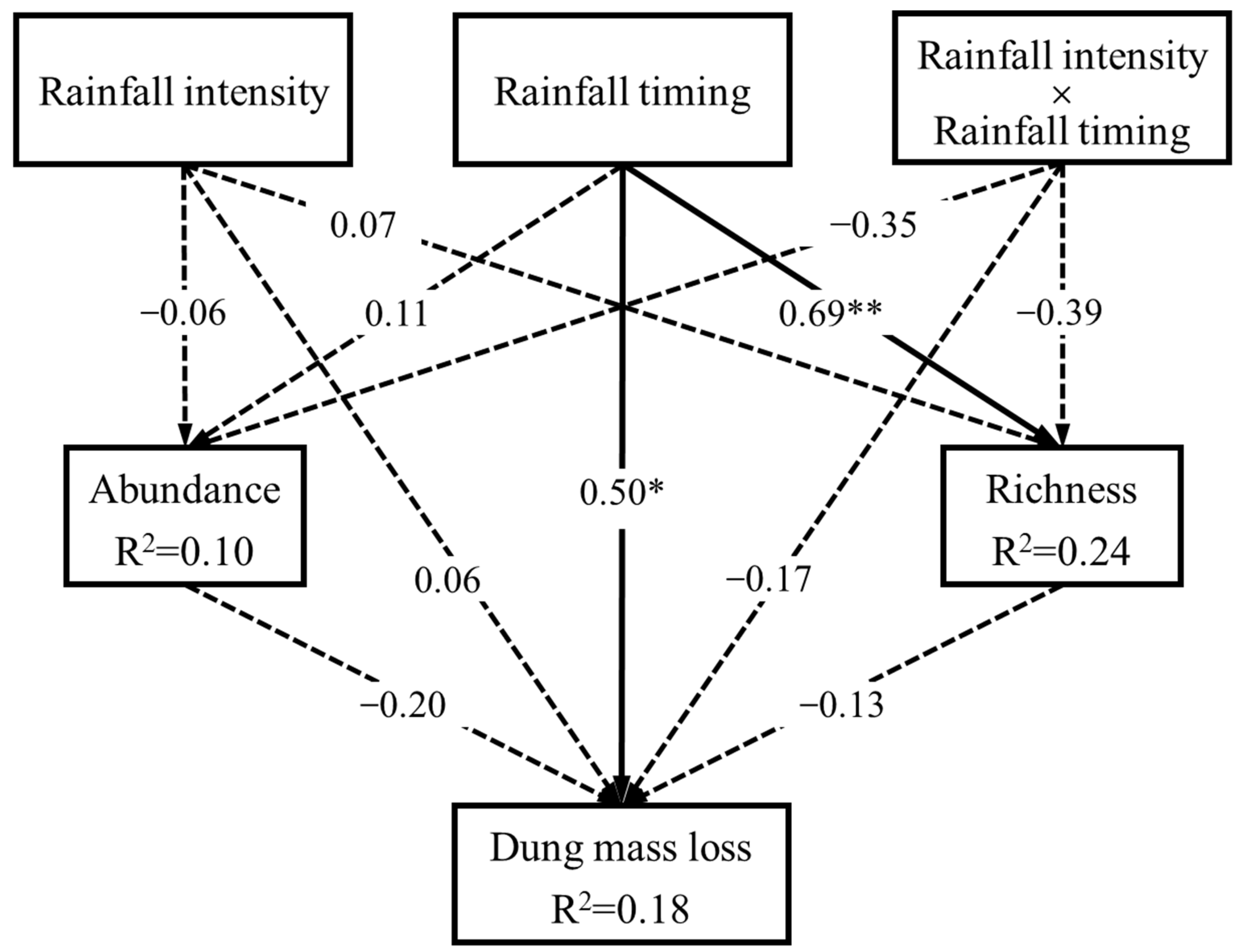
3. Results
3.1. Responses of Beetle Assemblage and Dung Decomposition
3.2. Relationships among Rainfall, Beetle Assemblage, and Dung Decomposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Gu, J. Ecological Responses, Adaptation and Mechanisms of Mangrove Wetland Ecosystem to Global Climate Change and Anthropogenic Activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sgro, C.M. Climate Change and Evolutionary Adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cao, J.; Bai, Y.; Zhou, X.; Ning, Z.; Yang, S.; Hu, L. Effects of Rainfall Amount and Frequency on Vegetation Growth in a Tibetan Alpine Meadow. Clim. Chang. 2013, 118, 197–212. [Google Scholar] [CrossRef]
- Gordon, H.B.; Whetton, P.H.; Pittock, A.B.; Fowler, A.M.; Haylock, M.R. Simulated Changes in Daily Rainfall Intensity Due to the Enhanced Greenhouse Effect: Implications for Extreme Rainfall Events. Clim. Dyn. 1992, 8, 83–102. [Google Scholar] [CrossRef]
- Westra, S.; Fowler, H.J.; Evans, J.P.; Alexander, L.V.; Berg, P.; Johnson, F.; Kendon, E.J.; Lenderink, G.; Roberts, N.M. Future Changes to the Intensity and Frequency of Short-Duration Extreme Rainfall. Rev. Geophys. 2014, 52, 522–555. [Google Scholar] [CrossRef]
- Qiu, T.; Song, C.; Clark, J.S.; Seyednasrollah, B.; Rathnayaka, N.; Li, J. Understanding the Continuous Phenological Development at Daily Time Step with a Bayesian Hierarchical Space-Time Model: Impacts of Climate Change and Extreme Weather Events. Remote Sens. Environ. 2020, 247, 111956. [Google Scholar] [CrossRef]
- Henen, B.T.; Peterson, C.C.; Wallis, I.R.; Berry, K.H.; Nagy, K.A. Effects of Climatic Variation on Field Metabolism and Water Relations of Desert Tortoises. Oecologia 1998, 117, 365–373. [Google Scholar] [CrossRef]
- Szarek, S.R.; Ting, I.P. Seasonal Patterns of Acid Metabolism and Gas Exchange in Opuntia Basilaris. Plant Physiol. 1974, 54, 76–81. [Google Scholar] [CrossRef]
- Previtali, M.A.; Lima, M.; Meserve, P.L.; Kelt, D.A.; Gutiérrez, J.R. Population Dynamics of Two Sympatric Rodents in a Variable Environment: Rainfall, Resource Availability, and Predation. Ecology 2009, 90, 1996–2006. [Google Scholar] [CrossRef]
- Pires, A.P.F.; Marino, N.A.C.; Srivastava, D.S.; Farjalla, V.F. Predicted Rainfall Changes Disrupt Trophic Interactions in a Tropical Aquatic Ecosystem. Ecology 2016, 97, 2750–2759. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Peralta, A.M.L.; Luzuriaga, A.L.; Prieto, M.; Escudero, A. Climate Change and Biocrust Disturbance Synergistically Decreased Taxonomic, Functional and Phylogenetic Diversity in Annual Communities on Gypsiferous Soils. Oikos 2022, 2022, 08809. [Google Scholar] [CrossRef]
- Amissah, L.; Mohren, G.M.J.; Bongers, F.; Hawthorne, W.D.; Poorter, L. Rainfall and Temperature Affect Tree Species Distribution in Ghana. J. Trop. Ecol. 2014, 30, 435–446. [Google Scholar] [CrossRef]
- Bongers, F.; Poorter, L.; Rompaey, R.S.A.R.; Parren, M.P.E. Distribution of Twelve Moist Forest Canopy Tree Species in Liberia and Côte D’ivoire: Response Curves to a Climatic Gradient. J. Veg. Sci. 1999, 10, 371–382. [Google Scholar] [CrossRef]
- Abalos, D.; Sanz-Cobena, A.; Andreu, G.; Vallejo, A. Rainfall Amount and Distribution Regulate Dmpp Effects on Nitrous Oxide Emissions under Semiarid Mediterranean Conditions. Agric. Ecosyst. Environ. 2017, 238, 36–45. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological Linkages between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the Dominant Controls on Litter Decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Cole, L.; Staddon, P.L.; Sleep, D.; Bardgett, R.D. Soil Animals Influence Microbial Abundance, but Not Plant-Microbial Competition for Soil Organic Nitrogen. Funct. Ecol. 2004, 18, 631–640. [Google Scholar] [CrossRef]
- Salamanca, E.F.; Kaneko, N.; Katagiri, S. Rainfall Manipulation Effects on Litter Decomposition and the Microbial Biomass of the Forest Floor. Appl. Soil Ecol. 2003, 22, 271–281. [Google Scholar] [CrossRef]
- Joly, F.X.; Weibel, A.K.; Coulis, M.; Throop, H.L. Rainfall Frequency, Not Quantity, Controls Isopod Effect on Litter Decomposition. Soil Biol. Biochem. 2019, 135, 154–162. [Google Scholar] [CrossRef]
- DeVault, T.L.; Rhodes, J.; Olin, E.; Shivik, J.A. Scavenging by Vertebrates: Behavioral, Ecological, and Evolutionary Perspectives on an Important Energy Transfer Pathway in Terrestrial Ecosystems. Oikos 2003, 102, 225–234. [Google Scholar] [CrossRef]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rodenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial Gross Carbon Dioxide Uptake: Global Distribution and Covariation with Climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity Meets Decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, J.; Lartigue, J. Patterns of Herbivory and Decomposition in Aquatic and Terrestrial Ecosystems. Ecol. Monogr. 2004, 74, 237–259. [Google Scholar] [CrossRef]
- Parmenter, R.R.; Macmahon, J.A. Carrion Decomposition and Nutrient Cycling in a Semiarid Shrub–Steppe Ecosystem. Ecol. Monogr. 2009, 79, 637–661. [Google Scholar] [CrossRef]
- Barton, P.S.; Evans, M.J.; Foster, C.N.; Pechal, J.L.; Bump, J.K.; Quaggiotto, M.M.; Benbow, M.E. Towards Quantifying Carrion Biomass in Ecosystems. Trends Ecol. Evol. 2019, 34, 950–961. [Google Scholar] [CrossRef]
- Barton, P.S.; Cunningham, S.A.; Lindenmayer, D.B.; Manning, A.D. The Role of Carrion in Maintaining Biodiversity and Ecological Processes in Terrestrial Ecosystems. Oecologia 2013, 171, 761–772. [Google Scholar] [CrossRef]
- Moore, J.C.; Berlow, E.L.; Coleman, D.C.; Ruiter, P.C.; Dong, Q.; Hastings, A.; Johnson, N.C.; McCann, K.S.; Melville, K.; Morin, P.J.; et al. Detritus, Trophic Dynamics and Biodiversity. Ecol. Lett. 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Liu, Y.; Cheng, J.; Wang, Y.; Liu, J.; Wang, X.; Li, Y. Herbivore Dung Promotes Plant Litter Decomposition Rate in a Semi-Arid Grassland Ecosystem. Ecosystems 2023, 26, 661–674. [Google Scholar] [CrossRef]
- Cole, L.; Bardgett, R.D.; Ineson, P.; Adamson, J.K. Relationships between Enchytraeid Worms (Oligochaeta), Climate Change, and the Release of Dissolved Organic Carbon from Blanket Peat in Northern England. Soil Biol. Biochem. 2002, 34, 599–607. [Google Scholar] [CrossRef]
- Noriega, J.A.; Santos, A.M.C.; Calatayud, J.; Chozas, S.; Hortal, J. Short- and Long-Term Temporal Changes in the Assemblage Structure of Amazonian Dung Beetles. Oecologia 2021, 195, 719–736. [Google Scholar] [CrossRef]
- Clein, J.S.; Schimel, J.P. Reduction in Microbial Activity in Birch Litter Due to Drying and Rewetting Events. Soil Biol. Biochem. 1994, 26, 403–406. [Google Scholar] [CrossRef]
- Dickinson, C.H.; Underhay, V.S.H.; Ross, V. Effect of Season, Soil Fauna and Water Content on the Decomposition of Cattle Dung Pats. New Phytol. 1981, 88, 129–141. [Google Scholar] [CrossRef]
- Medina, A.M.; Lopes, P.P. Seasonality in the Dung Beetle Community in a Brazilian Tropical Dry Forest: Do Small Changes Make a Difference? J. Insect Sci. 2014, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Novais, S.M.; Evangelista, L.A.; Reis-Júnior, R.; Neves, F.S. How Does Dung Beetle (Coleoptera: Scarabaeidae) Diversity Vary Along a Rainy Season in a Tropical Dry Forest? J. Insect Sci. 2016, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Salunke, P.; Jain, S.; Mishra, S.K. Performance of the Cmip5 Models in the Simulation of the Himalaya-Tibetan Plateau Monsoon. Theor. Appl. Climatol. 2018, 137, 909–928. [Google Scholar] [CrossRef]
- Duan, A.; Wu, G.; Zhang, Q.; Liu, Y. New Proofs of the Recent Climate Warming over the Tibetan Plateau as a Result of the Increasing Greenhouse Gases Emissions. Chin. Sci. Bull. 2006, 51, 1396–1400. [Google Scholar] [CrossRef]
- Feng, X.; Shen, H.; Li, W.; Wang, Q.; Duan, L.; Li, H. Spatiotemporal Changes for Extreme Precipitation in Wet Season over the Qinghai-Tibetan Plateau and the Surroundings During 1961–2017. Plateau Meteorol. 2020, 39, 694–705. [Google Scholar] [CrossRef]
- Zhang, R.; Su, F.; Jiang, Z.; Gao, X.; Guo, D.; Ni, J.; You, Q.; Cuo, L.; Zhou, B. An Overview of Projected Climate and Environmental Changes across the Tibetan Plateau in the 21st Century. Chin. Sci. Bull. 2015, 60, 3036–3047. [Google Scholar] [CrossRef]
- Wu, X.; Sun, S. The Roles of Beetles and Flies in Yak Dung Removal in an Alpine Meadow of Eastern Qinghai-Tibetan Plateau. Ecoscience 2010, 17, 146–155. [Google Scholar] [CrossRef]
- Kou, L.; Sun, W.; Wei, X.; Wu, X.; Sun, S. Drainage Increases Species Richness and Density of Soil Macro-Invertebrates in the Zoige Peatland of Eastern Tibetan Plateau. Pedobiologia 2021, 89, 150773. [Google Scholar] [CrossRef]
- Mohr, C.O. Cattle Droppings as Ecological Units. Ecol. Monogr. 1943, 13, 275–298. [Google Scholar] [CrossRef]
- Wu, X.; Li, G.; Sun, S. Effect of Rainfall Regimes on the Decomposition Rate of Yak Dung in an Alpine Meadow of Northwest Sichuan Province, China. Acta Ecol. Sin. 2011, 31, 28–36. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks CA, USA, 2019; ISBN 978-154-433-647-3. [Google Scholar]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Lefcheck, J.S. Piecewisesem: Piecewise Structural Equation Modelling in R for Ecology, Evolution, and Systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- RR Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 3 September 2023).
- Hoter, P. Effect of Dung-Beetles (Aphodius spp.) and Earthworms on the Disappearance of Cattle Dung. Oikos 1979, 32, 393–402. [Google Scholar] [CrossRef]
- Freymann, P.B.; Buitenwerf, R.; Desouza, O.; Olff, H. The Importance of Termites (Isoptera) for the Recycling of Herbivore Dung in Tropical Ecosystems: A Review. Eur. J. Entomol. 2008, 105, 165–173. [Google Scholar] [CrossRef]
- Herrick, J.E.; Lal, R. Dung Decomposition and Pedoturbation in a Seasonally Dry Tropical Pasture. Biol. Fertil. Soils 1996, 23, 177–181. [Google Scholar] [CrossRef]
- Janzen, D.H. Seasonal Change in Abundance of Large Nocturnal Dung Beetles (Scarabaeidae) in a Costa Rican Deciduous Forest and Adjacent Horse Pasture. Oikos 1983, 41, 274–283. [Google Scholar] [CrossRef]
- Noriega, J.A.; March-Salas, M.; Castillo, S.; García, Q.H.; Hortal, J.; Santos, A.M.C. Human Perturbations Reduce Dung Beetle Diversity and Dung Removal Ecosystem Function. Biotropica 2021, 53, 753–766. [Google Scholar] [CrossRef]
- Weithmann, S.; von Hoermann, C.; Schmitt, T.; Steiger, S.; Ayasse, M. The Attraction of the Dung Beetle Anoplotrupes Stercorosus (Coleoptera: Geotrupidae) to Volatiles from Vertebrate Cadavers. Insects 2020, 11, 476. [Google Scholar] [CrossRef]
- Dormont, L.; Epinat, G.; Lumaret, J.P. Trophic Preferences Mediated by Olfactory Cues in Dung Beetles Colonizing Cattle and Horse Dung. Environ. Entomol. 2004, 33, 370–377. [Google Scholar] [CrossRef]
- Vallat, A.; Gu, H.; Dorn, S. How Rainfall, Relative Humidity and Temperature Influence Volatile Emissions from Apple Trees in Situ. Phytochemistry 2005, 66, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Gotcha, N.; Cuthbert, R.N.; Machekano, H.; Nyamukondiwa, C. Density-Dependent Ecosystem Service Delivery under Shifting Temperatures by Dung Beetles. Sci. Total Environ. 2022, 807, 150575. [Google Scholar] [CrossRef]
- Underhay, V.H.S.; Dickinson, C.H. Water, Mineral and Energy Fluctuations in Decomposing Cattle Dung Pats. Grass Forage Sci. 1978, 33, 189–196. [Google Scholar] [CrossRef]
| df | Richness | Abundance | Dung Mass Loss | ||||
|---|---|---|---|---|---|---|---|
| SS | F | SS | F | SS | F | ||
| Timing | 4 | 0.06 | 3.43 * | 0.35 | 3.83 ** | 14,350 | 7.70 *** |
| Intensity | 1 | 0.00 | 0.55 | 0.12 | 5.37 * | 61 | 0.13 |
| Timing × Intensity | 4 | 0.02 | 1.31 | 0.38 | 4.27 ** | 2401 | 1.29 |
| Residuals | 40 | 0.18 | 0.90 | 18,627 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Tang, W.; Wu, Y.; He, S.; Wu, X. The Influences of Rainfall Intensity and Timing on the Assemblage of Dung Beetles and the Rate of Dung Removal in an Alpine Meadow. Biology 2023, 12, 1496. https://doi.org/10.3390/biology12121496
Sun W, Tang W, Wu Y, He S, Wu X. The Influences of Rainfall Intensity and Timing on the Assemblage of Dung Beetles and the Rate of Dung Removal in an Alpine Meadow. Biology. 2023; 12(12):1496. https://doi.org/10.3390/biology12121496
Chicago/Turabian StyleSun, Wenxiao, Wenting Tang, Yashi Wu, Shuaibing He, and Xinwei Wu. 2023. "The Influences of Rainfall Intensity and Timing on the Assemblage of Dung Beetles and the Rate of Dung Removal in an Alpine Meadow" Biology 12, no. 12: 1496. https://doi.org/10.3390/biology12121496





