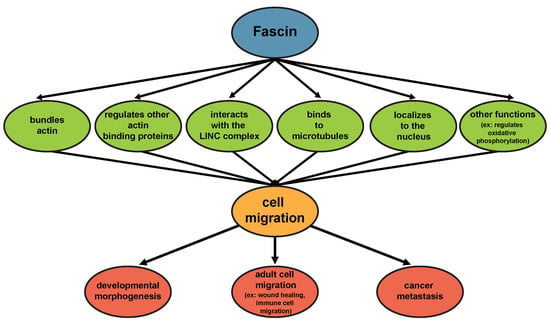
Fascin in Cell Migration: More Than an Actin Bundling Protein
Abstract
:Simple Summary
Abstract
1. Introduction
2. Fascin Structure, Expression, and Functions
2.1. Structure of Fascin
2.2. Expression and Bundling Function of Fascin
2.3. Non-Canonical Roles of Fascin
3. Regulation of Fascin
3.1. Post-Translational Modifications
3.2. Regulation by Prostaglandin Signaling
3.3. Protein–Protein Interactions
3.4. Transcriptional Regulation
4. Fascin and Disease
4.1. Fascin and Cancer
4.2. Fascin and Other Diseases
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- De Arcangelis, A.; Georges-Labouesse, E.; Adams, J.C. Expression of fascin-1, the gene encoding the actin-bundling protein fascin-1, during mouse embryogenesis. Gene Expr. Patterns 2004, 4, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Machesky, L.M. Fascin1 in carcinomas: Its regulation and prognostic value. Int. J. Cancer 2015, 137, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Jayo, A.; Parsons, M. Fascin: A key regulator of cytoskeletal dynamics. Int. J. Biochem. Cell Biol. 2010, 42, 1614–1617. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kim, D.J.; Adams, J.C. The roles of fascins in health and disease. J. Pathol. 2011, 224, 289–300. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Parsons, M.; Adams, J.C. Dual Actin-bundling and Protein Kinase C-binding Activities of Fascin Regulate Carcinoma Cell Migration Downstream of Rac and Contribute to Metastasis. Mol. Biol. Cell 2007, 18, 4591–4602. [Google Scholar] [CrossRef] [Green Version]
- Van Audenhove, I.; Denert, M.; Boucherie, C.; Pieters, L.; Cornelissen, M.; Gettemans, J. Fascin Rigidity and L-plastin Flexibility Cooperate in Cancer Cell Invadopodia and Filopodia. J. Biol. Chem. 2016, 291, 9148–9160. [Google Scholar] [CrossRef] [Green Version]
- Vignjevic, D.M.; Kojima, S.-I.; Aratyn, Y.; Danciu, O.; Svitkina, T.; Borisy, G.G. Role of fascin in filopodial protrusion. J. Cell Biol. 2006, 174, 863–875. [Google Scholar] [CrossRef] [Green Version]
- Elkhatib, N.; Neu, M.B.; Zensen, C.; Schmoller, K.M.; Louvard, D.; Bausch, A.R.; Betz, T.; Vignjevic, D.M. Fascin Plays a Role in Stress Fiber Organization and Focal Adhesion Disassembly. Curr. Biol. 2014, 24, 1492–1499. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.C. Characterization of Cell–Matrix Adhesion Requirements for the Formation of Fascin Microspikes. Mol. Biol. Cell 1997, 8, 2345–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.C.; Clelland, J.D.; Collett, G.D.; Matsumura, F.; Yamashiro, S.; Zhang, L. Cell-Matrix Adhesions Differentially Regulate Fascin Phosphorylation. Mol. Biol. Cell 1999, 10, 4177–4190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Dawson, J.C.; Forerovargas, M.G.; Spence, H.J.; Yu, X.; König, I.; Anderson, K.I.; Machesky, L.M. The Actin-Bundling Protein Fascin Stabilizes Actin in Invadopodia and Potentiates Protrusive Invasion. Curr. Biol. 2010, 20, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliewe, F.; Scharf, C.; Rogge, H.; Darm, K.; Lindenmeyer, M.T.; Amann, K.; Cohen, C.D.; Endlich, K.; Endlich, N. Studying the role of fascin-1 in mechanically stressed podocytes. Sci. Rep. 2017, 7, 9916. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, M.; Elia, L.; Condorelli, G.; Courtneidge, S.A. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J. Cell Biol. 2010, 189, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.-R.; Tao, L.-H.; Shen, Z.-Y.; Lv, Z.; Xu, L.; Li, E.-M. Fascin Expression in Human Embryonic, Fetal, and Normal Adult Tissue. J. Histochem. Cytochem. 2008, 56, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Harker, A.J.; Katkar, H.H.; Bidone, T.C.; Aydin, F.; Voth, G.A.; Applewhite, D.A.; Kovar, D.R. Ena/VASP processive elongation is modulated by avidity on actin filaments bundled by the filopodia cross-linker fascin. Mol. Biol. Cell 2019, 30, 851–862. [Google Scholar] [CrossRef]
- Winkelman, J.D.; Bilancia, C.G.; Peifer, M.; Kovar, D.R. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc. Natl. Acad. Sci. USA 2014, 111, 4121–4126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villari, G.; Jayo, A.; Zanet, J.; Fitch, B.; Serrels, B.; Frame, M.C.; Stramer, B.M.; Goult, B.T.; Parsons, M. A direct interaction between fascin and microtubules contributes to adhesion dynamics and cell migration. J. Cell Sci. 2015, 128, 4601–4614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayo, A.; Malboubi, M.; Antoku, S.; Chang, W.; Ortiz-Zapater, E.; Groen, C.; Pfisterer, K.; Tootle, T.; Charras, G.; Gundersen, G.G.; et al. Fascin Regulates Nuclear Movement and Deformation in Migrating Cells. Dev. Cell 2016, 38, 371–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groen, C.M.; Jayo, A.; Parsons, M.; Tootle, T.L. Prostaglandins regulate nuclear localization of Fascin and its function in nucleolar architecture. Mol. Biol. Cell 2015, 26, 1901–1917. [Google Scholar] [CrossRef] [Green Version]
- Kane, R.E. Preparation and purification of polymerized actin from sea urchin egg extracts. J. Cell Biol. 1975, 66, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Cant, K.; Cooley, L. Single Amino Acid Mutations in Drosophila Fascin Disrupt Actin Bundling Function in Vivo. Genetics 1996, 143, 249–258. [Google Scholar] [PubMed]
- Cant, K.; Knowles, B.A.; Mooseker, M.S.; Cooley, L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J. Cell Biol. 1994, 125, 369–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holthuis, J.C.; Schoonderwoert, V.T.; Martens, G.J. A vertebrate homolog of the actin-bundling protein fascin. Biochim. Biophys. Acta 1994, 1219, 184–188. [Google Scholar] [CrossRef]
- Edwards, R.A.; Herrera-Sosa, H.; Otto, J.; Bryan, J. Cloning and Expression of a Murine Fascin Homolog from Mouse Brain. J. Biol. Chem. 1995, 270, 10764–10770. [Google Scholar] [CrossRef] [Green Version]
- Duh, F.-M.; Latif, F.; Weng, Y.; Geil, L.; Modi, W.; Stackhouse, T.; Matsumura, F.; Duan, D.R.; Linehan, W.M.; Lerman, M.I.; et al. cDNA Cloning and Expression of the Human Homolog of the Sea Urchin fascin and Drosophila singed Genes Which Encodes an Actin-Bundling Protein. DNA Cell Biol. 1994, 13, 821–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashiro-Matsumura, S.; Matsumura, F. Purification and characterization of an F-actin-bundling 55-kilodalton protein from HeLa cells. J. Biol. Chem. 1985, 260, 5087–5097. [Google Scholar]
- Murzin, A.G.; Lesk, A.M.; Chothia, C. Beta-Trefoil fold. Patterns of structure and sequence in the Kunitz inhibitors interleukins-1 beta and 1 alpha and fibroblast growth factors. J. Mol. Biol. 1992, 223, 531–543. [Google Scholar] [CrossRef]
- Ponting, C.P.; Russell, R.B. Identification of distant homologues of fibroblast growth factors suggests a common ancestor for all beta-trefoil proteins. J. Mol. Biol. 2000, 302, 1041–1047. [Google Scholar] [CrossRef]
- Yang, S.; Huang, F.-K.; Huang, J.; Chen, S.; Jakoncic, J.; Leo-Macias, A.; Diaz-Avalos, R.; Chen, L.; Zhang, J.J.; Huang, X.-Y. Molecular Mechanism of Fascin Function in Filopodial Formation. J. Biol. Chem. 2013, 288, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Aramaki, S.; Mayanagi, K.; Jin, M.; Aoyama, K.; Yasunaga, T. Filopodia formation by crosslinking of F-actin with fascin in two different binding manners. Cytoskeleton (Hoboken) 2016, 73, 365–374. [Google Scholar] [CrossRef]
- Jansen, S.; Collins, A.; Yang, C.; Rebowski, G.; Svitkina, T.; Dominguez, R. Mechanism of Actin Filament Bundling by Fascin. J. Biol. Chem. 2011, 286, 30087–30096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin-Jones, J.; Burnside, B. Retina-Specific Protein Fascin 2 Is an Actin Cross-linker Associated with Actin Bundles in Photoreceptor Inner Segments and Calycal Processes. Investig. Opthalmol. Vis. Sci. 2007, 48, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Perrin, B.J.; Strandjord, D.M.; Narayanan, P.; Henderson, D.M.; Johnson, K.R.; Ervasti, J.M. Beta-Actin and fascin-2 cooperate to maintain stereocilia length. J. Neurosci. 2013, 33, 8114–8121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubb, B.E.; Bardien-Kruger, S.; Kashork, C.D.; Shaffer, L.G.; Ramagli, L.S.; Xu, J.; Siciliano, M.J.; Bryan, J. Characterization of Human Retinal Fascin Gene (FSCN2) at 17q25: Close Physical Linkage of Fascin and Cytoplasmic Actin Genes. Genomics 2000, 65, 146–156. [Google Scholar] [CrossRef]
- Gamundi, M.J.; Hernan, I.; Maseras, M.; Baiget, M.; Ayuso, C.; Borrego, S.; Antiñolo, G.; Millán, J.M.; Valverde, D.; Carballo, M. Sequence variations in the retinal fascin FSCN2 gene in a Spanish population with autosomal dominant retinitis pigmentosa or macular degeneration. Mol. Vis. 2005, 11, 922–928. [Google Scholar]
- Yokokura, S.; Wada, Y.; Nakai, S.; Sato, H.; Yao, R.; Yamanaka, H.; Ito, S.; Sagara, Y.; Takahashi, M.; Nakamura, Y.; et al. Targeted Disruption of FSCN2 Gene Induces Retinopathy in Mice. Investig. Opthalmol. Vis. Sci. 2005, 46, 2905–2915. [Google Scholar] [CrossRef] [Green Version]
- Tubb, B.; Mulholland, D.J.; Vogl, W.; Lan, Z.-J.; Niederberger, C.; Cooney, A.; Bryan, J. Testis Fascin (FSCN3): A Novel Paralog of the Actin-Bundling Protein Fascin Expressed Specifically in the Elongate Spermatid Head. Exp. Cell Res. 2002, 275, 92–109. [Google Scholar] [CrossRef]
- Yamakita, Y.; Matsumura, F.; Yamashiro, S. Fascin1 is dispensable for mouse development but is favorable for neonatal survival. Cell Motil. Cytoskelet. 2009, 66, 524–534. [Google Scholar] [CrossRef] [Green Version]
- Yamakita, Y.; Matsumura, F.; Lipscomb, M.W.; Chou, P.-C.; Werlen, G.; Burkhardt, J.K.; Yamashiro, S. Fascin1 Promotes Cell Migration of Mature Dendritic Cells. J. Immunol. 2011, 186, 2850–2859. [Google Scholar] [CrossRef] [Green Version]
- Boer, E.; Howell, E.D.; Schilling, T.F.; Jette, C.A.; Stewart, R.A. Fascin1-Dependent Filopodia are Required for Directional Migration of a Subset of Neural Crest Cells. PLoS Genet. 2015, 11, e1004946. [Google Scholar] [CrossRef]
- Ma, Y.; Li, A.; Faller, W.J.; Libertini, S.; Fiorito, F.; Gillespie, D.A.; Sansom, O.J.; Yamashiro, S.; Machesky, L.M. Fascin 1 is transiently expressed in mouse melanoblasts during development and promotes migration and proliferation. Development 2013, 140, 2203–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Reynolds, L.E.; Li, A.; Stevenson, R.P.; Hodivala-Dilke, K.M.; Yamashiro, S.; Machesky, L.M. Fascin 1 is dispensable for developmental and tumour angiogenesis. Biol. Open 2013, 2, 1187–1191. [Google Scholar] [CrossRef] [Green Version]
- Mosialos, G.; Birkenbach, M.; Ayehunie, S.; Matsumura, F.; Pinkus, G.S.; Kieff, E.; Langhoff, E. Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am. J. Pathol. 1996, 148, 593–600. [Google Scholar] [PubMed]
- Sonego, M.; Gajendra, S.; Parsons, M.; Ma, Y.; Hobbs, C.; Zentar, M.P.; Williams, G.; Machesky, L.M.; Doherty, P.; Lalli, G. Fascin regulates the migration of subventricular zone-derived neuroblasts in the postnatal brain. J. Neurosci. 2013, 33, 12171–12185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeRosier, D.J.; Edds, K.T. Evidence for fascin cross-links between the actin filaments in coelomocyte filopodia. Exp. Cell Res. 1980, 126, 490–494. [Google Scholar] [CrossRef]
- Zanet, J.; Stramer, B.M.; Millard, T.; Martin, P.; Payre, F.; Plaza, S. Fascin is required for blood cell migration during Drosophila embryogenesis. Development 2009, 136, 2557–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, M.C.; Anliker, K.K.; Tootle, T.L. Fascin regulates protrusions and delamination to mediate invasive, collective cell migration in vivo. Dev. Dyn. 2020, 249, 961–982. [Google Scholar] [CrossRef] [PubMed]
- Bear, J.E.; Gertler, F.B. Ena/VASP: Towards resolving a pointed controversy at the barbed end. J. Cell Sci. 2009, 122, 1947–1953. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Huang, C.; Gunda, V.; Sun, J.; Chellappan, S.P.; Li, Z.; Izumi, V.; Fang, B.; Koomen, J.; Singh, P.K.; et al. Fascin Controls Metastatic Colonization and Mitochondrial Oxidative Phosphorylation by Remodeling Mitochondrial Actin Filaments. Cell Rep. 2019, 28, 2824–2836.e8. [Google Scholar] [CrossRef]
- Lombardi, M.L.; Jaalouk, D.E.; Shanahan, C.M.; Burke, B.; Roux, K.J.; Lammerding, J. The Interaction between Nesprins and Sun Proteins at the Nuclear Envelope Is Critical for Force Transmission between the Nucleus and Cytoskeleton. J. Biol. Chem. 2011, 286, 26743–26753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardi, M.L.; Lammerding, J. Keeping the LINC: The importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function. Biochem. Soc. Trans. 2011, 39, 1729–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, H.; Bhate, A.V.; Gangadaran, P.; Sawant, S.; Salot, S.; Sehgal, L.; Dange, P.P.; Chaukar, D.A.; D’Cruz, A.; Kannan, S.; et al. Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma. BMC Cancer 2012, 12, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, T.; Swift, J.; Irianto, J.; Shin, J.-W.; Spinler, K.R.; Athirasala, A.; Diegmiller, R.; Dingal, P.D.P.; Ivanovska, I.L.; Discher, D.E. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 2014, 204, 669–682. [Google Scholar] [CrossRef] [Green Version]
- Kelpsch, D.J.; Groen, C.M.; Fagan, T.N.; Sudhir, S.; Tootle, T.L. Fascin regulates nuclear actin during Drosophila oogenesis. Mol. Biol. Cell 2016, 27, 2965–2979. [Google Scholar] [CrossRef]
- Kelpsch, D.J.; Tootle, T.L. Nuclear Actin: From Discovery to Function. Anat. Rec. (Hoboken) 2018, 301, 1999–2013. [Google Scholar] [CrossRef] [Green Version]
- Vartiainen, M.K.; Guettler, S.; Larijani, B.; Treisman, R. Nuclear Actin Regulates Dynamic Subcellular Localization and Activity of the SRF Cofactor MAL. Science 2007, 316, 1749–1752. [Google Scholar] [CrossRef] [Green Version]
- Gau, D.; Roy, P. SRF’ing and SAP’ing—The role of MRTF proteins in cell migration. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Feric, M.; Vaidya, N.; Harmon, T.S.; Mitrea, D.M.; Zhu, L.; Richardson, T.M.; Kriwacki, R.W.; Pappu, R.V.; Brangwynne, C.P. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 2016, 165, 1686–1697. [Google Scholar] [CrossRef] [Green Version]
- Percipalle, P. Co-transcriptional nuclear actin dynamics. Nucleus 2013, 4, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Cui, J.; Zhang, Y.; Niu, M.; Xue, X.; Yin, H.; Tang, Y.; Dai, L.; Dai, F.; Guo, Y.; et al. Mass spectrometry-based proteomic analysis of FSCN1-interacting proteins in laryngeal squamous cell carcinoma cells. IUBMB Life 2019, 71, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Bijian, K.; Qiu, D.; Da Silva, S.D.; Marques, M.; Chang, C.-H.; Nassour, H.; Ramotar, D.; Damaraju, S.; Mackey, J.; et al. Insights into a novel nuclear function for Fascin in the regulation of the amino-acid transporter SLC3A2. Sci. Rep. 2016, 6, 36699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, S.; Yamakita, Y.; Yamashiro, S.; Matsudaira, P.T.; Gnarra, J.R.; Obinata, T.; Matsumura, F. Identification of an Actin Binding Region and a Protein Kinase C Phosphorylation Site on Human Fascin. J. Biol. Chem. 1997, 272, 2527–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakita, Y.; Ono, S.; Matsumura, F.; Yamashiro, S. Phosphorylation of Human Fascin Inhibits Its Actin Binding and Bundling Activities. J. Biol. Chem. 1996, 271, 12632–12638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anilkumar, N.; Parsons, M.; Monk, R.; Ng, T.; Adams, J.C. Interaction of fascin and protein kinase Calpha: A novel intersection in cell adhesion and motility. EMBO J. 2003, 22, 5390–5402. [Google Scholar] [CrossRef] [Green Version]
- Zanet, J.; Jayo, A.; Plaza, S.; Millard, T.; Parsons, M.; Stramer, B. Fascin promotes filopodia formation independent of its role in actin bundling. J. Cell Biol. 2012, 197, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Lu, S.; Mulaj, M.; Fang, B.; Keeley, T.; Wan, L.; Hao, J.; Muschol, M.; Sun, J.; Yang, S. Monoubiquitination Inhibits the Actin Bundling Activity of Fascin. J. Biol. Chem. 2016, 291, 27323–27333. [Google Scholar] [CrossRef] [Green Version]
- Bos, C.L.; Richel, D.J.; Ritsema, T.; Peppelenbosch, M.P.; Versteeg, H.H. Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 2004, 36, 1187–1205. [Google Scholar] [CrossRef]
- Tootle, T.L. Genetic insights into the in vivo functions of prostaglandin signaling. Int. J. Biochem. Cell Biol. 2013, 45, 1629–1632. [Google Scholar] [CrossRef]
- Peppelenbosch, M.P.; Tertoolen, L.G.; Hage, W.J.; De Laat, S.W. Epidermal growth factor—Induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell 1993, 74, 565–575. [Google Scholar] [CrossRef]
- Groen, C.M.; Spracklen, A.J.; Fagan, T.N.; Tootle, T.L. DrosophilaFascin is a novel downstream target of prostaglandin signaling during actin remodeling. Mol. Biol. Cell 2012, 23, 4567–4578. [Google Scholar] [CrossRef] [PubMed]
- Tootle, T.L.; Spradling, A.C. Drosophila Pxt: A cyclooxygenase-like facilitator of follicle maturation. Development 2008, 135, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, K.L.; Fujino, H.; Srinivasan, D.; Regan, J.W. Activation of FP Prostanoid Receptor Isoforms Leads to Rho-mediated Changes in Cell Morphology and in the Cell Cytoskeleton. J. Biol. Chem. 1999, 274, 35944–35949. [Google Scholar] [CrossRef] [Green Version]
- Bulin, C.; Albrecht, U.; Bode, J.; Weber, A.-A.; Schrör, K.; Levkau, B.; Fischer, J.W. Differential Effects of Vasodilatory Prostaglandins on Focal Adhesions, Cytoskeletal Architecture, and Migration in Human Aortic Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2005, 25, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Dormond, O.; Bezzi, M.; Mariotti, A.; Rüegg, C. Prostaglandin E2 promotes integrin alpha Vbeta 3-dependent endothelial cell adhesion, rac-activation, and spreading through cAMP/PKA-dependent signaling. J. Biol. Chem. 2002, 277, 45838–45846. [Google Scholar] [CrossRef] [Green Version]
- Birukova, A.A.; Zagranichnaya, T.; Fu, P.; Alekseeva, E.; Chen, W.; Jacobson, J.R.; Birukov, K.G. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA—And Epac1/Rap1-dependent Rac activation. Exp. Cell Res. 2007, 313, 2504–2520. [Google Scholar] [CrossRef] [Green Version]
- Spracklen, A.J.; Kelpsch, D.J.; Chen, X.; Spracklen, C.N.; Tootle, T.L. Prostaglandins temporally regulate cytoplasmic actin bundle formation during Drosophila oogenesis. Mol. Biol. Cell 2014, 25, 397–411. [Google Scholar] [CrossRef]
- Spracklen, A.J.; Lamb, M.C.; Groen, C.M.; Tootle, T.L. Pharmaco-Genetic Screen to Uncover Actin Regulators Targeted by Prostaglandins During Drosophila Oogenesis. G3 (Bethesda) 2019, 9, 3555–3565. [Google Scholar] [CrossRef] [Green Version]
- Tootle, T.L.; Williams, D.; Hubb, A.; Frederick, R.; Spradling, A. Drosophila Eggshell Production: Identification of New Genes and Coordination by Pxt. PLoS ONE 2011, 6, e19943. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Clipstone, N.A. Prostaglandin F2alpha inhibits adipocyte differentiation via a G alpha q-calcium-calcineurin-dependent signaling pathway. J. Cell Biochem. 2007, 100, 161–173. [Google Scholar] [CrossRef]
- Parsons, M.; Adams, J.C. Rac regulates the interaction of fascin with protein kinase C in cell migration. J. Cell Sci. 2008, 121, 2805–2813. [Google Scholar] [CrossRef] [Green Version]
- Jayo, A.; Parsons, M.; Adams, J.C. A novel Rho-dependent pathway that drives interaction of fascin-1 with p-Lin-11/Isl-1/Mec-3 kinase (LIMK) 1/2 to promote fascin-1/actin binding and filopodia stability. BMC Biol. 2012, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, R.; Breitsprecher, D.; Collins, A.; Corrêa, I.R.; Xu, M.-Q.; Goode, B.L. The Formin Daam1 and Fascin Directly Collaborate to Promote Filopodia Formation. Curr. Biol. 2013, 23, 1373–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shonukan, T.; Bagayogo, I.; McCrea, P.; Chao, M.V.; Hempstead, B.; Shonukan, O.; McCrea, P. Neurotrophin-induced melanoma cell migration is mediated through the actin-bundling protein fascin. Oncogene 2003, 22, 3616–3623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Fonovic, M.; Suyama, K.; Bogyo, M.; Scott, M.P. Rab35 Controls Actin Bundling by Recruiting Fascin as an Effector Protein. Science 2009, 325, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Ross, X.-L.; Pautz, A.; Reske-Kunz, A.B.; Ross, R. The Human Fascin Gene Promoter Is Highly Active in Mature Dendritic Cells Due to a Stage-Specific Enhancer. J. Immunol. 2003, 171, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Jonuleit, H.; Bros, M.; Ross, X.-L.; Enk, A.H.; Knop, J.; Reske-Kunz, A.B.; Yamashiro, S.; Matsumura, F. Expression of the Actin-Bundling Protein Fascin in Cultured Human Dendritic Cells Correlates with Dendritic Morphology and Cell Differentiation. J. Investig. Dermatol. 2000, 115, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Geyeregger, R.; Zeyda, M.; Bauer, W.M.; Kriehuber, E.; Säemann, M.D.; Zlabinger, G.J.; Maurer, D.; Stulnig, T. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood 2007, 109, 4288–4295. [Google Scholar] [CrossRef]
- Borghese, L.; Fletcher, G.; Mathieu, J.; Atzberger, A.; Eades, W.C.; Cagan, R.L.; Rørth, P. Systematic Analysis of the Transcriptional Switch Inducing Migration of Border Cells. Dev. Cell 2006, 10, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Silver, D.L.; Geisbrecht, E.R.; Montell, D.J. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development 2005, 132, 3483–3492. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, Y.; Loftis, D.W.; Adams, J.C. Fascin-1 Promoter Activity Is Regulated by CREB and the Aryl Hydrocarbon Receptor in Human Carcinoma Cells. PLoS ONE 2009, 4, e5130. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Hashizume, R.; Ji, H.; Tubb, B.E.; Patrick, C.W.; Yu, D.; Money, E.E.; McCrea, P.D. C-erbB-2/ HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene 2000, 19, 4864–4875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Jin, L.; Alesi, G.N.; Kim, Y.-M.; Fan, J.; Seo, J.H.; Wang, N.; Tucker, M.; Gu, T.-L.; Lee, B.H.; et al. The Prometastatic Ribosomal S6 Kinase 2-cAMP Response Element-binding Protein (RSK2-CREB) Signaling Pathway Up-regulates the Actin-binding Protein Fascin-1 to Promote Tumor Metastasis. J. Biol. Chem. 2013, 288, 32528–32538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Choi, I.; Cheong, T.; Lee, S.; Lotan, R.; Park, S.H.; Chun, K.-H. Galectin-3 Increases Gastric Cancer Cell Motility by Up-regulating Fascin-1 Expression. Gastroenterology 2010, 138, 1035–1045.e2. [Google Scholar] [CrossRef] [PubMed]
- Vignjevic, D.M.; Schoumacher, M.; Gavert, N.; Janssen, K.-P.; Jih, G.; Laé, M.; Louvard, D.; Ben-Ze’Ev, A.; Robine, S. Fascin, a Novel Target of beta--Catenin-TCF Signaling, Is Expressed at the Invasive Front of Human Colon Cancer. Cancer Res. 2007, 67, 6844–6853. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; He, H.; Pillai, S.; Xiong, Y.; Challa, S.; Xu, L.; Chellappan, S.; Yang, S. GATA3 Transcription Factor Abrogates Smad4 Transcription Factor-mediated Fascin Overexpression, Invadopodium Formation, and Breast Cancer Cell Invasion. J. Biol. Chem. 2013, 288, 36971–36982. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; He, H.; Xiong, Y.; Lu, S.; Shen, J.; Cheng, A.; Chang, W.-C.; Hou, M.-F.; Lancaster, J.M.; Kim, M.; et al. Fascin protein is critical for transforming growth factor beta protein-induced invasion and filopodia formation in spindle-shaped tumor cells. J. Biol. Chem. 2011, 286, 38865–38875. [Google Scholar] [CrossRef] [Green Version]
- Snyder, M.; Huang, X.-Y.; Zhang, J.J. Signal Transducers and Activators of Transcription 3 (STAT3) Directly Regulates Cytokine-induced Fascin Expression and Is Required for Breast Cancer Cell Migration. J. Biol. Chem. 2011, 286, 38886–38893. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Qian, C.J.; Ye, B.; Zhao, Z.Q.; Wei, J.; Liang, Y.; Zhang, X. Signal. transducer and activator of transcription 3 signaling upregulates fascin via nuclear factor-kappaB in gastric cancer: Implications in cell invasion and migration. Oncol. Lett. 2014, 7, 902–908. [Google Scholar] [CrossRef] [Green Version]
- Chivomaru, T.; Enokida, H.; Tatarano, S.; Kawahara, K.; Nishiyama, K.; Seki, N.; Nakagawa, M. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br. J. Cancer 2010, 102, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhu, J.; Ou, C.; Deng, Z.; Chen, M.; Huang, W.; Liu, L.-Z. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. Br. J. Cancer 2014, 110, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Fuse, M.; Seki, N.; Nohata, N.; Kojima, S.; Sakamoto, S.; Chiyomaru, T.; Kawakami, K.; Enokida, H.; Nakagawa, M.; Naya, Y.; et al. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int. J. Oncol. 2011, 38, 1093–1101. [Google Scholar] [PubMed]
- Gao, R.; Zhang, N.; Yang, J.; Zhu, Y.; Zhang, Z.; Wang, J.; Xu, X.; Li, Z.; Liu, X.; Li, Z.; et al. Long non-coding RNA ZEB1-AS1 regulates miR-200b/FSCN1 signaling and enhances migration and invasion induced by TGF-beta1 in bladder cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Zhang, C.; Li, W.; Li, H.; Sang, J.; Zhao, Q.; Bo, Y.; Luo, H.; Zheng, X.; Lu, Y.; et al. Promoter Methylation-Regulated miR-145-5p Inhibits Laryngeal Squamous Cell Carcinoma Progression by Targeting FSCN1. Mol. Ther. 2019, 27, 365–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götte, M.; Mohr, C.; Koo, C.Y.; Stock, C.; Vaske, A.-K.; Viola, M.; Ibrahim, S.A.; Peddibhotla, S.; Teng, Y.H.-F.; Low, J.-Y.; et al. miR-145-dependent targeting of Junctional Adhesion Molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene 2010, 29, 6569–6580. [Google Scholar] [CrossRef] [Green Version]
- Kano, M.; Seki, N.; Kikkawa, N.; Fujimura, L.; Hoshino, I.; Akutsu, Y.; Chiyomaru, T.; Enokida, H.; Nakagawa, M.; Matsubara, H. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int. J. Cancer 2010, 127, 2804–2814. [Google Scholar] [CrossRef]
- Liu, R.; Liao, J.; Yang, M.; Sheng, J.; Yang, H.; Wang, Y.; Pan, E.; Guo, W.; Pu, Y.; Kim, S.J.; et al. The Cluster of miR-143 and miR-145 Affects the Risk for Esophageal Squamous Cell Carcinoma through Co-Regulating Fascin Homolog 1. PLoS ONE 2012, 7, e33987. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, C.-Q.; Xiang, R.; Liu, X.; Ye, S.; Yang, X.-Q.; Zhang, G.-H.; Xu, X.; Zhu, T.; Wu, Q. Loss of miR-133a expression associated with poor survival of breast cancer and restoration of miR-133a expression inhibited breast cancer cell growth and invasion. BMC Cancer 2012, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Kang, X.; Xia, X.; Wo, L.; Gu, X.; Hu, Y.; Xie, X.; Chang, H.; Lou, L.; Shen, X. miR-145 suppresses breast cancer cell migration by targeting FSCN-1 and inhibiting epithelial-mesenchymal transition. Am. J. Transl. Res. 2016, 8, 3106–3114. [Google Scholar]
- Arlt, M.J.E.; Kuzmanov, A.; Snedeker, J.G.; Fuchs, B.; Silvan, U.; Sabile, A.A. Fascin-1 enhances experimental osteosarcoma tumor formation and metastasis and is related to poor patient outcome. BMC Cancer 2019, 19, 83. [Google Scholar] [CrossRef]
- Lee, H.J.; An, H.J.; Kim, T.H.; Kim, G.; Kang, H.; Heo, J.H.; Kwon, A.-Y.; Kim, S. Fascin expression is inversely correlated with breast cancer metastasis suppressor 1 and predicts a worse survival outcome in node-negative breast cancer patients. J. Cancer 2017, 8, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Q.; Li, Y.; Huang, B.-F.; Zhao, Y.-M.; Yuan, H.; Guo, D.; Su, C.-M.; Hu, G.-N.; Wang, Q.; Long, T.; et al. EGFR conjunct FSCN1 as a Novel Therapeutic Strategy in Triple-Negative Breast Cancer. Sci. Rep. 2017, 7, 15654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarrió, D.; Rodriguez-Pinilla, S.M.; Hardisson, D.; Cano, A.; Moreno-Bueno, G.; Palacios, J. Epithelial-Mesenchymal Transition in Breast Cancer Relates to the Basal-like Phenotype. Cancer Res. 2008, 68, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esnakula, A.K.; Ricks-Santi, L.; Kwagyan, J.; Kanaan, Y.M.; DeWitty, R.L.; Wilson, L.L.; Gold, B.; Frederick, W.A.I.; Naab, T.J. Strong association of fascin expression with triple negative breast cancer and basal-like phenotype in African-American women. J. Clin. Pathol. 2014, 67, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Yang, Z.; Huang, X.; Zhang, Z.; Li, J.; Ju, J.; Zhang, H.; Ma, J. Ilamycin C induces apoptosis and inhibits migration and invasion in triple-negative breast cancer by suppressing IL-6/STAT3 pathway. J. Hematol. Oncol. 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.-K.; Han, S.; Xing, B.; Huang, J.; Liu, B.; Bordeleau, F.; Reinhart-King, C.A.; Zhang, J.J.; Huang, X.-Y. Targeted inhibition of fascin function blocks tumour invasion and metastatic colonization. Nat. Commun. 2015, 6, 7465. [Google Scholar] [CrossRef] [Green Version]
- Alburquerque-González, B.; Bernabé-García, M.; Montoro-García, S.; Rodrigues, P.C.; Sanz, J.R.; López-Calderón, F.F.; Luque, I.; Nicolas, F.J.; Cayuela, M.L.; Salo, T.; et al. New role of the antidepressant imipramine as a Fascin1 inhibitor in colorectal cancer cells. Exp. Mol. Med. 2020, 52, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massagué, J. Genes that mediate breast cancer metastasis to lung. Nature 2005, 436, 518–524. [Google Scholar] [CrossRef]
- Li, A.; Morton, J.P.; Ma, Y.; Karim, S.A.; Zhou, Y.; Faller, W.J.; Woodham, E.F.; Morris, H.; Stevenson, R.P.; Juin, A.; et al. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology 2014, 146, 1386–1396.e1. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jia, L.; Ren, D.; Liu, C.; Gong, Y.; Wang, N.; Zhang, X.; Zhao, Y. Axl mediates tumor invasion and chemosensitivity through PI3K/Akt signaling pathway and is transcriptionally regulated by slug in breast carcinoma. IUBMB Life 2014, 66, 507–518. [Google Scholar] [CrossRef]
- Lin, S.; Taylor, M.D.; Singh, P.K.; Yang, S. How does fascin promote cancer metastasis? FEBS J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hegmans, J.P.; Bard, M.P.; Hemmes, A.; Luider, T.M.; Kleijmeer, M.J.; Prins, J.-B.; Zitvogel, L.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic Analysis of Exosomes Secreted by Human Mesothelioma Cells. Am. J. Pathol. 2004, 164, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-Y.; Oskarsson, T.; Acharyya, S.; Nguyen, D.X.; Zhang, X.H.-F.; Norton, L.; Massagué, J. Tumor Self-Seeding by Circulating Cancer Cells. Cell 2009, 139, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoumacher, M.; El-Marjou, F.; Laé, M.; Kambou, N.; Louvard, D.; Robine, S.; Vignjevic, D.M. Conditional expression of fascin increases tumor progression in a mouse model of intestinal cancer. Eur. J. Cell Biol. 2014, 93, 388–395. [Google Scholar] [CrossRef]
- Xing, P.; Li, J.-G.; Jin, F.; Zhao, T.-T.; Liu, Q.; Dong, H.-T.; Wei, X.-L. Fascin, an actin-bundling protein, promotes breast cancer progression in vitro. Cell Biochem. Funct. 2011, 29, 303–310. [Google Scholar] [CrossRef]
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and Their Role in Cellular Stress in Cancer. Front. Oncol. 2014, 4, 153. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.O.; Shin, S.; Mercurio, A.M. Hypoxia Stimulates Carcinoma Invasion by Stabilizing Microtubules and Promoting the Rab11 Trafficking of the 6 4 Integrin. Cancer Res. 2005, 65, 2761–2769. [Google Scholar] [CrossRef] [Green Version]
- Heinz, L.S.; Muhs, S.; Schiewek, J.; Grüb, S.; Nalaskowski, M.; Lin, Y.-N.; Wikman, H.; Oliveira-Ferrer, L.; Lange, T.; Wellbrock, J.; et al. Strong fascin expression promotes metastasis independent of its F-actin bundling activity. Oncotarget 2017, 8, 110077–110091. [Google Scholar] [CrossRef]
- Hein, N.; Hannan, K.M.; George, A.J.; Sanij, E.; Hannan, R.D. The nucleolus: An emerging target for cancer therapy. Trends Mol. Med. 2013, 19, 643–654. [Google Scholar] [CrossRef]
- Ruggero, D. Revisiting the Nucleolus: From Marker to Dynamic Integrator of Cancer Signaling. Sci. Signal. 2012, 5, pe38. [Google Scholar] [CrossRef] [Green Version]
- Quin, J.E.; Devlin, J.R.; Cameron, D.; Hannan, K.M.; Pearson, R.B.; Hannan, R.D. Targeting the nucleolus for cancer intervention. Biochim. Biophys. Acta 2014, 1842, 802–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisvert, F.-M.; Van Koningsbruggen, S.; Navascués, J.; Lamond, A.I. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 2007, 8, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.P.Z.P.; Spencer, V.A.; Mori, H.; Carvalho, H.F.; Bissell, M.J.; Bruni-Cardoso, A. Laminin-111 and the Level of Nuclear Actin Regulate Epithelial Quiescence via Exportin-6. Cell Rep. 2017, 19, 2102–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, V.A.; Costes, S.; Inman, J.L.; Xu, R.; Chen, J.; Hendzel, M.J.; Bissell, M.J. Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. J. Cell Sci. 2011, 124, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Q.; Hill, M.C.; Chen, F.; Mo, Q.; Ku, A.T.; Ramos, C.; Sock, E.; Lefebvre, V.; Nguyen, H. SOX11 and SOX4 drive the reactivation of an embryonic gene program during murine wound repair. Nat. Commun. 2019, 10, 4042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, M.-J.; Lee, C.; Kim, J.; Shin, H.-S.; Yu, M.-H. Proteomic analysis of stargazer mutant mouse neuronal proteins involved in absence seizure. J. Neurochem. 2008, 104, 1260–1270. [Google Scholar] [CrossRef]
- Castao, E.M.; Maarouf, C.L.; Wu, T.; Leal, M.C.; Whiteside, C.M.; Lue, L.-F.; Kokjohn, T.A.; Sabbagh, M.N.; Beach, T.G.; Roher, A.E.; et al. Alzheimer disease periventricular white matter lesions exhibit specific proteomic profile alterations. Neurochem. Int. 2013, 62, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Cohan, C.S.; Welnhofer, E.A.; Zhao, L.; Matsumura, F.; Yamashiro, S. Role of the actin bundling protein fascin in growth cone morphogenesis: Localization in filopodia and lamellipodia. Cell Motil. Cytoskelet. 2001, 48, 109–120. [Google Scholar] [CrossRef]
- Nagel, J.; Delandre, C.; Zhang, Y.; Forstner, F.; Moore, A.W.; Tavosanis, G. Fascin controls neuronal class-specific dendrite arbor morphology. Development 2012, 139, 2999–3009. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamb, M.C.; Tootle, T.L. Fascin in Cell Migration: More Than an Actin Bundling Protein. Biology 2020, 9, 403. https://doi.org/10.3390/biology9110403
Lamb MC, Tootle TL. Fascin in Cell Migration: More Than an Actin Bundling Protein. Biology. 2020; 9(11):403. https://doi.org/10.3390/biology9110403
Chicago/Turabian StyleLamb, Maureen C., and Tina L. Tootle. 2020. "Fascin in Cell Migration: More Than an Actin Bundling Protein" Biology 9, no. 11: 403. https://doi.org/10.3390/biology9110403
APA StyleLamb, M. C., & Tootle, T. L. (2020). Fascin in Cell Migration: More Than an Actin Bundling Protein. Biology, 9(11), 403. https://doi.org/10.3390/biology9110403