Development of an LDL Receptor-Targeted Peptide Susceptible to Facilitate the Brain Access of Diagnostic or Therapeutic Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phage Display Experiments
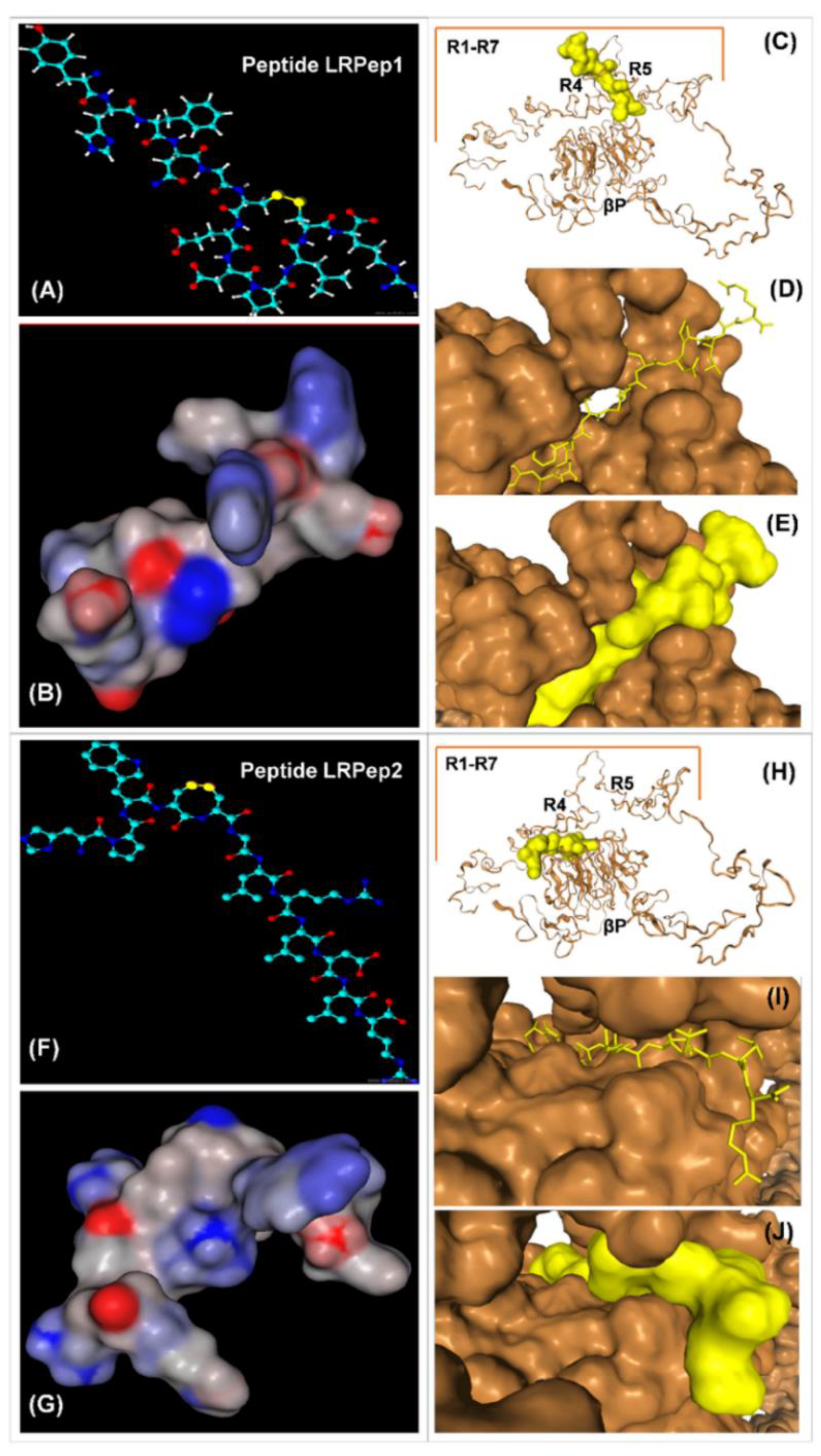
2.2. Docking of the Selected Peptides to ED-LDLR
2.3. Cell Culture
2.4. Evaluation of the Endocytosis Potential of Peptides LRPep1 and LRPep2
2.5. Evaluation of the LDLR Expression in Cells and Colocalization of LRPep2 with LDLR
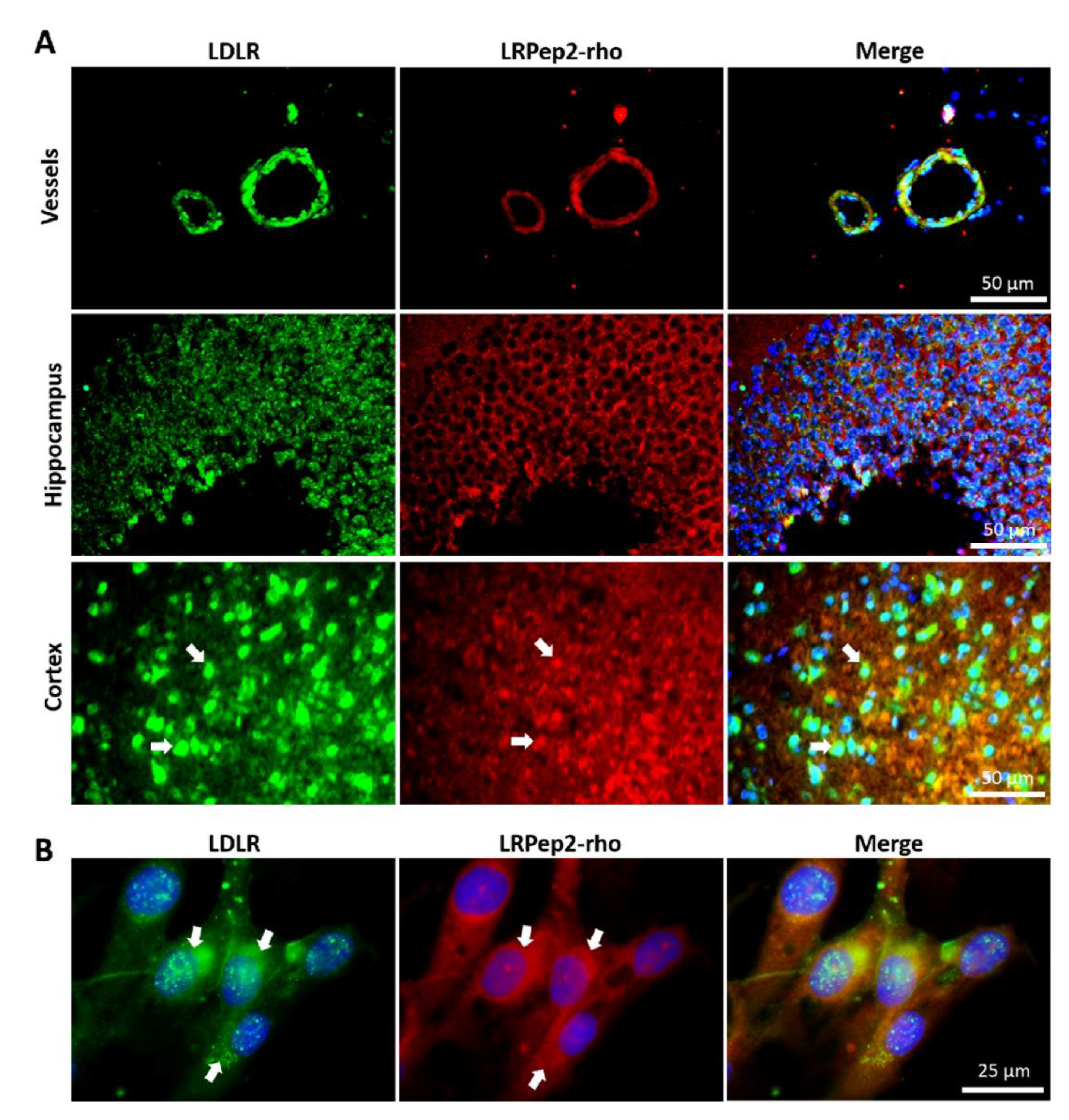
2.6. Evaluation of LDLR Expression on Mouse Brain Slices and Colocalization of LRPep2 with LDLR
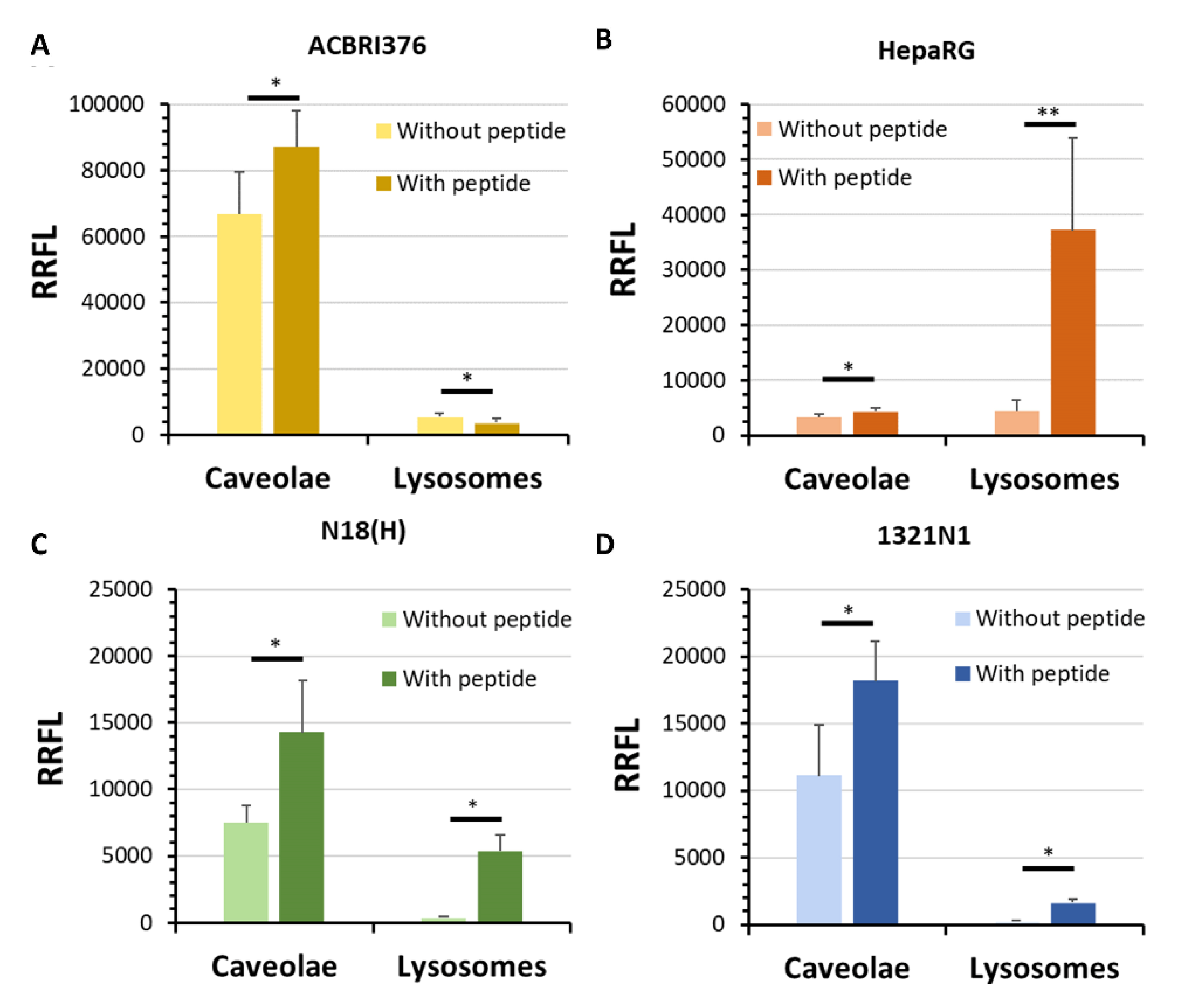
2.7. Colocalization of the Peptide LRPep2 with Caveolae and Lysosomes
2.8. Synthesis of USPIO Derivatives
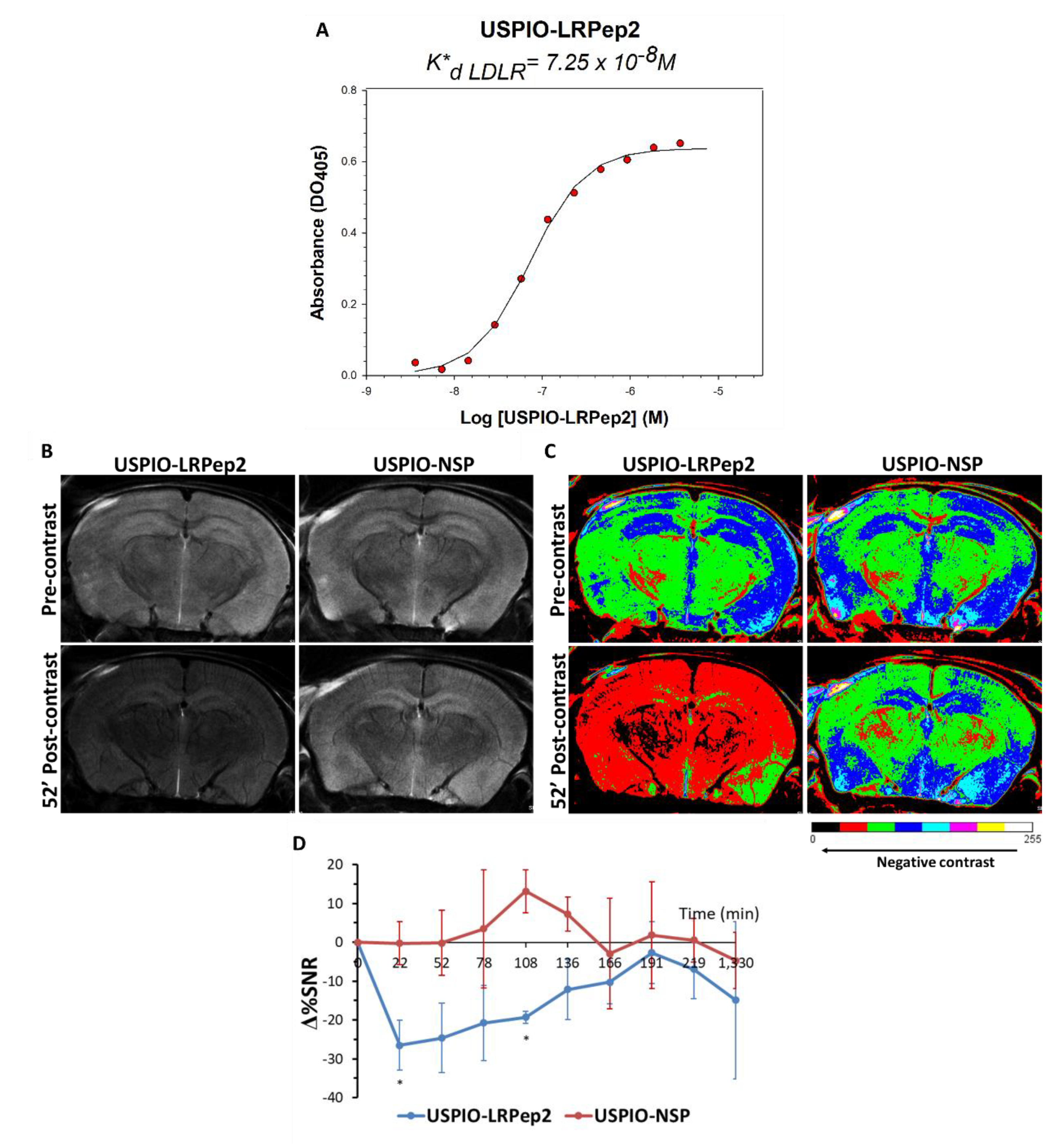
2.9. Evaluation of the Affinity of USPIO-LRPep2 by ELISA
2.10. MRI Experiments and Contrast Analysis Measurement
2.11. Organ Collection for Immunohistochemistry or Biodistribution Studies
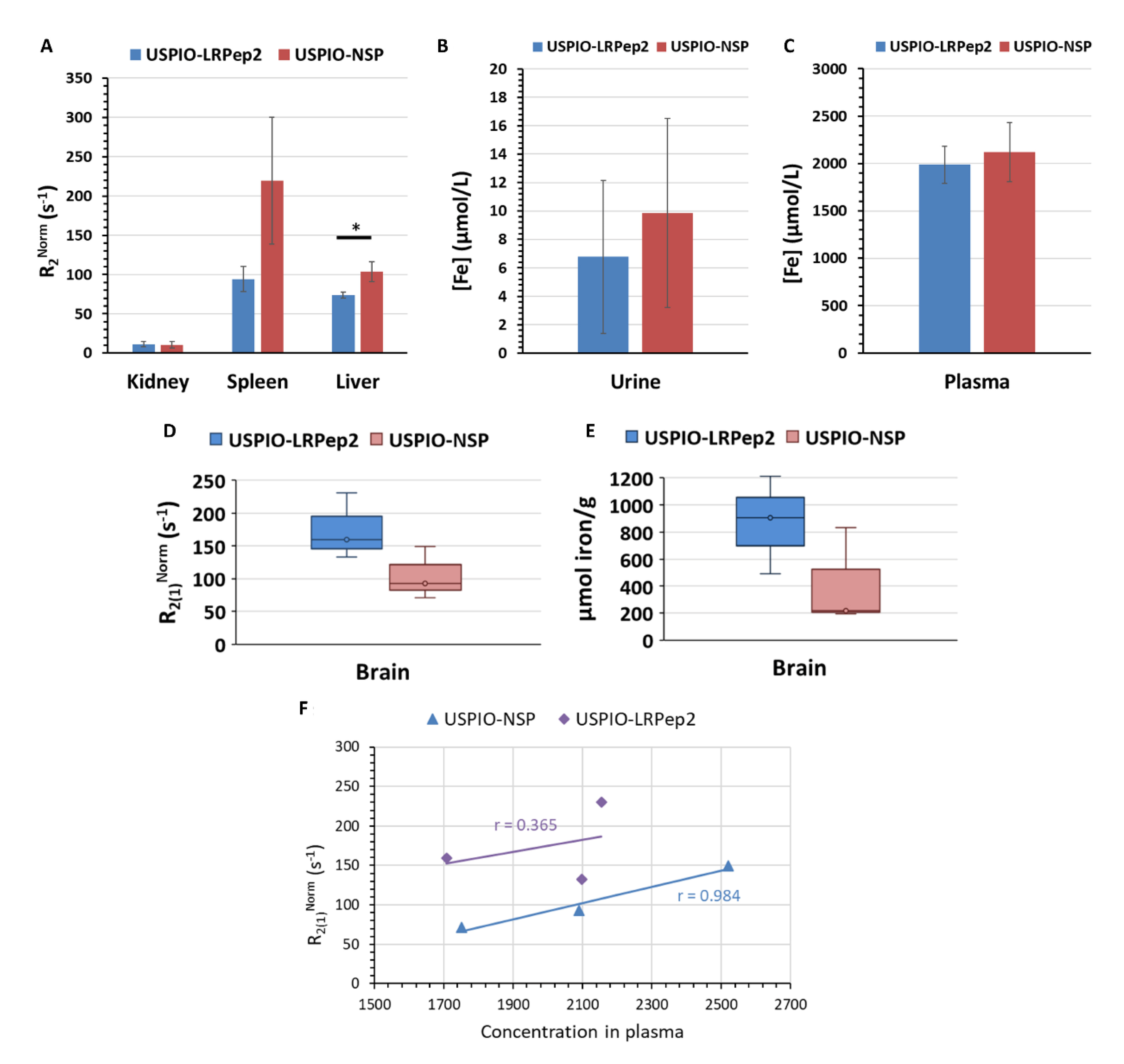
2.12. Evaluation of the Biodistribution of UPSIO Derivatives by NMR Relaxometry
2.13. Detection of USPIO Derivatives on Mouse Brains by Perls’-DAB Staining of Iron
2.14. FLI Experiments and Fluorescence Measurement
2.15. Statistical Analysis
3. Results and Discussion
3.1. Selection of the LDLR-Targeted Peptides
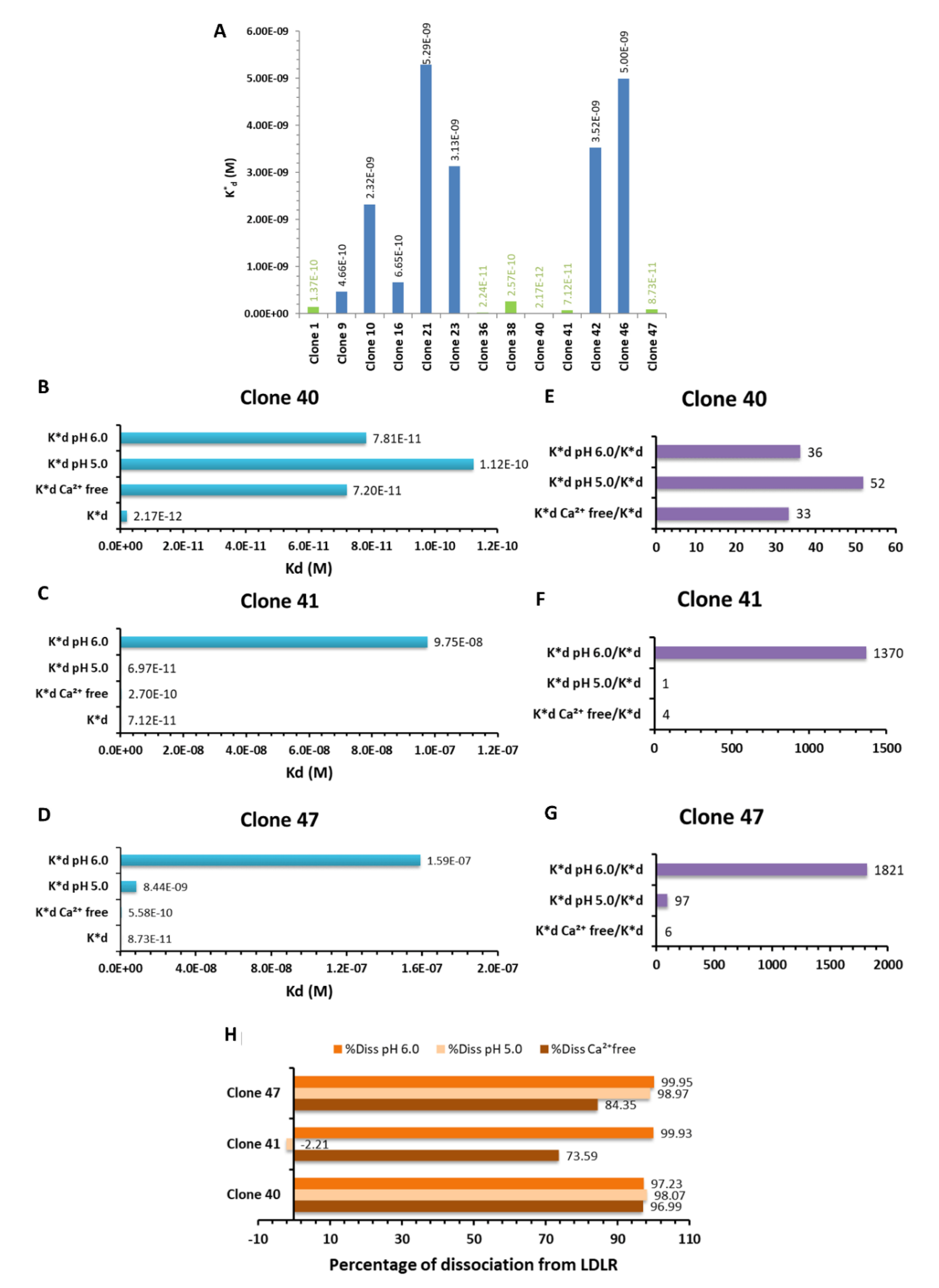
3.2. Analysis of Selected Peptides
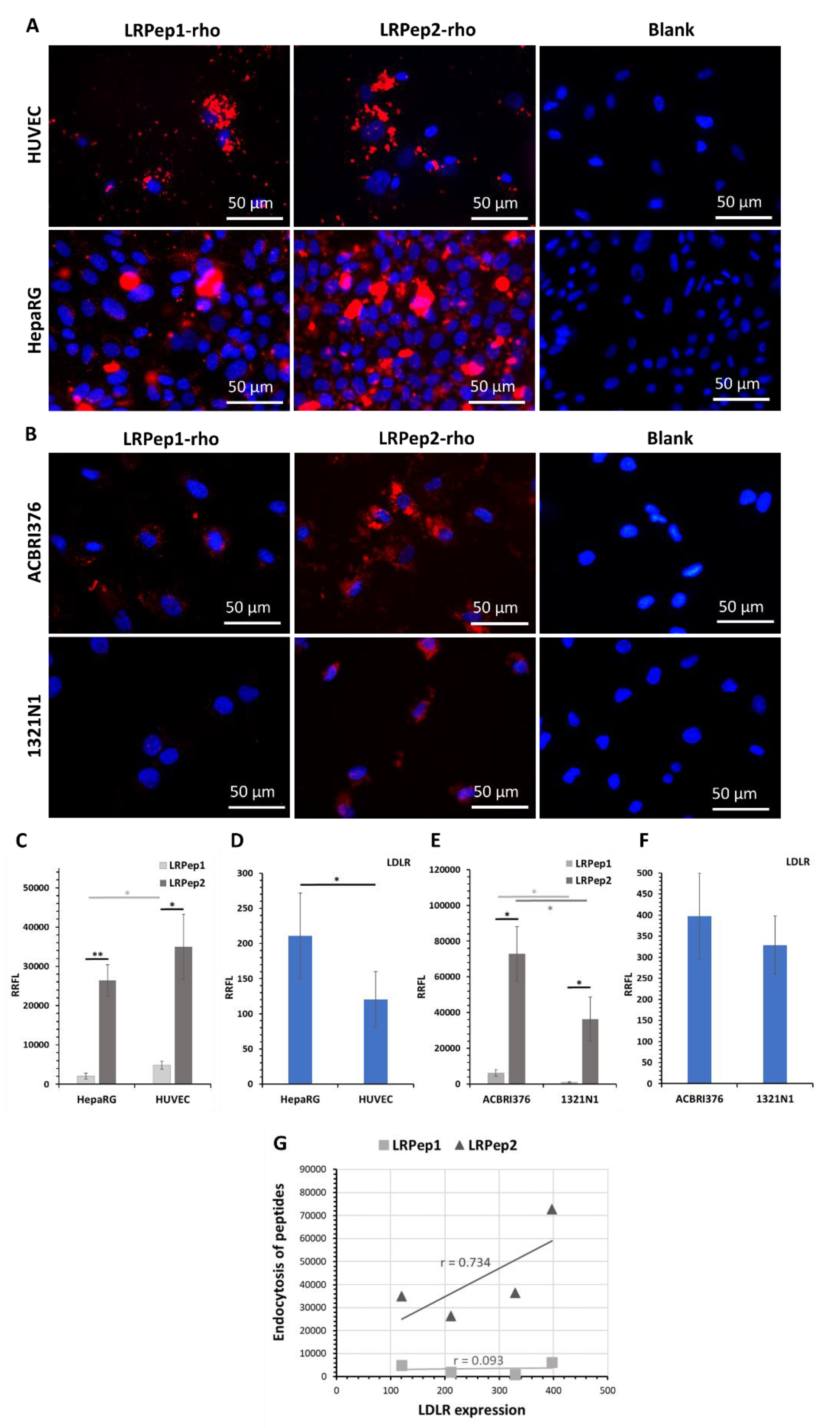
3.3. In Vitro Evaluation of the Endocytosis Potential of Peptides LRPep1 and LRPep2
3.4. Colocalization of LRPep2 with LDLR on Mouse Brain Slices and Endothelial Cells
3.5. In Vitro Study of the Endocytosis Mechanism of Peptide LRPep2
3.6. In Vivo MRI Evaluation of USPIO-LRPep2
3.7. In Vivo Biodistribution of USPIO Derivatives
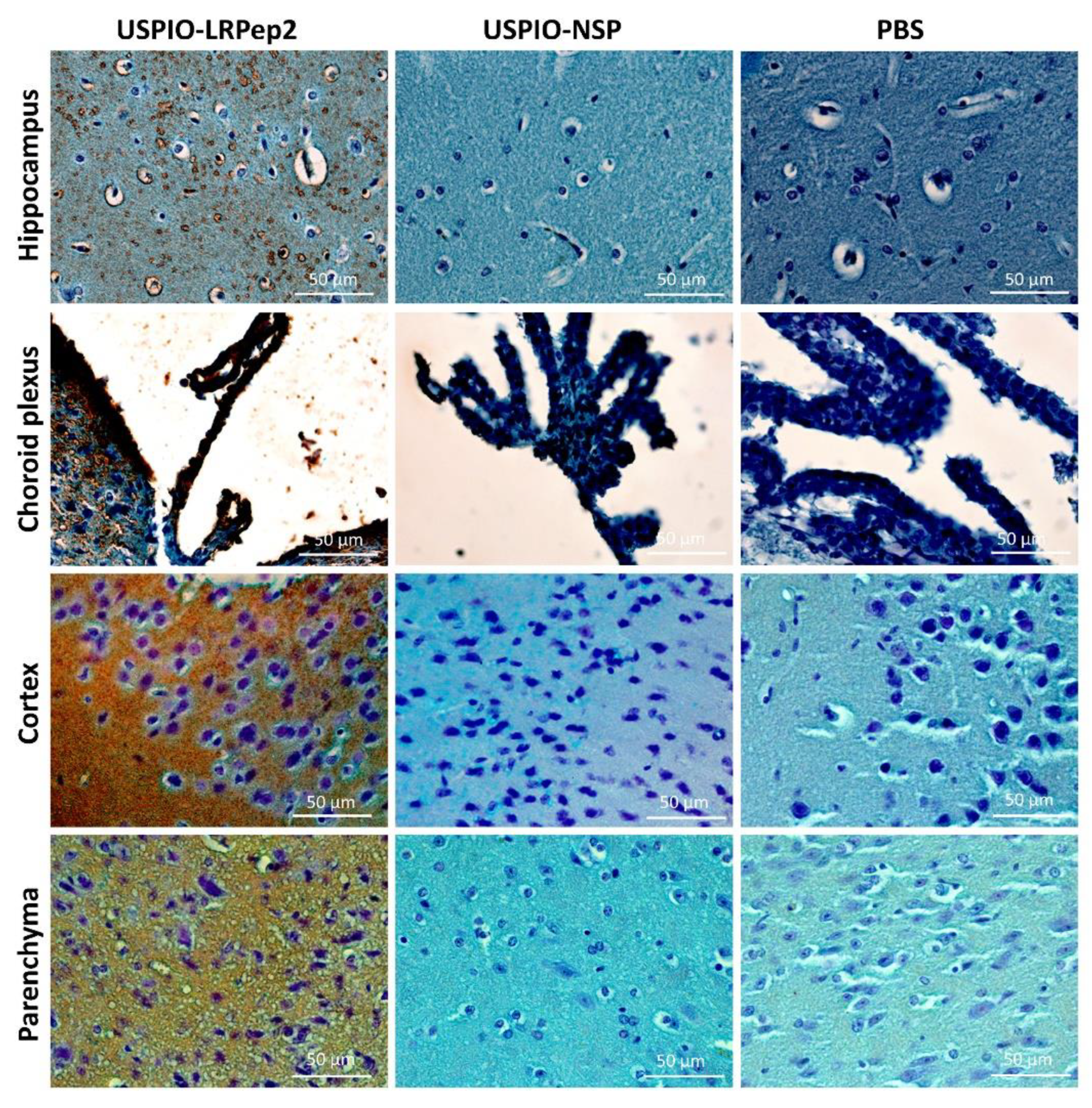
3.8. Detection of USPIO Derivatives in Mouse Brains by Perls’-DAB Staining
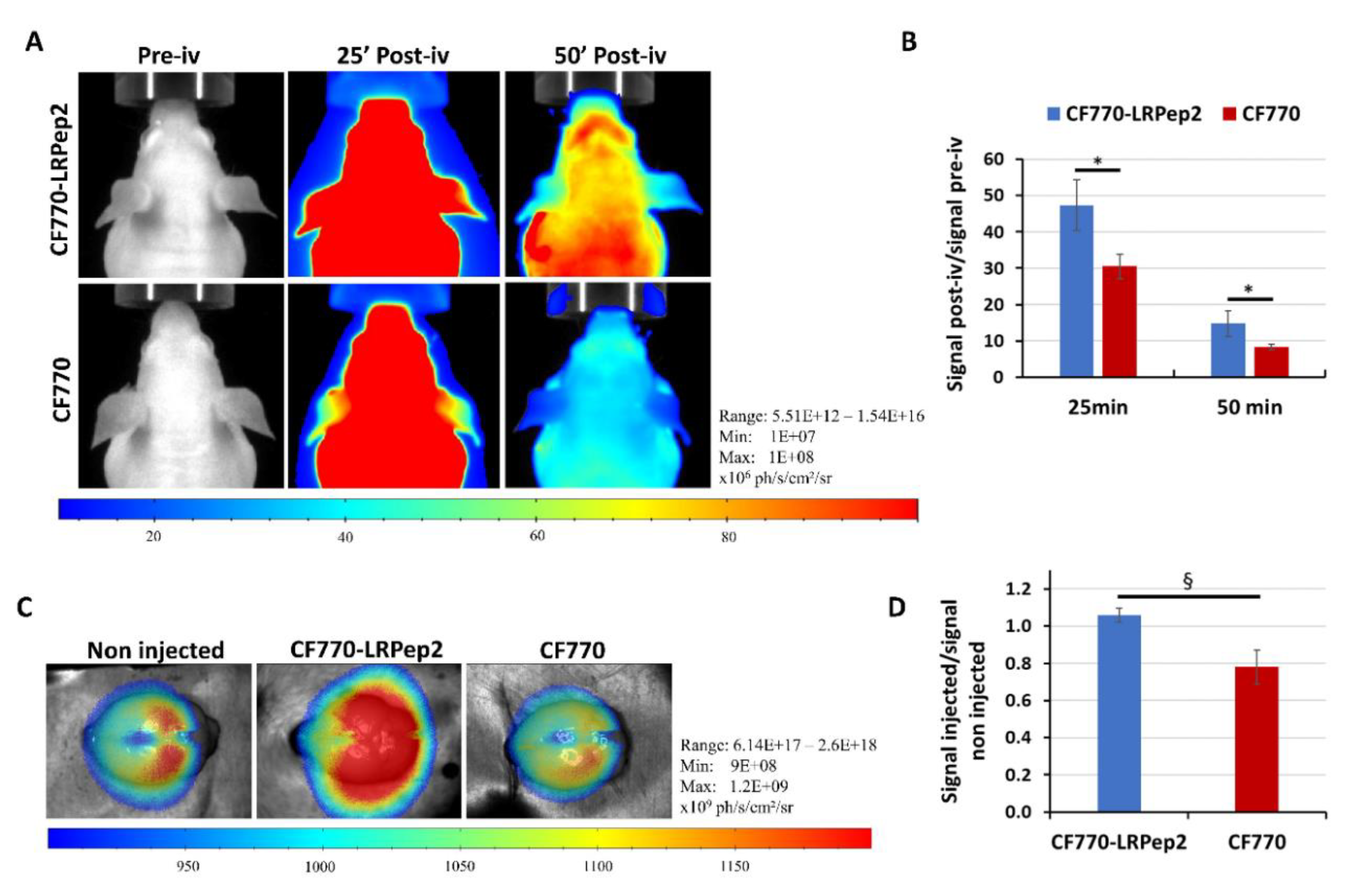
3.9. In Vivo Fluorescence Evaluation of CF770-LRPep2
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnár, Z.; O’Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery-recent developments and future prospects. J. Control. Release Off. J. Control. Release Soc. 2012, 161, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Brasnjevic, I.; Steinbusch, H.W.M.; Schmitz, C.; Martinez-Martinez, P. European NanoBioPharmaceutics Research Initiative Delivery of peptide and protein drugs over the blood-brain barrier. Prog. Neurobiol. 2009, 87, 212–251. [Google Scholar] [CrossRef]
- Jones, A.R.; Shusta, E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Zou, D.; Wang, W.; Yin, Y.; Yin, T.; Hao, S.; Wang, B.; Wang, G.; Wang, Y. Non-invasive approaches for drug delivery to the brain based on the receptor mediated transport. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1316–1327. [Google Scholar] [CrossRef]
- Go, G.-W.; Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J. Biol. Med. 2012, 85, 19–28. [Google Scholar]
- Dehouck, B.; Fenart, L.; Dehouck, M.P.; Pierce, A.; Torpier, G.; Cecchelli, R. A new function for the LDL receptor: Transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 1997, 138, 877–889. [Google Scholar] [CrossRef]
- Candela, P.; Gosselet, F.; Miller, F.; Buee-Scherrer, V.; Torpier, G.; Cecchelli, R.; Fenart, L. Physiological pathway for low-density lipoproteins across the blood-brain barrier: Transcytosis through brain capillary endothelial cells in vitro. Endothel. J. Endothel. Cell Res. 2008, 15, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Molino, Y.; David, M.; Varini, K.; Jabès, F.; Gaudin, N.; Fortoul, A.; Bakloul, K.; Masse, M.; Bernard, A.; Drobecq, L.; et al. Use of LDL receptor-targeting peptide vectors for in vitro and in vivo cargo transport across the blood-brain barrier. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1807–1827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.B.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Vanlandewijck, M.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 2018, 5, 180160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulgar, V.M. Transcytosis to Cross the Blood Brain Barrier, New Advancements and Challenges. Front. Neurosci. 2018, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Petrenko, V.A. Phage Display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef]
- Kristensen, M.; Brodin, B. Routes for Drug Translocation Across the Blood-Brain Barrier: Exploiting Peptides as Delivery Vectors. J. Pharm. Sci. 2017. [Google Scholar] [CrossRef] [Green Version]
- Thayer, A.M. Improving peptides. Chem. Eng. News Arch. 2011, 89, 13–20. [Google Scholar] [CrossRef]
- Larbanoix, L.; Burtea, C.; Laurent, S.; Van Leuven, F.; Toubeau, G.; Vander Elst, L.; Muller, R.N. Potential amyloid plaque-specific peptides for the diagnosis of Alzheimer’s disease. Neurobiol. Aging 2010, 31, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.-Y. HPEPDOCK: A web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Ansciaux, E.; Saidi, E.; Larbanoix, L.; Stanicki, D.; Nonclercq, D.; Vander Elst, L.; Laurent, S.; Muller, R.N.; Burtea, C. Validation by Magnetic Resonance Imaging of the Diagnostic Potential of a Heptapeptide-Functionalized Imaging Probe Targeted to Amyloid-β and Able to Cross the Blood-Brain Barrier. J. Alzheimers Dis. JAD 2017, 60, 1547–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burtea, C.; Laurent, S.; Port, M.; Lancelot, E.; Ballet, S.; Rousseaux, O.; Toubeau, G.; Vander Elst, L.; Corot, C.; Muller, R.N. Magnetic resonance molecular imaging of vascular cell adhesion molecule-1 expression in inflammatory lesions using a peptide-vectorized paramagnetic imaging probe. J. Med. Chem. 2009, 52, 4725–4742. [Google Scholar] [CrossRef] [PubMed]
- Stanicki, D.; Boutry, S.; Laurent, S.; Wacheul, L.; Nicolas, E.; Crombez, D.; Vander Elst, L.; Lafontaine, D.L.J.; Muller, R.N. Carboxy-silane coated iron oxide nanoparticles: A convenient platform for cellular and small animal imaging. J. Mater. Chem. B 2013, 2, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Bridot, J.-L.; Stanicki, D.; Laurent, S.; Boutry, S.; Gossuin, Y.; Leclère, P.; Lazzaroni, R.; Vander Elst, L.; Muller, R.N. New carboxysilane-coated iron oxide nanoparticles for nonspecific cell labelling. Contrast Media Mol. Imaging 2013, 8, 466–474. [Google Scholar] [CrossRef]
- Mireles, L.-K.; Sacher, E.; Yahia, L.; Laurent, S.; Stanicki, D. A comparative physicochemical, morphological and magnetic study of silane-functionalized superparamagnetic iron oxide nanoparticles prepared by alkaline coprecipitation. Int. J. Biochem. Cell Biol. 2016, 75, 203–211. [Google Scholar] [CrossRef]
- Burtea, C.; Laurent, S.; Mahieu, I.; Larbanoix, L.; Roch, A.; Port, M.; Rousseaux, O.; Ballet, S.; Murariu, O.; Toubeau, G.; et al. In vitro biomedical applications of functionalized iron oxide nanoparticles, including those not related to magnetic properties. Contrast Media Mol. Imaging 2011, 6, 236–250. [Google Scholar] [CrossRef]
- Dupont, W.; Plummer, W. Power and Sample Size Calculations for studies Involving Linear Regression. Control. Clin. Trials 1998, 19, 589–601. [Google Scholar] [CrossRef]
- Burtea, C.; Ballet, S.; Laurent, S.; Rousseaux, O.; Dencausse, A.; Gonzalez, W.; Port, M.; Corot, C.; Vander Elst, L.; Muller, R.N. Development of a magnetic resonance imaging protocol for the characterization of atherosclerotic plaque by using vascular cell adhesion molecule-1 and apoptosis-targeted ultrasmall superparamagnetic iron oxide derivatives. Arterioscler. Thromb. Vasc. Biol. 2012, 32, e36–e48. [Google Scholar] [CrossRef] [Green Version]
- Burtea, C.; Laurent, S.; Crombez, D.; Delcambre, S.; Sermeus, C.; Millard, I.; Rorive, S.; Flamez, D.; Beckers, M.-C.; Salmon, I.; et al. Development of a peptide-functionalized imaging nanoprobe for the targeting of (FXYD2)γa as a highly specific biomarker of pancreatic beta cells. Contrast Media Mol. Imaging 2015, 10, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Ansciaux, E.; Burtea, C.; Laurent, S.; Crombez, D.; Nonclercq, D.; Vander Elst, L.; Muller, R.N. In vitro and in vivo characterization of several functionalized ultrasmall particles of iron oxide, vectorized against amyloid plaques and potentially able to cross the blood-brain barrier: Toward earlier diagnosis of Alzheimer’s disease by molecular imaging. Contrast Media Mol. Imaging 2015, 10, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Burtea, C.; Laurent, S.; Sanli, T.; Fanfone, D.; Devalckeneer, A.; Sauvage, S.; Beckers, M.-C.; Rorive, S.; Salmon, I.; Vander Elst, L.; et al. Screening for peptides targeted to IL-7Rα for molecular imaging of rheumatoid arthritis synovium. Arthritis Res. Ther. 2016, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Henry, L.; Ho, Y.K.; Pownall, H.J.; Rudenko, G. Mechanism of LDL binding and release probed by structure-based mutagenesis of the LDL receptor. J. Lipid Res. 2010, 51, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.A.; Halliday, G.M.; Garner, B. Apolipoprotein-E forms dimers in human frontal cortex and hippocampus. BMC Neurosci. 2010, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Al-Ali, H.; Khachfe, H.M. The N-terminal domain of apolipoprotein B-100: Structural characterization by homology modeling. BMC Biochem. 2007, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesk, A.M. Introduction to Protein Sciences-Architecture, Function, Genomics; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Zhao, Z.; Michaely, P. The role of calcium in lipoprotein release by the low-density lipoprotein receptor. Biochemistry 2009, 48, 7313–7324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussard, C. La Biochimie-Tome 1. Biochimie Structurale et Métabolique-Médecine Pharmacie, Sciences; DeBoeck Université: Paris, Bruxelles, 1999. [Google Scholar]
- Uniprot APOB-Apolipoprotein B-100 Precursor-Homo Sapiens (Human)-APOB Gene & Protein. Available online: https://www.uniprot.org/uniprot/P04114#sequences (accessed on 11 April 2019).
- Uniprot APOE-Apolipoprotein E Precursor-Homo Sapiens (Human)-APOE Gene & Protein. Available online: https://www.uniprot.org/uniprot/P02649#sequences (accessed on 11 April 2019).
- Alves, A.C.; Etxebarria, A.; Soutar, A.K.; Martin, C.; Bourbon, M. Novel functional APOB mutations outside LDL-binding region causing familial hypercholesterolaemia. Hum. Mol. Genet. 2014, 23, 1817–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prévost, M.; Raussens, V. Apolipoprotein E-low density lipoprotein receptor binding: Study of protein-protein interaction in rationally selected docked complexes. Proteins 2004, 55, 874–884. [Google Scholar] [CrossRef]
- Lalazar, A.; Weisgraber, K.H.; Rall, S.C.; Giladi, H.; Innerarity, T.L.; Levanon, A.Z.; Boyles, J.K.; Amit, B.; Gorecki, M.; Mahley, R.W. Site-specific mutagenesis of human apolipoprotein E. Receptor binding activity of variants with single amino acid substitutions. J. Biol. Chem. 1988, 263, 3542–3545. [Google Scholar]
- Blanco, F.J.; Villegas, S.; Benítez, S.; Bancells, C.; Diercks, T.; Ordóñez-Llanos, J.; Sánchez-Quesada, J.L. 2D-NMR reveals different populations of exposed lysine residues in the apoB-100 protein of electronegative and electropositive fractions of LDL particles. J. Lipid Res. 2010, 51, 1560–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Ehling, M.; Kato, K.; Kanai, K.; van Lessen, M.; Frye, M.; Zeuschner, D.; Nakayama, M.; Vestweber, D.; Adams, R.H. Integrin β1 controls VE-cadherin localization and blood vessel stability. Nat. Commun. 2015, 6, 6429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelton, J.C.; Wright, C.E.; Leitges, M.; Bautch, V.L. Multiple endothelial cells constitute the tip of developing blood vessels and polarize to promote lumen formation. Dev. Camb. Engl. 2014, 141, 4121–4126. [Google Scholar] [CrossRef] [Green Version]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- van de Sluis, B.; Wijers, M.; Herz, J. News on the molecular regulation and function of hepatic low-density lipoprotein receptor and LDLR-related protein 1. Curr. Opin. Lipidol. 2017, 28, 241–247. [Google Scholar] [CrossRef]
- Sukhbaatar, N.; Weichhart, T. Iron Regulation: Macrophages in Control. Pharm. Basel Switz. 2018, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Xiong, X.-Q.; Zhao, L.; Xu, T.-T.; Bi, H.; Fu, R.; Wang, Q.-H. Biodistribution and Toxicity Assessment of Superparamagnetic Iron Oxide Nanoparticles In Vitro and In Vivo. Curr. Med. Sci. 2018, 38, 1096–1102. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Appeldoorn, C.C.M.; Dorland, R.; van Kregten, J.; Manca, F.; Vugts, D.J.; Windhorst, B.; van Dongen, G.A.M.S.; de Vries, H.E.; Maussang, D.; et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS ONE 2014, 9, e82331. [Google Scholar] [CrossRef] [Green Version]
- Shanehsazzadeh, S.; Oghabian, M.A.; Lahooti, A.; Abdollahi, M.; Abolghasem Haeri, S.; Amanlou, M.; Daha, F.J.; Allen, B.J. Estimated background doses of [67Ga]-DTPA-USPIO in normal Balb/c mice as a potential therapeutic agent for liver and spleen cancers. Nucl. Med. Commun. 2013, 34, 915–925. [Google Scholar] [CrossRef]
- Brunnquell, C.L.; Hernandez, R.; Graves, S.A.; Smit-Oistad, I.; Nickles, R.J.; Cai, W.; Meyerand, M.E.; Suzuki, M. Uptake and retention of manganese contrast agents for PET and MRI in the rodent brain. Contrast Media Mol. Imaging 2016, 11, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Gouritin, B.; Villarroya, H.; Eclancher, F.; Giannavola, C.; Klein, C.; Andreux, J.P.; Couvreur, P. Quantification and localization of PEGylated polycyanoacrylate nanoparticles in brain and spinal cord during experimental allergic encephalomyelitis in the rat. Eur. J. Neurosci. 2002, 15, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.; Yan, F.; Wang, Y.; Sun, Y.; Yang, N.; Ye, L. The blood-brain barrier penetration and distribution of PEGylated fluorescein-doped magnetic silica nanoparticles in rat brain. Biochem. Biophys. Res. Commun. 2010, 394, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Fan, L.; Zha, Y.; Guo, L.; Zhang, Q.; Chen, J.; Pang, Z.; Wang, Y.; Jiang, X.; et al. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 2011, 32, 4943–4950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalraj, R.K.; Zhu, H.; Kelly, J.F.; Mendiondo, M.; Pulliam, J.F.; Bennett, D.A.; Estus, S. Genetic association of low density lipoprotein receptor and Alzheimer’s disease. Neurobiol. Aging 2005, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Deane, R.; Gottesdiener, A.J.; Verghese, P.B.; Stewart, F.R.; West, T.; Paoletti, A.C.; Kasper, T.R.; DeMattos, R.B.; Zlokovic, B.V.; et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15502–15507. [Google Scholar] [CrossRef] [Green Version]
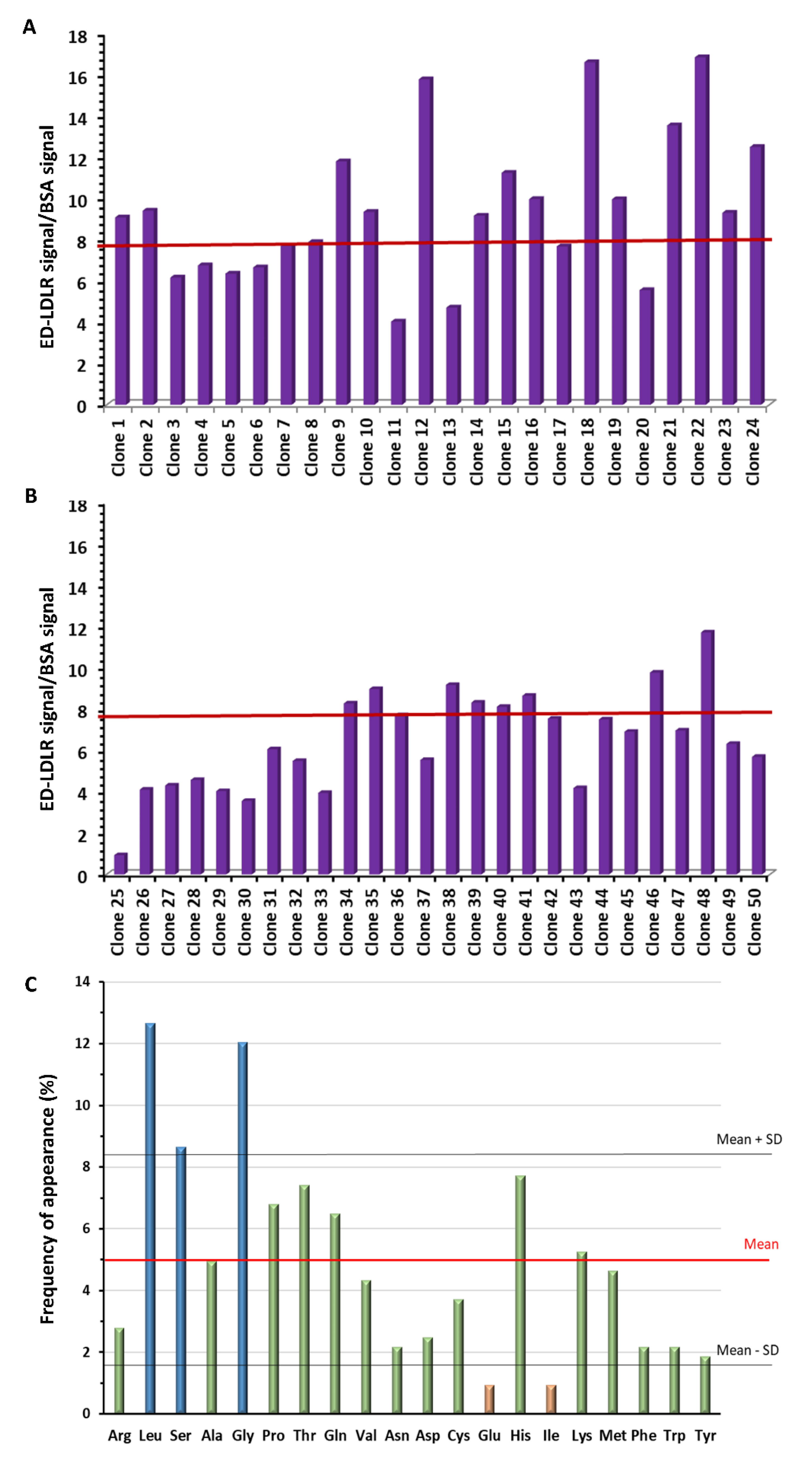
| Clones | Sequences | P (k > 0) (%) |
|---|---|---|
| 1 | GHIPTCLTPMCR | 40.9 |
| 9 | HSIRDGFRSTPV | 99.9 |
| 10 | TGQTVTGLSYIF | 99.4 |
| 16 | KVVSLSALQSMT | 100 |
| 21 | WTSQPHLQHVDD | 91.5 |
| 17, 23 | KVWSLVNQGGQF | 39.3 |
| 2, 7, 8, 12, 14, 18, 22, 24, 35, 36, 44, 48 | AHLPTSMLKGQG | 99.9 |
| 38 | GHLAVNMPRASL | 100 |
| 40 | HHTGCLSPLSCS | 99.9 |
| 34, 41 | YHFNGCEDPLCR | 6.1 |
| 42 | HWKVTTWNSSTV | 89.8 |
| 46 | SGVYKVAYDWQH | 33.9 |
| 47 | HPWCCGLRLDLR | 38.3 |
| Clones | K*d | IC50 ApoB | IC50 ApoE | Ratio ApoB | Ratio ApoE |
|---|---|---|---|---|---|
| 1 | 1.36 × 10−10 | 6.23 × 10−19 | 1.67 × 10−20 | 4.55 × 10−9 | 1.22 × 10−10 |
| 36 | 2.23 × 10−11 | 8.31 × 10−19 | 1.71 × 10−8 | 3.71 × 10−8 | 763.97 |
| 38 | 2.41 × 10−10 | 3.49 × 10−9 | 2.57 × 10−9 | 14.48 | 10.66 |
| 40 | 2.17 × 10−12 | 2.95 × 10−9 | 3.33 × 10−9 | 1361.51 | 1536.90 |
| 41 | 7.12 × 10−11 | 2.32 × 10−11 | 1.90 × 10−10 | 0.33 | 2.67 |
| 47 | 8.73 × 10−11 | 3.54 × 10−9 | 1.26 × 10−7 | 40.54 | 1443.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, S.; Larbanoix, L.; Verteneuil, S.; Stanicki, D.; Nonclercq, D.; Vander Elst, L.; Laurent, S.; Muller, R.N.; Burtea, C. Development of an LDL Receptor-Targeted Peptide Susceptible to Facilitate the Brain Access of Diagnostic or Therapeutic Agents. Biology 2020, 9, 161. https://doi.org/10.3390/biology9070161
André S, Larbanoix L, Verteneuil S, Stanicki D, Nonclercq D, Vander Elst L, Laurent S, Muller RN, Burtea C. Development of an LDL Receptor-Targeted Peptide Susceptible to Facilitate the Brain Access of Diagnostic or Therapeutic Agents. Biology. 2020; 9(7):161. https://doi.org/10.3390/biology9070161
Chicago/Turabian StyleAndré, Séverine, Lionel Larbanoix, Sébastien Verteneuil, Dimitri Stanicki, Denis Nonclercq, Luce Vander Elst, Sophie Laurent, Robert N. Muller, and Carmen Burtea. 2020. "Development of an LDL Receptor-Targeted Peptide Susceptible to Facilitate the Brain Access of Diagnostic or Therapeutic Agents" Biology 9, no. 7: 161. https://doi.org/10.3390/biology9070161






