Plant Biomass Conversion to Vehicle Liquid Fuel as a Path to Sustainability
Abstract
:1. Introduction
2. Materials and Methods
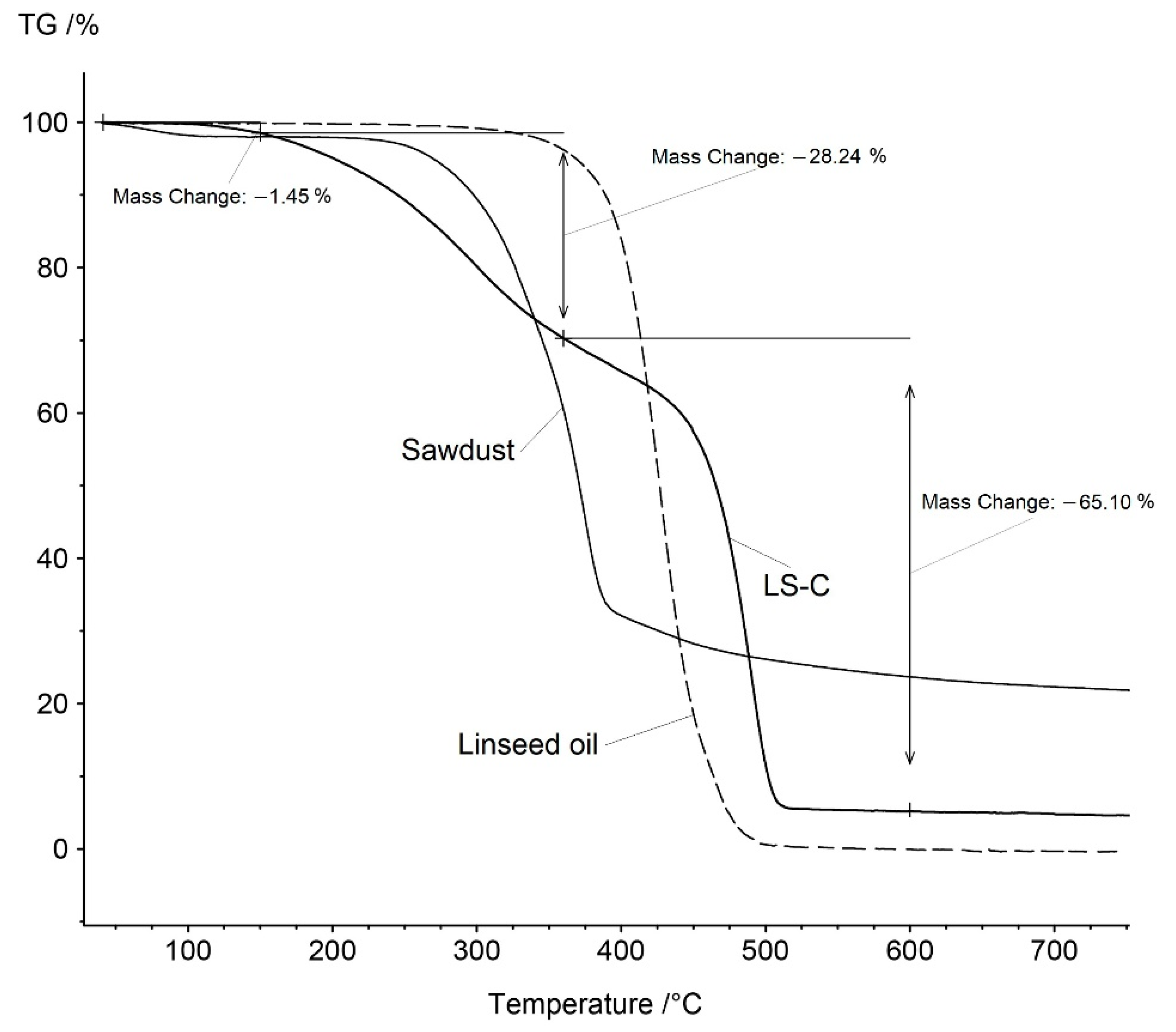
3. Results
3.1. Distillation of the Product
3.2. Gross Calorific Value
4. Discussion
4.1. Chemical Structure and Modification of Liquid Biofuels
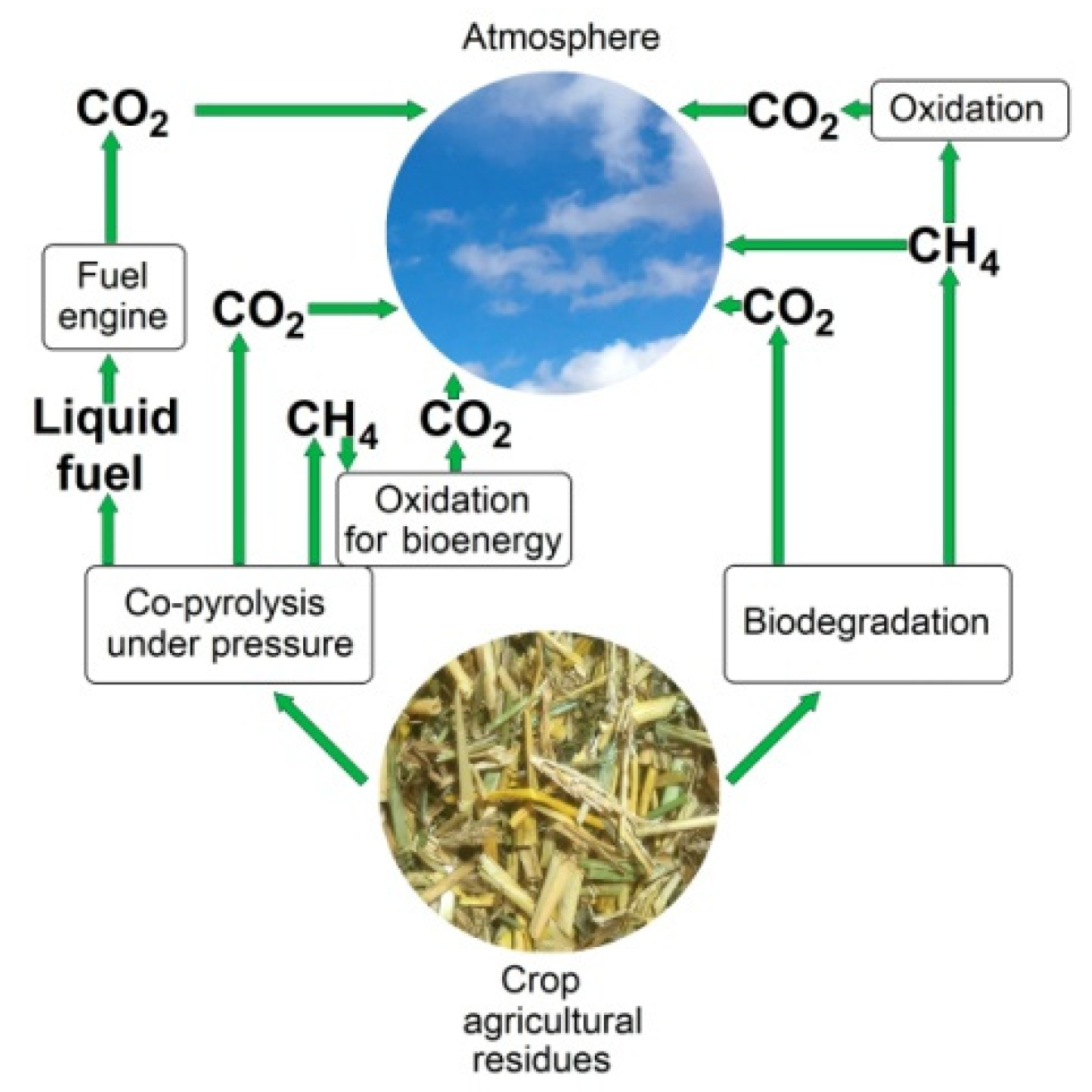
4.2. Carbon Footprint of Liquid Fuels
4.3. Resources for Liquid Biofuels
4.4. Energy Conversion and Logistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ilari, A.; Duca, D.; Boakye-Yiadom, K.A.; Gasperini, T.; Toscano, G. Carbon Footprint and Feedstock Quality of a Real Biomass Power Plant Fed with Forestry and Agricultural Residues. Resources 2022, 11, 7. [Google Scholar] [CrossRef]
- Hoeft, F. Internal combustion engine to electric vehicle retrofitting: Potential customer’s needs, public perception and business model implications. Transp. Res. Interdiscip. Perspect. 2021, 9, 100330. [Google Scholar] [CrossRef]
- Towoju, O.A.; Ishola, F.A. A case for the internal combustion engine powered vehicle. Energy Rep. 2020, 6, 315–321. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. Biomethane: A Renewable Resource as Vehicle Fuel. Resources 2017, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Atabani, A.; Mahlia, T.; Badruddin, I.A.; Masjuki, H.; Chong, W.; Lee, K.T. Investigation of physical and chemical properties of potential edible and non-edible feedstocks for biodiesel production, a comparative analysis. Renew. Sustain. Energy Rev. 2013, 21, 749–755. [Google Scholar] [CrossRef]
- Sivaramakrishnan, K.; Ravikumar, P. Determination of Higher heating value of biodiesels. Int. J. Eng. Sci. Technol. 2011, 3, 7981–7987. [Google Scholar]
- Suris, A.L. Heat of combustion of liquid halogen-organic compounds. Chem. Pet. Eng. 2007, 43, 20–22. [Google Scholar] [CrossRef]
- Tait, J. The ethics of biofuels. GCB Bioenergy 2011, 3, 271–275. [Google Scholar] [CrossRef]
- Long, F.; Liu, W.; Jiang, X.; Zhai, Q.; Cao, X.; Jiang, J.; Xu, J. State-of-the-art technologies for biofuel production from triglycerides: A review. Renew. Sustain. Energy Rev. 2021, 148, 111269. [Google Scholar] [CrossRef]
- Ketov, A.; Korotaev, V.; Sliusar, N.; Bosnic, V.; Krasnovskikh, M.; Gorbunov, A. Baseline Data of Low-Density Polyethylene Continuous Pyrolysis for Liquid Fuel Manufacture. Recycling 2022, 7, 2. [Google Scholar] [CrossRef]
- Murata, K.; Sato, K.; Sakata, Y. Effect of pressure on thermal degradation of polyethylene. J. Anal. Appl. Pyrolysis 2004, 71, 569–589. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of Vegetable Oils with Ethanol and Characterization of the Key Fuel Properties of Ethyl Esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Silva, R.V.; Pereira, V.; Stelzer, K.T.; Almeida, T.A.; Romeiro, G.A.; Azevedo, D.A. Comprehensive study of the liquid products from slow pyrolysis of crambe seeds: Bio-oil and organic compounds of the aqueous phase. Biomass Bioenergy 2019, 123, 78–88. [Google Scholar] [CrossRef]
- Buffi, M.; Cappelletti, A.; Rizzo, A.M.; Martelli, F.; Chiaramonti, D. Combustion of fast pyrolysis bio-oil and blends in a micro gas turbine. Biomass Bioenergy 2018, 115, 174–185. [Google Scholar] [CrossRef]
- van de Beld, B.; Holle, E.; Florijn, J. The use of a fast pyrolysis oil—Ethanol blend in diesel engines for chp applications. Biomass Bioenergy 2018, 110, 114–122. [Google Scholar] [CrossRef]
- Chang, S.H. Bio-oil derived from palm empty fruit bunches: Fast pyrolysis, liquefaction and future prospects. Biomass Bioenergy 2018, 119, 263–276. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Wei, Q. Improving the quality of fast pyrolysis bio-oil by reduced pressure distillation. Biomass Bioenergy 2011, 35, 1804–1810. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Liakos, D.; Pfisterer, U.; Moustaka-Gouni, M.; Karonis, D.; Bezergianni, S. Impact of hydrogenation on miscibility of fast pyrolysis bio-oil with refinery fractions towards bio-oil refinery integration. Biomass Bioenergy 2021, 151, 106171. [Google Scholar] [CrossRef]
- Kim, S.W.; Koo, B.S.; Ryu, J.W.; Lee, J.S.; Kim, C.J.; Lee, D.H.; Choi, S. Bio-oil from the pyrolysis of palm and Jatropha wastes in a fluidized bed. Fuel Process. Technol. 2013, 108, 118–124. [Google Scholar] [CrossRef]
- García, L. Hydrogen production by steam reforming of natural gas and other nonrenewable feedstocks. In Compendium of Hydrogen Energy; Woodhead Publishing: Cambridge, UK, 2015; pp. 83–107. [Google Scholar] [CrossRef]
- De Richter, R.; Ming, T.; Caillol, S.; Liu, W. Fighting global warming by GHG removal: Destroying CFCs and HCFCs in solar-wind power plant hybrids producing renewable energy with no-intermittency. Int. J. Greenh. Gas Control. 2016, 49, 449–472. [Google Scholar] [CrossRef]
- Stegarescu, G.; Escuer-Gatius, J.; Soosaar, K.; Kauer, K.; Tõnutare, T.; Astover, A.; Reintam, E. Effect of Crop Residue Decomposition on Soil Aggregate Stability. Agriculture 2020, 10, 527. [Google Scholar] [CrossRef]
- Kashyap, R.; Chugh, P.; Nandakumar, T. Opportunities & Challenges in Capturing Landfill Gas from an Active and Un-scientifically Managed Land Fill Site—A Case Study. Procedia Environ. Sci. 2016, 35, 348–367. [Google Scholar] [CrossRef]
- Pehme, K.-M.; Orupõld, K.; Kuusemets, V.; Tamm, O.; Jani, Y.; Tamm, T.; Kriipsalu, M. Field Study on the Efficiency of a Methane Degradation Layer Composed of Fine Fraction Soil from Landfill Mining. Sustainability 2020, 12, 6209. [Google Scholar] [CrossRef]
- Business and Economic Data for 200 Countries, Germany Economic Indicators. Available online: https://www.theglobaleconomy.com/Germany/ (accessed on 2 August 2022).
- Konvalina, P.; Stehno, Z.; Capouchová, I.; Zechner, E.; Berger, S.; Grausgruber, H.; Moudrý, J. Differences in grain/straw ratio, protein content and yield in landraces and modern varieties of different wheat species under organic farming. Euphytica 2014, 199, 31–40. [Google Scholar] [CrossRef]
- Yorgun, S.; Şensöz, S.; Koçkar, Ö.M. Characterization of the pyrolysis oil produced in the slow pyrolysis of sunflower-extracted bagasse. Biomass Bioenergy 2001, 20, 141–148. [Google Scholar] [CrossRef]
- Lavanya, M.; Meenakshisundaram, A.; Renganathan, S.; Chinnasamy, S.; Lewis, D.M.; Nallasivam, J.; Bhaskar, S. Hydrothermal liquefaction of freshwater and marine algal biomass: A novel approach to produce distillate fuel fractions through blending and co-processing of biocrude with petrocrude. Bioresour. Technol. 2016, 203, 228–235. [Google Scholar] [CrossRef]
- Chaiwong, K.; Kiatsiriroat, T.; Vorayos, N.; Thararax, C. Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenergy 2013, 56, 600–606. [Google Scholar] [CrossRef]
- Janssen, P.J.D.; Lambreva, M.D.; Plumere, N.; Bartolucci, C.; Antonacci, A.; Buonasera, K.; Rea, G. Photosynthesis at the forefront of a sustainable life. Front. Chem. 2014, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- MacKay, D.J.C. Solar energy in the context of energy use, energy transportation and energy storage, Philosophical Transactions of the Royal Society A: Mathematical. Phys. Eng. Sci. 2013, 371, 1996. [Google Scholar] [CrossRef]
- Doll, C.G.; Plymale, A.E.; Cooper, A.; Kutnyakov, I.; Swita, M.; Lemmon, T.; Wang, H. Determination of low-level biogenic gasoline, jet fuel, and diesel in blends using the direct liquid scintillation counting method for 14C content. Fuel 2021, 291, 120084. [Google Scholar] [CrossRef]
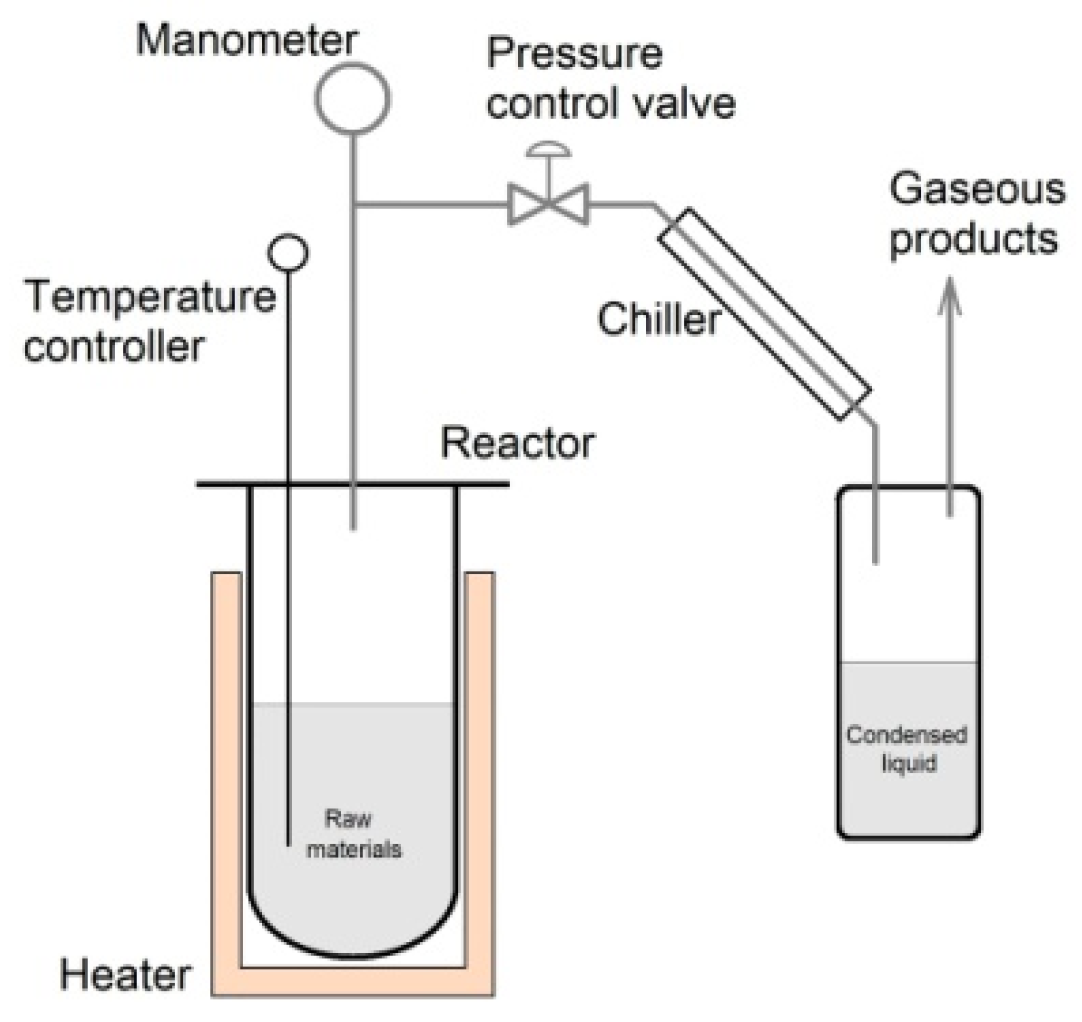
| Sample Designation | Raw Material | Type of the Process | Pressure (MPa) | Duration (min) | Temperature (°C) |
|---|---|---|---|---|---|
| TE | Linseed oil and ethanol | transesterification | - | 90 | 80 |
| L-V | Linseed oil | pyrolysis, non-regulated pressure | Up to 9.0 | 30 | 590 |
| L-C | Linseed oil | pyrolysis, regulated pressure | 3.0 | 40 | 590 |
| LS-V | Linseed oil and sawdust (1:1 wt.) | pyrolysis, non-regulated pressure | Up to 9.0 | 30 | 590 |
| LS-C | Linseed oil and sawdust (1:1 wt.) | pyrolysis, regulated pressure | 3.0 | 40 | 590 |
| Sample Designation | Raw Material | Type of the Process | Gross Calorific Value (MJ/kg) |
|---|---|---|---|
| LO | Linseed oil | raw linseed oil | 48.84 |
| TE | Linseed oil and ethanol | transesterification | 45.75 |
| L-V | Linseed oil | pyrolysis, non-regulated pressure | 51.19 |
| L-C | Linseed oil | pyrolysis, regulated pressure | 53.55 |
| LS-V | Linseed oil and sawdust (1:1 wt.) | pyrolysis, non-regulated pressure | 44.99 |
| LS-C | Linseed oil and sawdust (1:1 wt.) | pyrolysis, regulated pressure | 49.06 |
| FD | fossil diesel fuel | 57.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketov, A.; Sliusar, N.; Tsybina, A.; Ketov, I.; Chudinov, S.; Krasnovskikh, M.; Bosnic, V. Plant Biomass Conversion to Vehicle Liquid Fuel as a Path to Sustainability. Resources 2022, 11, 75. https://doi.org/10.3390/resources11080075
Ketov A, Sliusar N, Tsybina A, Ketov I, Chudinov S, Krasnovskikh M, Bosnic V. Plant Biomass Conversion to Vehicle Liquid Fuel as a Path to Sustainability. Resources. 2022; 11(8):75. https://doi.org/10.3390/resources11080075
Chicago/Turabian StyleKetov, Aleksandr, Natalia Sliusar, Anna Tsybina, Iurii Ketov, Sergei Chudinov, Marina Krasnovskikh, and Vladimir Bosnic. 2022. "Plant Biomass Conversion to Vehicle Liquid Fuel as a Path to Sustainability" Resources 11, no. 8: 75. https://doi.org/10.3390/resources11080075







