Groundwater Resources of the Transboundary Quaternary Aquifer of the Lake Chad Basin: Towards a Better Management via Isotope Hydrology
Abstract
:1. Introduction
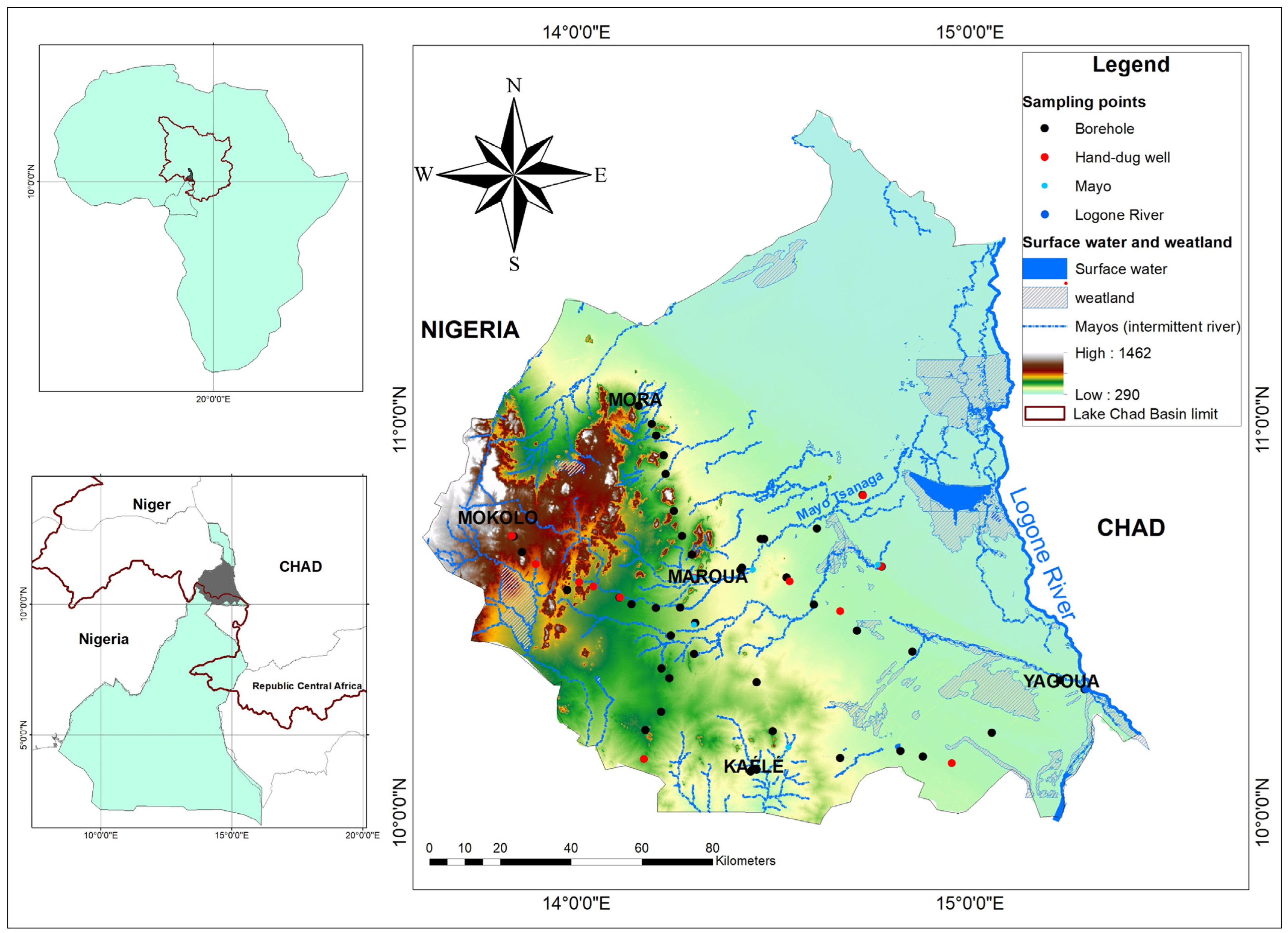
2. Study Area
2.1. Location and Geomorphology
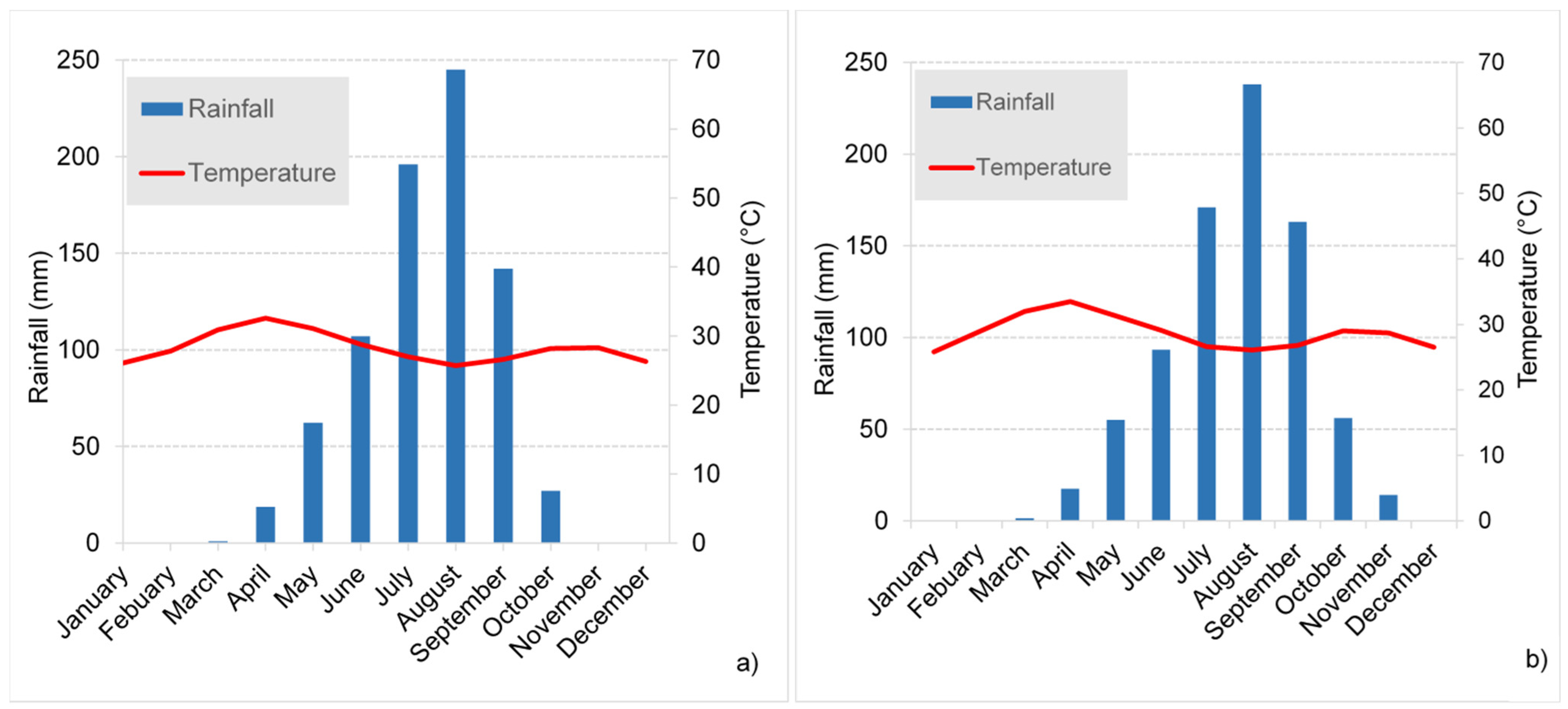
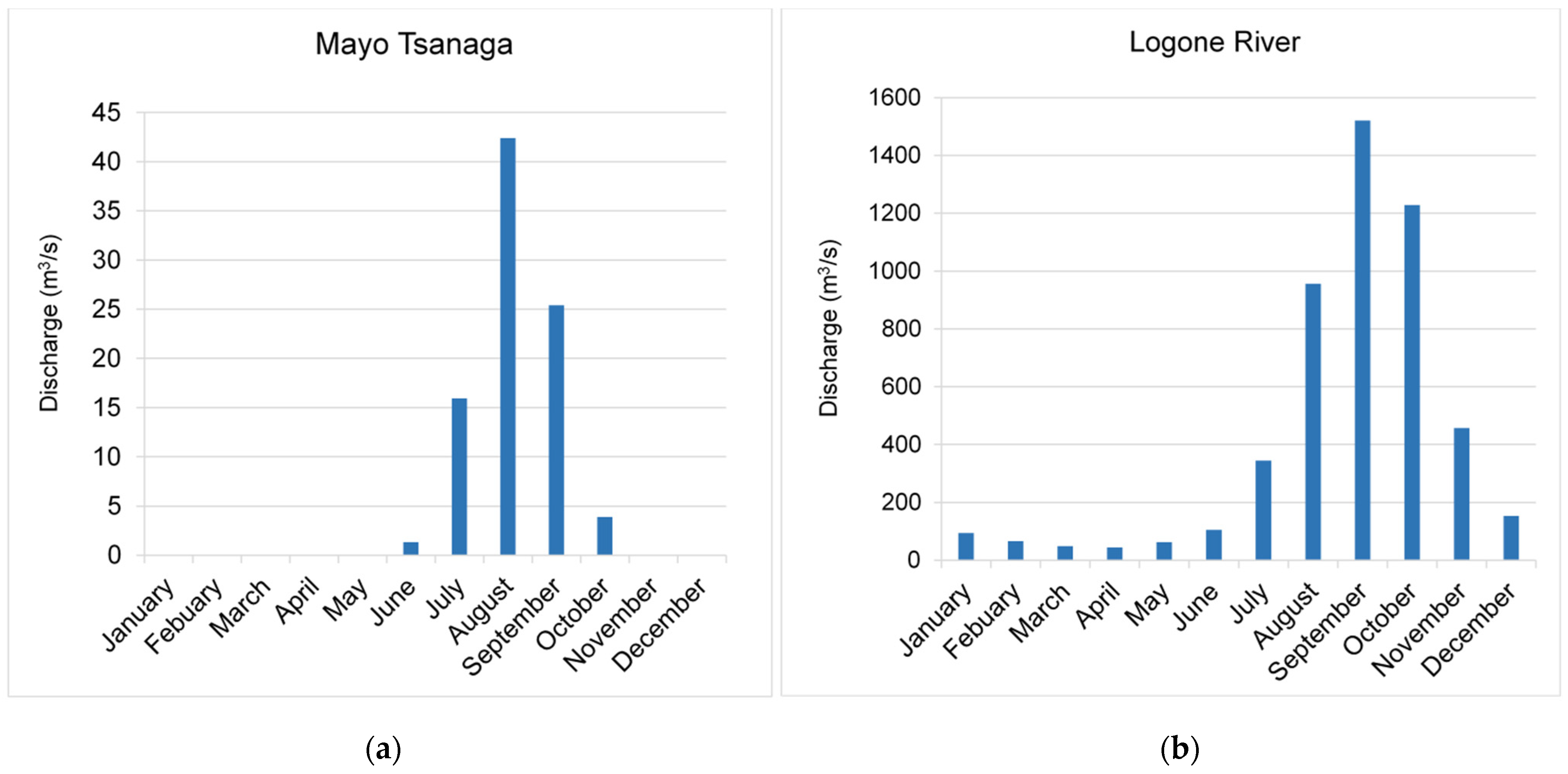
2.2. Hydroclimatology
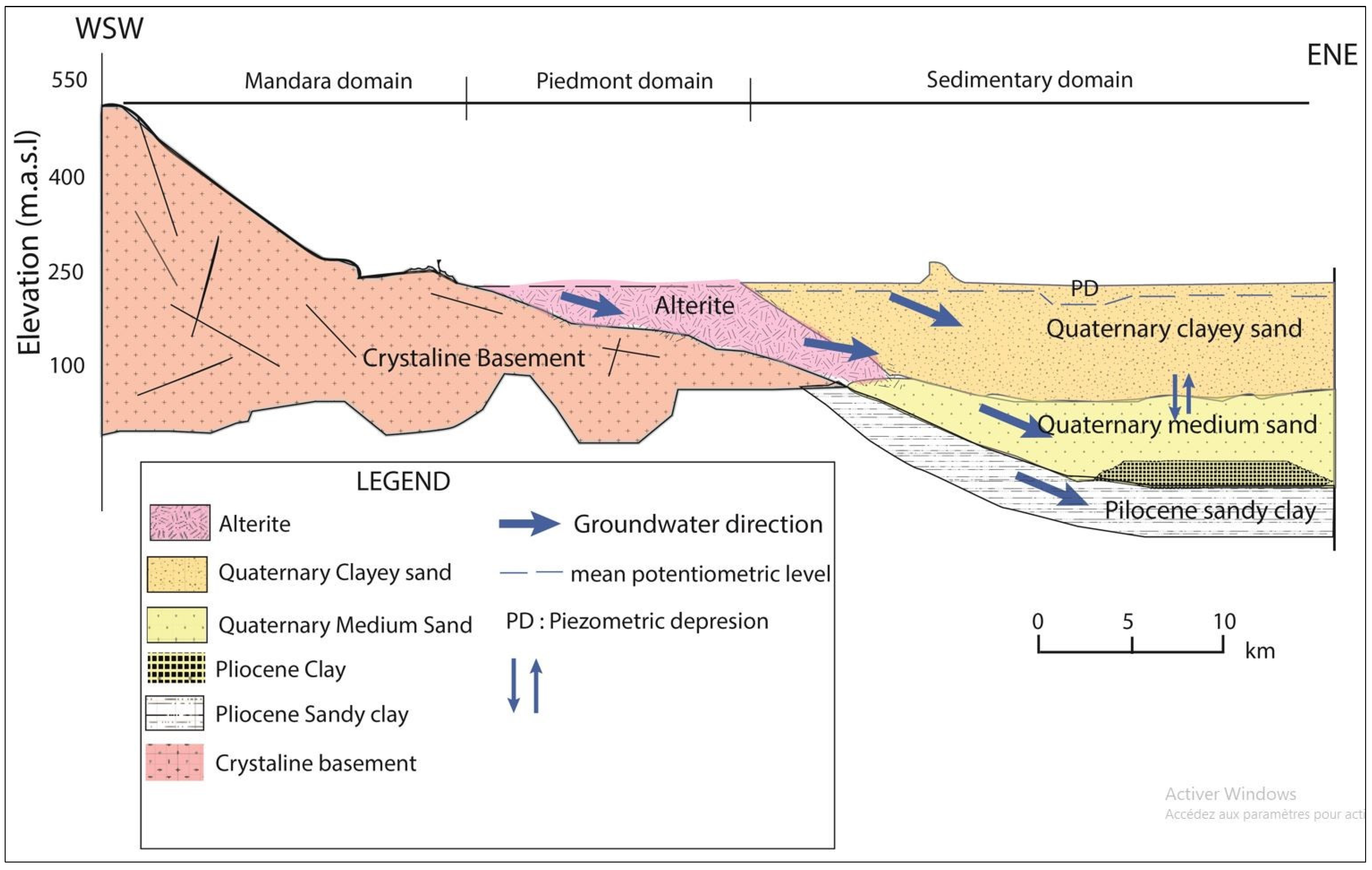
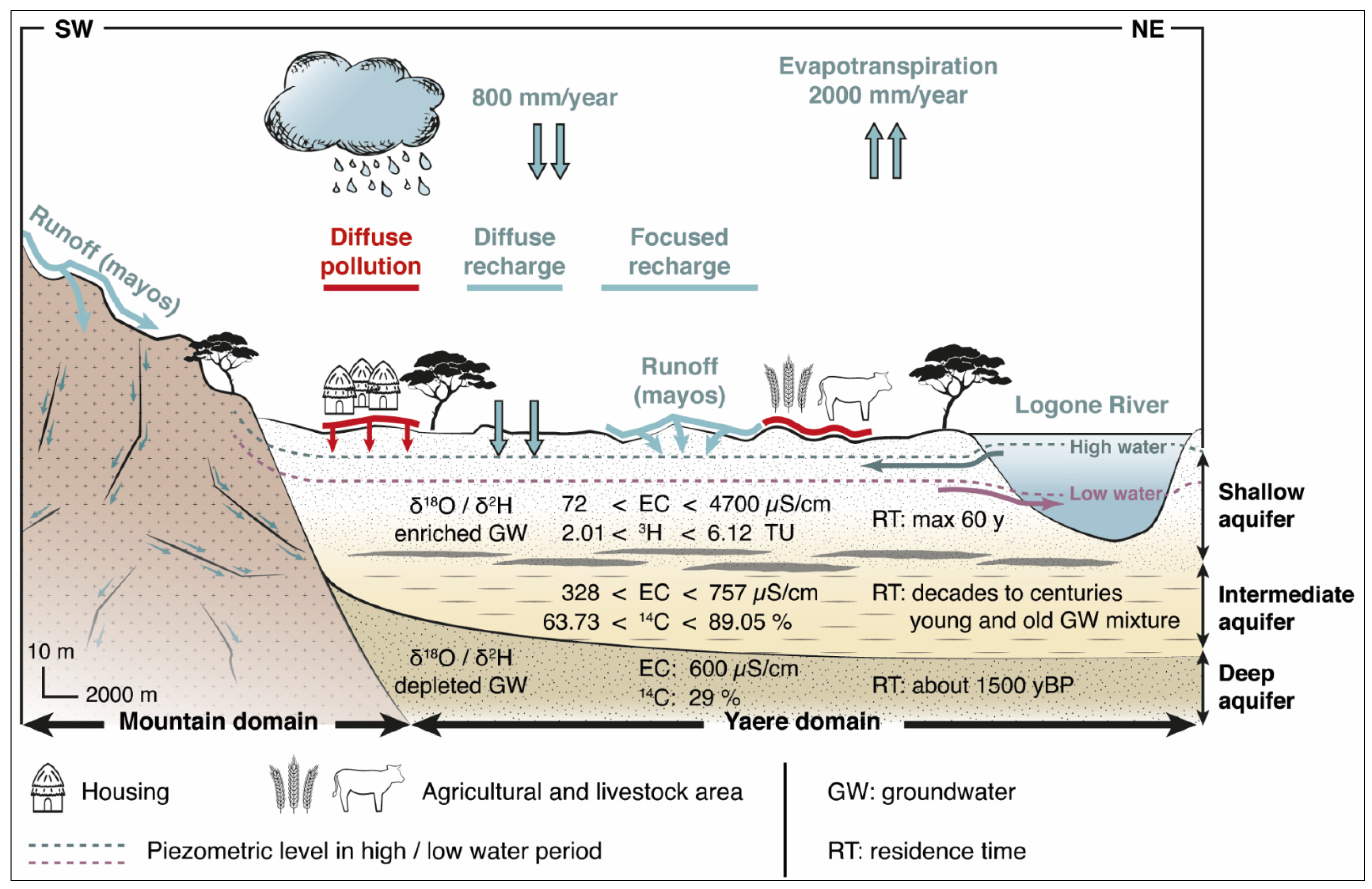
2.3. Geological and Hydrogeological Settings
3. Sampling and Analytical Methods
3.1. Sampling Network and Protocol
3.2. Chemical and Isotope Tracer Analyses
4. Results and Discussions
4.1. Groundwater Recharge
4.1.1. Rainfall Input Signal
4.1.2. Processes during Infiltration and Groundwater Recharge Modes
4.2. Delineation of Groundwater Flow Paths
4.3. Ambient Background Levels (ABL) and Threshold Values (TV)
4.4. Conceptual Scheme of the Southern Lake Chad Quaternary Aquifer Functioning and Recommendations for Management Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. The Sahel: One Region, Many Crises. Africa Renewal. 2013. Available online: https://www.un.org/africarenewal/magazine/december-2013/sahel-one-region-many-crises (accessed on 15 August 2023).
- Gbohoui, Y.P.; Paturel, J.-E.; Tazen, F.; Mounirou, L.A.; Yonaba, R.; Karambiri, H.; Yacouba, H. Impacts of climate and environmental changes on water resources: A multi-scale study based on Nakanbé nested watersheds in West African Sahel. J. Hydrol. Reg. Stud. 2021, 35, 100828. [Google Scholar] [CrossRef]
- TC Lake Chad Basin: Groundwater Management. Available online: https://www.bgr.bund.de/EN/Themen/Wasser/Projekte/laufend/TZ/Tschad/tschad-II_fb_en.html (accessed on 23 April 2021).
- Jones, P.D.; Hulme, M. Calculating Regional Climatic Time Series for Temperature and Precipitation: Methods and Illustrations. Int. J. Climatol. 1996, 16, 361–377. [Google Scholar] [CrossRef]
- Hulme, M.; Doherty, R.; Ngara, T.; New, M.; Lister, D. African climate change: 1900–2100. Clim. Res. 2001, 17, 145–168. [Google Scholar] [CrossRef]
- Bello, M.; Ketchemen-Tandia, B.; Nlend, B.; Huneau, F.; Fouepe, A.; Fantong, W.Y.; Ngo Boum-Nkot, S.; Garel, E.; Celle-Jeanton, H. Shallow Groundwater Quality Evolution after 20 Years of Exploitation in the Southern Lake Chad: Hydrochemistry and Stable Isotopes Survey in the Far North of Cameroon. Environ. Earth Sci. 2019, 78, 474. [Google Scholar] [CrossRef]
- Bon, A.F.; Abderamane, H.; Ewodo Mboudou, G.; Aoudou Doua, S.; Banakeng, L.A.; Bontsong Boyomo, S.B.; Piih, S.L.; Wangbara Damo, B. Parametrization of Groundwater Quality of the Quaternary Aquifer in N’Djamena (Chad), Lake Chad Basin: Application of Numerical and Multivariate Analyses. Environ. Sci. Pollut. Res. 2021, 28, 12300–12320. [Google Scholar] [CrossRef]
- Mahamat Nour, A.; Vallet-Coulomb, C.; Gonçalves, J.; Sylvestre, F.; Deschamps, P. Rainfall-Discharge Relationship and Water Balance over the Past 60 Years within the Chari-Logone Sub-Basins, Lake Chad Basin. J. Hydrol. Reg. Stud. 2021, 35, 100824. [Google Scholar] [CrossRef]
- Seignobos, C. Répartition et Densités de La Population. In Atlas de la Province Extrême-Nord Cameroun; Iyébi-Mandjek, O., Ed.; Atlas et Cartes; IRD Éditions: Marseille, France, 2017; pp. 61–63. [Google Scholar]
- Ketchmen, B. Etude Hydrogéologie du Grand Yaéré (Extrême Nord du Cameroun). Synthèse Hydrogéologique et Étude de la Recharge par les Isotopes de l’Environnement. Ph.D. Thesis, University de Dakar, Dakar, Sénégal, 1992. [Google Scholar]
- Ngounou Ngatcha, B. Hydrogéologie d’Aquifères Complexes en Zone Semi-Aride: Les Aquifères Quaternaires du Grand Yaéré (Nord Cameroun). Ph.D. Thesis, Université de Grenoble, Saint-Martin-d’Hères, France, 1993. [Google Scholar]
- Huneau, F.; Dakoure, D.; Celle-Jeanton, H.; Vitvar, T.; Ito, M.; Traore, S.; Compaore, N.F.; Jirakova, H.; Le Coustumer, P. Flow Pattern and Residence Time of Groundwater within the South-Eastern Taoudeni Sedimentary Basin (Burkina Faso, Mali). J. Hydrol. 2011, 409, 423–439. [Google Scholar] [CrossRef]
- Nlend, B.; Celle-Jeanton, H.; Huneau, F.; Garel, E.; Ngo Boum-Nkot, S.; Etame, J. Shallow urban aquifers under hyper-recharge equatorial conditions and strong anthropogenic constrains. Implications in terms of groundwater resources potential and integrated water resources management strategies. Sci. Total Environ. 2020, 757, 143887. [Google Scholar] [CrossRef]
- IUCN. Le Bassin du Tchad, une Source de vie Pour l’homme, la Nature et la Paix. 2019. Available online: https://www.iucn.org/fr/news/eau/201910/le-bassin-du-tchad-une-source-de-vie-pour-lhomme-la-nature-et-la-paix (accessed on 18 January 2022).
- FAO (Food and Agricultural Organisation). Gestion des Inondations Dans la Région de l’Extrême-Nord au Cameroun: Les Riverains N’ont Plus Peur Des Pluies Diluviennes Depuis Que la Digue du Logone et le Barrage de Maga Ont Été Réhabilités, Projet. 2020. Available online: https://www.banquemondiale.org/fr/results/2020/11/10/flood-management-in-the-far-north-of-cameroon (accessed on 18 October 2023).
- Sultan, B.; Janicot, S. Abrupt Shift of the ITCZ over West Africa and Intra-Seasonal Variability. Geophys. Res. Lett. 2000, 27, 3353–3356. [Google Scholar] [CrossRef]
- DNM (Direction de la Météorologie Nationale). Données Pluviométriques des Stations de 1965–2005; DNM: Yaounde, Cameroon, 2009.
- L’Hôte, Y.; Mahé, G. Afrique de l’Ouest et Centrale: Précipitations Moyennes Annuelles (Période 1951–1989); Carte à l’échelle 1/6,000,000, 90 × 60 cm; Orstom: Paris, France, 1996. [Google Scholar]
- Nouaceur, Z. La reprise des pluies et la recrudescence des inondations en Afrique de l’Ouest sahélienne. Physio-Géo Géogr. Phys. Environ. 2020, 15, 89–109. [Google Scholar] [CrossRef]
- Mahamat Nour, A. Fonctionnement Hydrologique, Chimique et Isotopique du Principal Affluent du Lac Tchad: Le Système Chari-Logone. Ph.D. Thesis, Université Aix-Marseille, Marseille, France, 2019. Available online: http://www.theses.fr/2019AIXM0196 (accessed on 5 July 2021).
- DHATchad (Direction de l’Hydraulique et de l’Assainissement). Données Des débits à la Station de Yagoua de 1961–2013; DHATchad: Ndjamena, Chad, 2013.
- Bessoles, B.; Lasserre, M. Le complexe de base du Cameroun. Bull. Soc. Géol. Fr. 1977, S7-XIX, 1085–1092. [Google Scholar] [CrossRef]
- Dietrich, F.; Diaz, N.; Deschamps, P.; Ngounou Ngatcha, B.; Sebag, D.; Verrecchia, E.P. Origin of Calcium in Pedogenic Carbonate Nodules from Silicate Watersheds in the Far North Region of Cameroon: Respective Contribution of in Situ Weathering Source and Dust Input. Chem. Geol. 2017, 460, 54–69. [Google Scholar] [CrossRef]
- Schneider, J.-L.; Wolf, J.P. Carte Géologique et Hydrogéologique de 1/500 000 de la Republique du Tchad, Mémoire Explicatif; BRGM: Paris, France, 1992; p. 531. Available online: https://www.worldcat.org/title/carte-geologique-et-cartes-hydrogeologiques-a-11-500-000-de-la-republique-du-tchad-memoire-explicatif/oclc/26094222/ (accessed on 15 September 2023).
- Bouchez, C. Bilan et Dynamique Des Interactions Rivières-Lac(s)-Aquifères Dans le Bassin Hydrologique du Lac Tchad. Ph.D. Thesis, Aix Marseille Université, Marseille, France, 2015. Available online: https://hal.archives-ouvertes.fr/tel-01298153 (accessed on 21 April 2021).
- Pias, J. Les Formations Sédimentaires Tertiaires et Quaternaires de la Cuvette Tchadienne et Les Sols Qui en Dérivent; Mémoires ORSTOM; ORSTOM: Paris, France, 1970; p. 411. [Google Scholar]
- Ewodo Mboudou, G.; Bon, A.; Bineli, E.; Ntep, F.; Ombolo, A. Caracterisation de la Productivite des Aquiferes du Socle de la Region de l’extreme Nord, Cameroun. J. Cameroon Acad. Sci. 2018, 14, 25. [Google Scholar] [CrossRef]
- Tillement, B. Hydrogéologie du Nord-Cameroun. Ph.D. Thesis, Université Claude Bernard, Lyon, France, 1971. [Google Scholar]
- IAEA/WMO. Global Network of Isotopes in Precipitation. The GNIP Database. 2018. Available online: https://nucleus.iaea.org/wiser (accessed on 18 October 2018).
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Muller, D.; Blum, A.; Hookey, J.; Kunkei, R.; Scheidieder, A.; Tomlin, C.; Wendland, F. Final Proposal of a Methodology to Set Up Groundwater Threshold Values in Europe. Specific Targeted EU Research Project BRIDGE. J. Environ. Manag. 2009, 90, 1523. [Google Scholar]
- Gourcy, L.; Aranyossy, J.-F.; Olivry, J.-C.; Zuppi, G.M. Évolution spatio-temporelle des teneurs isotopiques (δ2H–δ18O) des eaux de la cuvette lacustre du fleuve Niger (Mali). Comptes Rendus l’Académie Sci.-Ser. IIA-Earth Planet. Sci. 2000, 331, 701–707. [Google Scholar] [CrossRef]
- Goni, I.; Fellman, E.; Edmunds, W. Rainfall Geochemistry in the Sahel Region of Northern Nigeria. Atmos. Environ. 2001, 35, 4331–4339. [Google Scholar] [CrossRef]
- Abderamane, H. Étude du Fonctionnement Hydrogéochimique du Système Aquifère du Chari Baguirmi (République du Tchad). Ph.D. Thesis, Université de Poitiers, Paris, France, 2012. [Google Scholar]
- Goni, I.B.; Taylor, R.G.; Favreau, G.; Shamsudduha, M.; Nazoumou, Y.; Nhatcha, B.N. Groundwater recharge from heavy rainfall in the southwestern Lake Chad Basin: Evidence from isotopic observations. Hydrol. Sci. J. 2021, 66, 1359–1371. [Google Scholar] [CrossRef]
- Allies, A.; Demarty, J.; Olioso, A.; Bouzou Moussa, I.; Issoufou, H.B.-A.; Velluet, C.; Bahir, M.; Maïnassara, I.; Oï, M.; Chazarin, J.-P.; et al. Evapotranspiration Estimation in the Sahel Using a New Ensemble-Contextual Method. Remote Sens. 2020, 12, 380. [Google Scholar] [CrossRef]
- Mahamat-Nour, A.; Huneau, F.; Mahamat, A.; Mahamat Saleh, H.; Ngo Boum-Nkot, S.; Nlend, B.; Djebebe-Ndjiguim, C.-L.; Foto, E.; Sanoussi, R.; Araguas-Araguas, L.; et al. Shallow Quaternary groundwater in the Lake Chad basin is resilient to climate change but requires sustainable management strategy: Results of isotopic investigation. Sci. Total Environ. 2022, 851, 158152. [Google Scholar] [CrossRef]
- Leduc, C.; Favreau, G.; Schroeter, P. Long-Term Rise in a Sahelian Water-Table: The Continental Terminal in South-West Niger. J. Hydrol. 2001, 243, 43–54. [Google Scholar] [CrossRef]
- Landwehr, J.; Coplen, T.B. Line-Conditioned Excess: A New Method for Characterizing Stable Hydrogen and Oxygen Isotope Ratios in Hydrologic Systems. In Proceedings of the International Conference on Isotopes in Environmental Studies, Vienna, Austria, 25–29 October 2006; pp. 132–135. [Google Scholar]
- Yahouza, L.; Issoufou, S.; Abdou Babaye, M.S.; Métral, B.; Ousmane, B. Contribution of Stable Isotopes of Water (18O and 2H) to the Characterization of Goulbi N’kaba Valley Aquifer, Region of Maradi in the Republic of Niger. Int. J. Hydrol. 2018, 2, 560–565. [Google Scholar] [CrossRef]
- Clark, I.; Fritz, P. Environmental Isotopes in Hydrogeology Lewis; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar] [CrossRef]
- Modibo Sidibé, A.; Lin, X.; Koné, S. Assessing Groundwater Mineralization Process, Quality, and Isotopic Recharge Origin in the Sahel Region in Africa. Water 2019, 11, 789. [Google Scholar] [CrossRef]
- Santoni, S.; Garel, E.; Gillon, M.; Marc, V.; Miller, J.; Babic, M.; Simler, R.; Travi, Y.; Leblanc, M.; Huneau, F. Assessing the Hydrogeological Resilience of a Groundwater-Dependent Mediterranean Peatland: Impact of Global Change and Role of Water Management Strategies. Sci. Total Environ. 2021, 768, 144721. [Google Scholar] [CrossRef]
- Mahlangu, S.; Lorentz, S.; Diamond, R.; Dippenaar, M. Surface Water-Groundwater Interaction Using Tritium and Stable Water Isotopes: A Case Study of Middelburg, South Africa. J. Afr. Earth Sci. 2020, 171, 103886. [Google Scholar] [CrossRef]
- Djebebe-Ndjiguim, C.-L. Définition du Potentiel Aquifère du Sous-Sol de la Région de Bangui (République Centrafricaine) à l’Aide d’Outils Géochimiques et Isotopiques. Aide à La Mise En Oeuvre d’un Plan de Diversification Des Ressources En Eau Potable à Partir Des Eaux Souterraines. Ph.D. Thesis, Corte, France, 12 December 2014. Available online: http://www.theses.fr/2014CORT0013 (accessed on 14 July 2022).
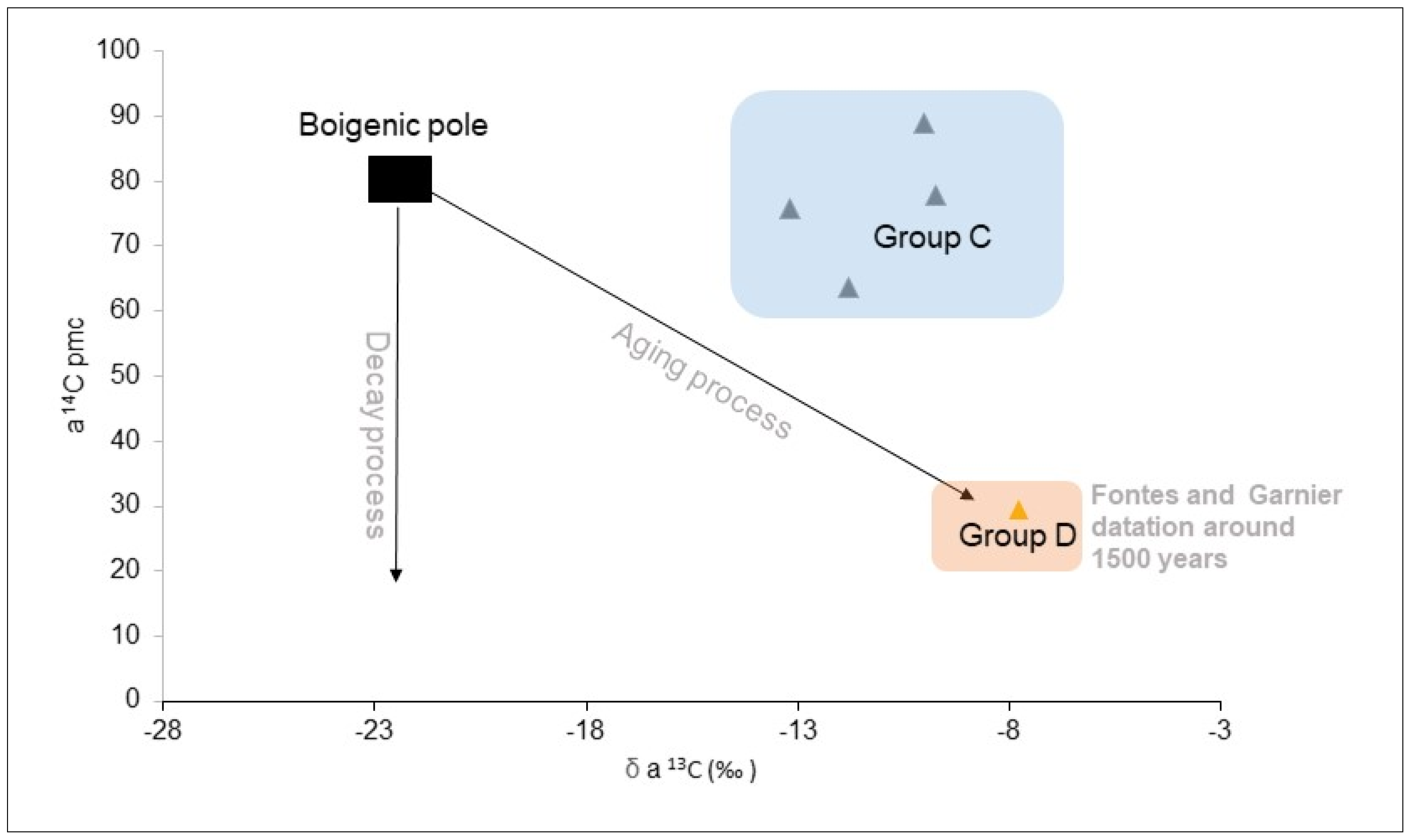
- Fontes, J.-C.; Garnier, J.-M. Determination of the Initial 14C Activity of the Total Dissolved Carbon: A Review of the Existing Models and a New Approach. Water Resour. Res. 1979, 15, 399–413. [Google Scholar] [CrossRef]
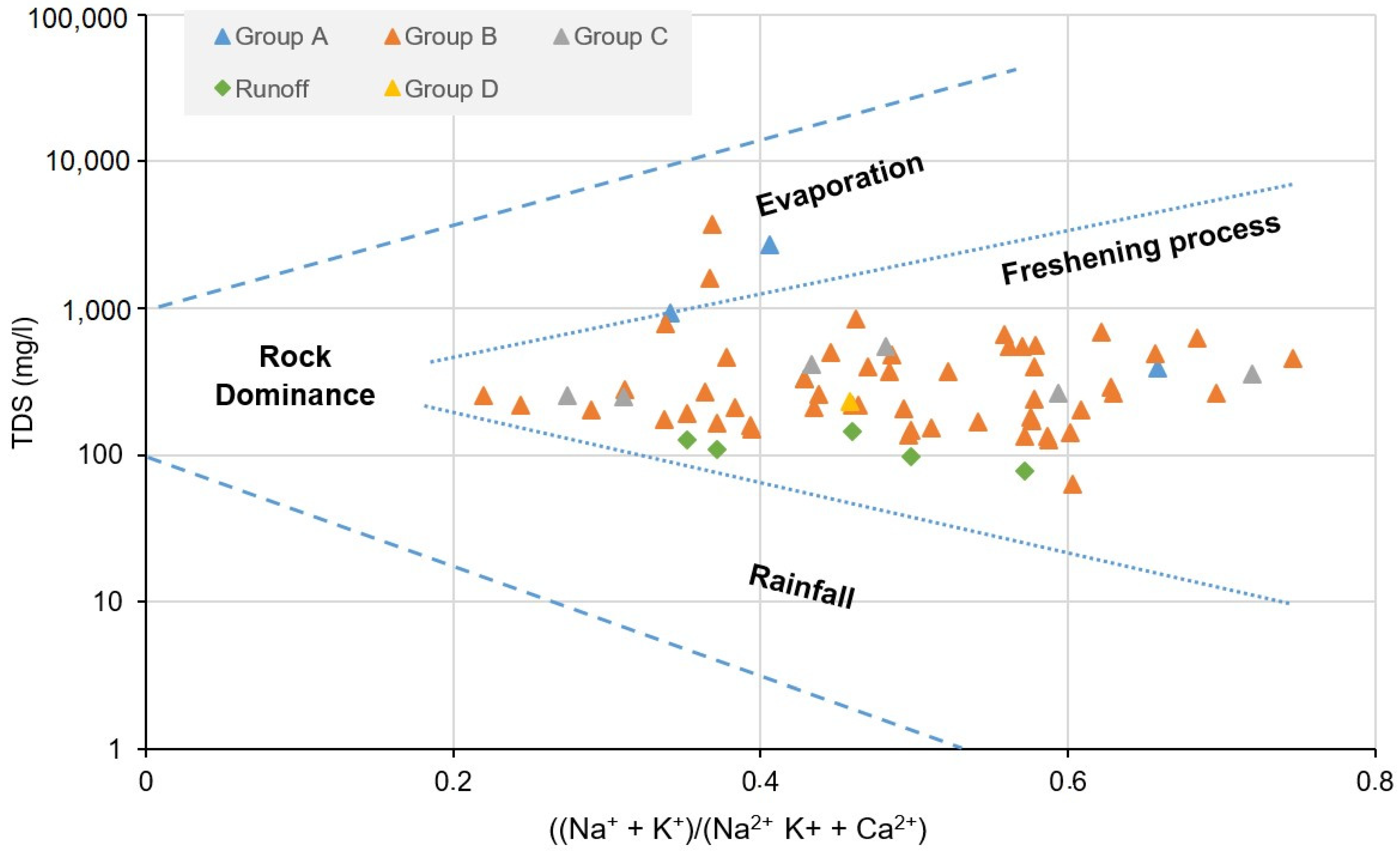
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Shand, P. Natural Groundwater Quality; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; ISBN 978-14051-5672-32. [Google Scholar]
- Sellerino, M.; Forte, G.; Ducci, D. Identification of the Natural Background Levels in the Phlaegrean Fields Groundwater Body (Southern Italy). J. Geochem. Explor. 2019, 200, 181–192. [Google Scholar] [CrossRef]
- Preziosi, E.; Giuliano, G.; Vivona, R. Natural Background Levels and Threshold Values Derivation for Naturally As, V and F Rich Groundwater Bodies: A Methodological Case Study in Central Italy. Environ. Earth Sci. 2010, 61, 885–897. [Google Scholar] [CrossRef]
- Rahman, A.; Mondal, N.C.; Tiwari, K.K. Anthropogenic Nitrate in Groundwater and Its Health Risks in the View of Background Concentration in a Semi-Arid Area of Rajasthan, India. Sci. Rep. 2021, 11, 9279. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.; Zhang, Y.; Liu, C. Groundwater recharge and hydrogeochemical evolution in the Ejina Basin, northwest China. J. Hydrol. 2013, 476, 72–86. [Google Scholar] [CrossRef]
- Cheo, A.E.; Voigt, H.-J.; Wendland, F. Modeling groundwater recharge through rainfall in the Far-North region of Cameroon. Groundw. Sustain. Dev. 2017, 5, 118–130. [Google Scholar] [CrossRef]
- Dogramaci, S.; Skrzypek, G.; Dodson, W.; Grierson, P. Stable isotope and hydrochemical evolution of groundwater in the semi-arid Hamersley Basin of subtropical northwest Australia. J. Hydrol. 2012, 475, 281–293. [Google Scholar] [CrossRef]





| Unit | Specific Discharge (m2/h) | Transmissivity (m2/s) | Hydraulic Conductivity (m/s) | References |
|---|---|---|---|---|
| Quaternary | 1.06 | 0.04 × 10−2 | 4 × 10−4 | [11] |
| Metamorphic | 0.27 | 3.71 × 10−5 | - | [27] |
| Granitic | 0.46 | 6.06 × 10−5 | - | [27] |
| Volcanic | 0.82 | 7.48 × 10−6 | - | [27] |
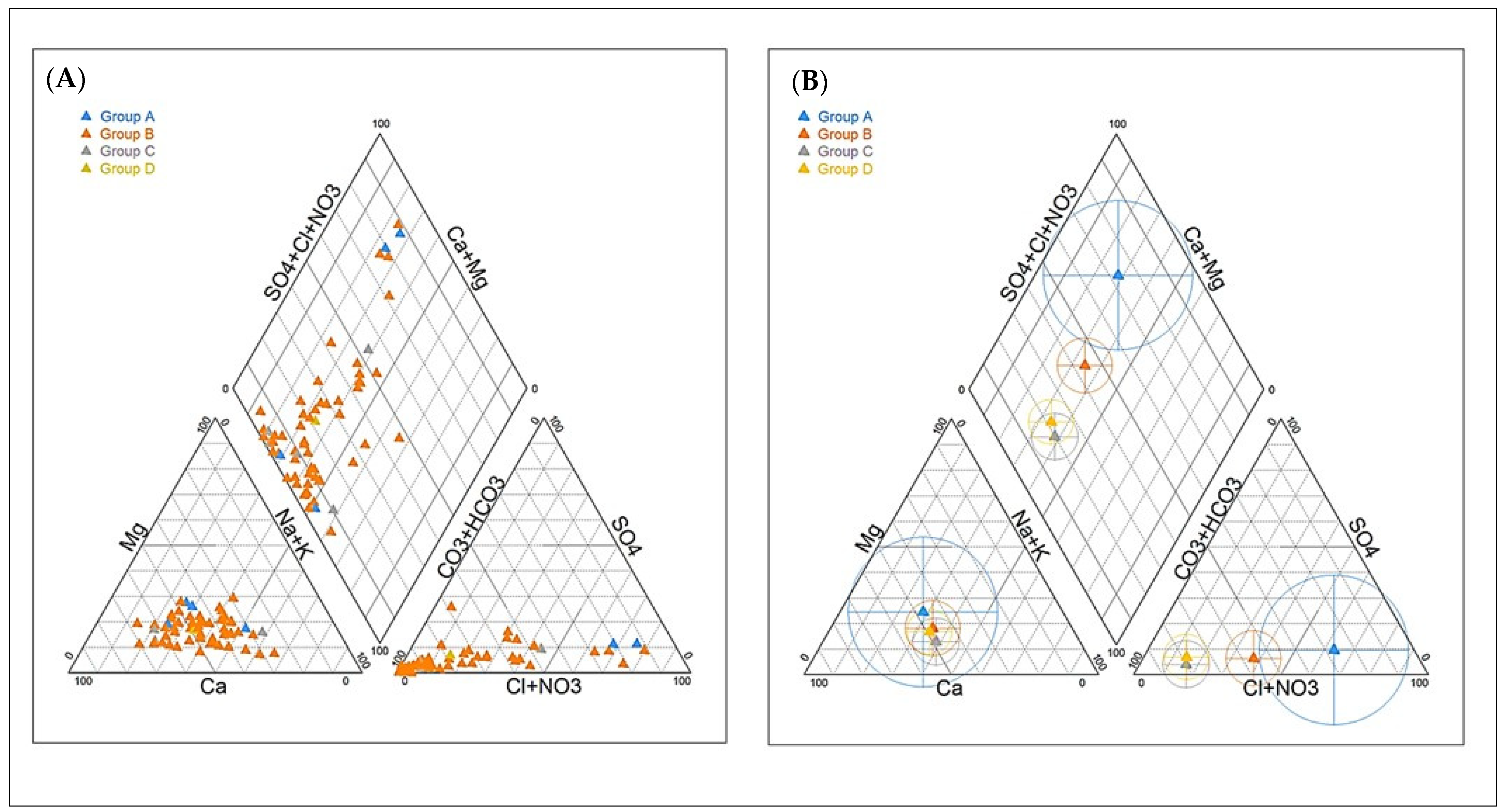
| Group A | Group B | Group C | Group D | Mayos | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean | Med | Max | Min | Mean | Med | Max | Min | Mean | Med | Max | F12 | Min | Mean | Med | Max | |
| pH | 6.6 | 7.0 | 7.0 | 7.3 | 6.2 | 7.0 | 6.8 | 8.4 | 6.6 | 7 | 7.0 | 7.2 | 6.8 | 6.8 | 7.5 | 7.5 | 8.4 |
| Redox | −30.8 | −9.7 | −11.5 | 14.7 | −89.4 | −7.1 | −20.0 | 37.2 | −25.1 | −6.3 | −9.2 | 23.1 | −7.7 | −89.4 | −26.2 | −22.9 | 13.4 |
| EC (µS/cm) | 468.1 | 1608.3 | 1119.7 | 3726.0 | 149.7 | 602.0 | 248.2 | 4769.0 | 328.1 | 519.1 | 473.1 | 757.5 | 462.3 | 152.9 | 221.5 | 221.6 | 291.9 |
| HCO3− (mg/L) | 158.4 | 243.9 | 262.3 | 292.8 | 73.2 | 180.5 | 118.9 | 378.2 | 176.5 | 211.4 | 195.2 | 280.6 | 207.4 | 91.5 | 113.4 | 115.9 | 140.3 |
| Cl− (mg/L) | 2.7 | 119.3 | 51.6 | 371.2 | 0.0 | 28.0 | 2.4 | 552.5 | 1.4 | 12.4 | 2.9 | 61.4 | 19.0 | 1.6 | 5.4 | 5.2 | 11.3 |
| NO3− (mg/L) | 0.0 | 368.9 | 178.5 | 1118.7 | 0.0 | 74.7 | 0.0 | 1520.5 | 0.0 | 22.3 | 10.5 | 93.5 | 6.2 | 0.0 | 5.0 | 0.3 | 14.8 |
| SO42− (mg/L) | 2.2 | 68.7 | 35.0 | 202.5 | 0.0 | 16.9 | 2.0 | 209.5 | 0.0 | 8.3 | 4.0 | 32.4 | 14.3 | 1.2 | 4.2 | 2.5 | 11.6 |
| Na+ (mg/L) | 30.2 | 94.7 | 63.7 | 221.2 | 9.1 | 40.7 | 14.4 | 248.5 | 14.7 | 39.4 | 39.5 | 65.6 | 33.6 | 9.1 | 12.9 | 11.4 | 18.8 |
| K+ (mg/L) | 0.0 | 8.1 | 4.4 | 23.7 | 0.0 | 6.1 | 1.8 | 101.5 | 0.0 | 1.6 | 1.2 | 3.8 | 4.2 | 3.9 | 5.3 | 5.1 | 7.3 |
| Mg2+ (mg/L) | 10.3 | 45.7 | 22.0 | 128.5 | 2.1 | 12.2 | 3.8 | 141.5 | 4.7 | 7.7 | 7.3 | 12.1 | 9.1 | 2.1 | 3.5 | 3.4 | 4.9 |
| Ca2+ (mg/L) | 30.5 | 144.8 | 94.9 | 358.7 | 12.1 | 52.7 | 21.7 | 600.5 | 24.5 | 43.9 | 41.5 | 73.3 | 44.8 | 14.1 | 22.7 | 24.2 | 28.8 |
| TDS (mg/L) | 333.5 | 1094.3 | 663.2 | 2717.5 | 126.5 | 412.1 | 178.1 | 3728.3 | 248.7 | 347.3 | 310.9 | 548.4 | 338.7 | 134.3 | 170.8 | 163.7 | 217.3 |
| δ 2H (‰) | −12.40 | −10.04 | −10.10 | −7.54 | −36.37 | −23.11 | −26.22 | −10.57 | −32.05 | −29.01 | −28.78 | −24.44 | −43.16 | −20.87 | −15.80 | −14.63 | −12.66 |
| δ 18O (‰) | −2.35 | −2.11 | −2.14 | −1.79 | −5.45 | −3.65 | −4.17 | −1.15 | −4.84 | −4.45 | −4.58 | −3.80 | −5.69 | −2.74 | −1.88 | −1.56 | −1.15 |
| 3H (TU) | 3 ± 0.3 | 4.8 ± 0.3 | 4.8 ± 0.3 | 6.1 ± 0.3 | 1.2 ± 0.3 | 3.2 ± 0.3 | 2.5 ± 0.3 | 6.1 ± 0.2 | 0.4 ± 0.3 | 1.9 ± 0.3 | 1.8 ± 0.3 | 4.5 ± 0.3 | 0.2 ± 0.3 | 5.1 ± 0.2 | 5.6 ± 0.3 | 5.6 ± 0.3 | 6.1 ± 0.3 |
| d-excess | 6.40 | 6.81 | 6.70 | 7.43 | −4.68 | 6.12 | 5.29 | 9.68 | 2.36 | 6.59 | 6.62 | 9.87 | 2.36 | −4.68 | −1.93 | −1.94 | 3.76 |
| LC-excess | −1.80 | −1.00 | −0.80 | −0.52 | −10.86 | −4.23 | −5.61 | −0.61 | −8.24 | −5.15 | −5.43 | −1.92 | −11.46 | −10.88 | −9.01 | −8.48 | −4.87 |
| Parameters | Ref | ABL | TV | Number of Samples above the ABL (%) | Number of Samples above the TV (%) | |||
|---|---|---|---|---|---|---|---|---|
| Max | Min | Shallow Ground Water (Groups A and B) | Deep Groundwater (Groups C and D) | Shallow Water (Groups A and B) | Deep Water (Groups C and D) | |||
| EC (µS/cm) | 250 | 597.3 | 516.1 | 250 | 33.9 | 28.5 | 73.2 | 28.5 |
| HCO3− (mg/L) | 200 | 280. | 203.3 | 200 | 8.9 | 0 | 57.1 | 37.5 |
| Cl− (mg/L) | 250 | 11.3 | 4.8 | 130 | 33.9 | 14.2 | 5.3 | 0 |
| NO3− (mg/L) | 50 | 8.2 | 4.4 | 27 | 44.6 | 25.1 | 35.7 | 14.2 |
| SO42− (mg/L) | 250 | 10.8 | 4.9 | 130 | 32.1 | 28.5 | 3.5 | 0 |
| Na+ (mg/L) | 200 | 51.0 | 38.5 | 125 | 30.3 | 28.5 | 5.3 | 0 |
| K+ (mg/L) | 100 | 20.7 | 10.4 | 60 | 5.3 | 0 | 1.7 | 0 |
| Mg2+ (mg/L) | 30 | 12.7 | 8.3 | 21 | 26.7 | 0 | 14.2 | 0 |
| Ca2+ (mg/L) | 75 | 47.3 | 40.5 | 61 | 30.3 | 28.5 | 16.0 | 14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.; Nlend, B.; Boum-Nkot, S.N.; Huneau, F.; Ndondo, G.N.; Garel, E.; Leydier, T.; Celle, H.; Djieugoue, B.; Ntamak-Nida, M.-J.; et al. Groundwater Resources of the Transboundary Quaternary Aquifer of the Lake Chad Basin: Towards a Better Management via Isotope Hydrology. Resources 2023, 12, 138. https://doi.org/10.3390/resources12120138
Song F, Nlend B, Boum-Nkot SN, Huneau F, Ndondo GN, Garel E, Leydier T, Celle H, Djieugoue B, Ntamak-Nida M-J, et al. Groundwater Resources of the Transboundary Quaternary Aquifer of the Lake Chad Basin: Towards a Better Management via Isotope Hydrology. Resources. 2023; 12(12):138. https://doi.org/10.3390/resources12120138
Chicago/Turabian StyleSong, Fricelle, Bertil Nlend, Suzanne Ngo Boum-Nkot, Frederic Huneau, Gustave Nkoue Ndondo, Emilie Garel, Thomas Leydier, Helene Celle, Boris Djieugoue, Marie-Joseph Ntamak-Nida, and et al. 2023. "Groundwater Resources of the Transboundary Quaternary Aquifer of the Lake Chad Basin: Towards a Better Management via Isotope Hydrology" Resources 12, no. 12: 138. https://doi.org/10.3390/resources12120138






