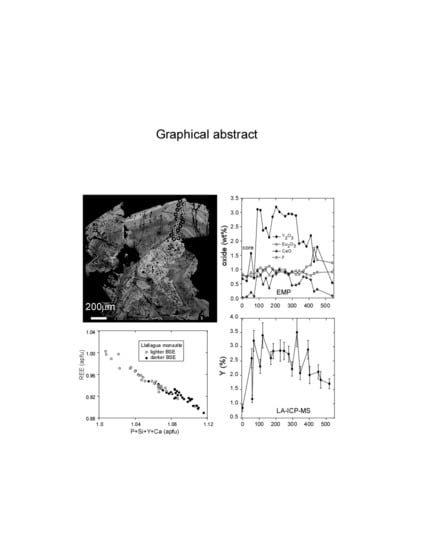
Speculations Linking Monazite Compositions to Origin: Llallagua Tin Ore Deposit (Bolivia)
Abstract
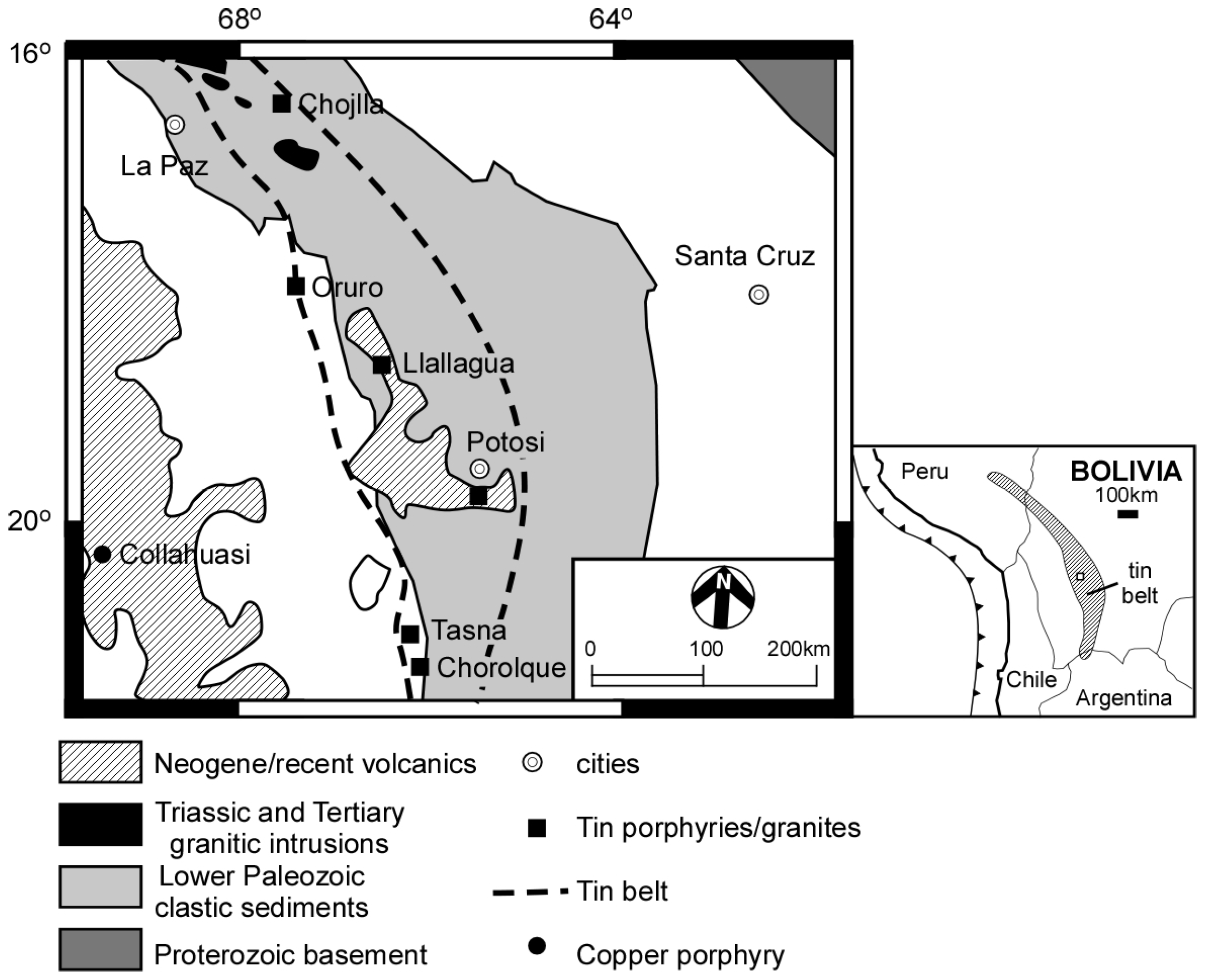
:1. Introduction
2. Analytical Approaches
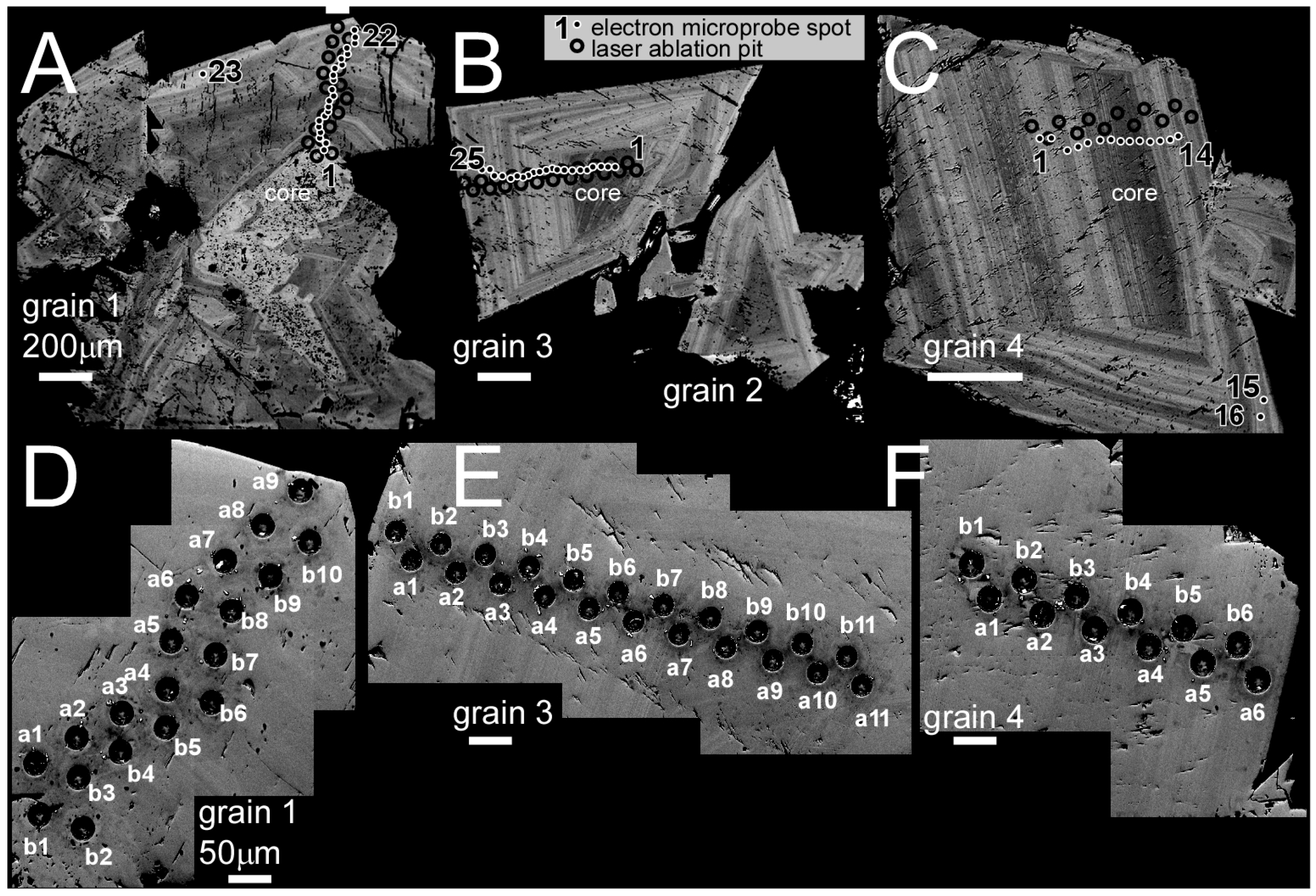
2.1. Sample Preparation and Imaging
2.2. Llallagua Monazite Electron Probe MicroAnalyses (EPMA)
2.3. Llallagua Monazite LA-ICP-MS Analyses
3. Results
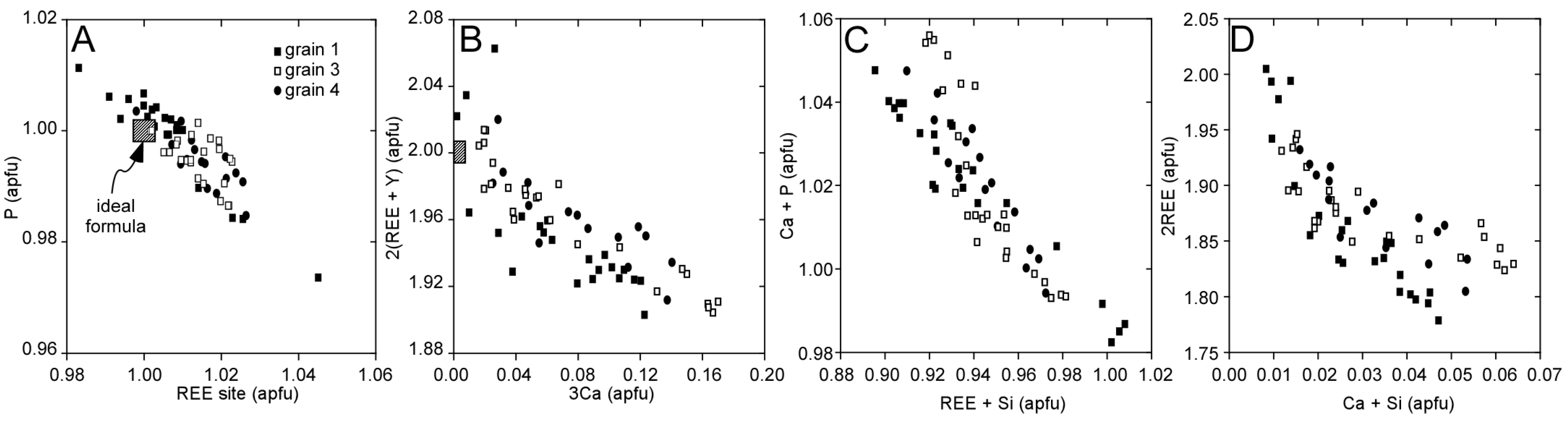
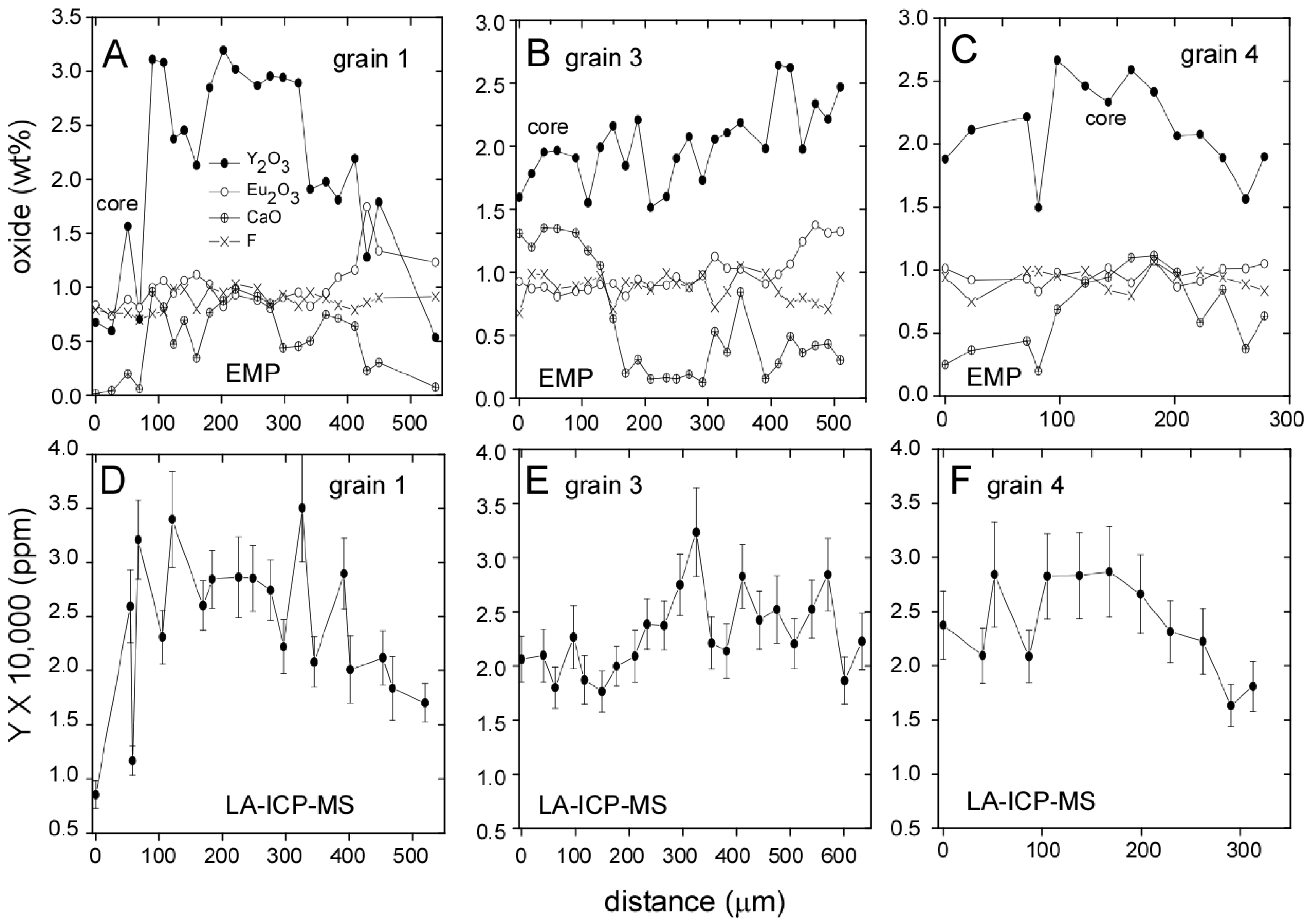
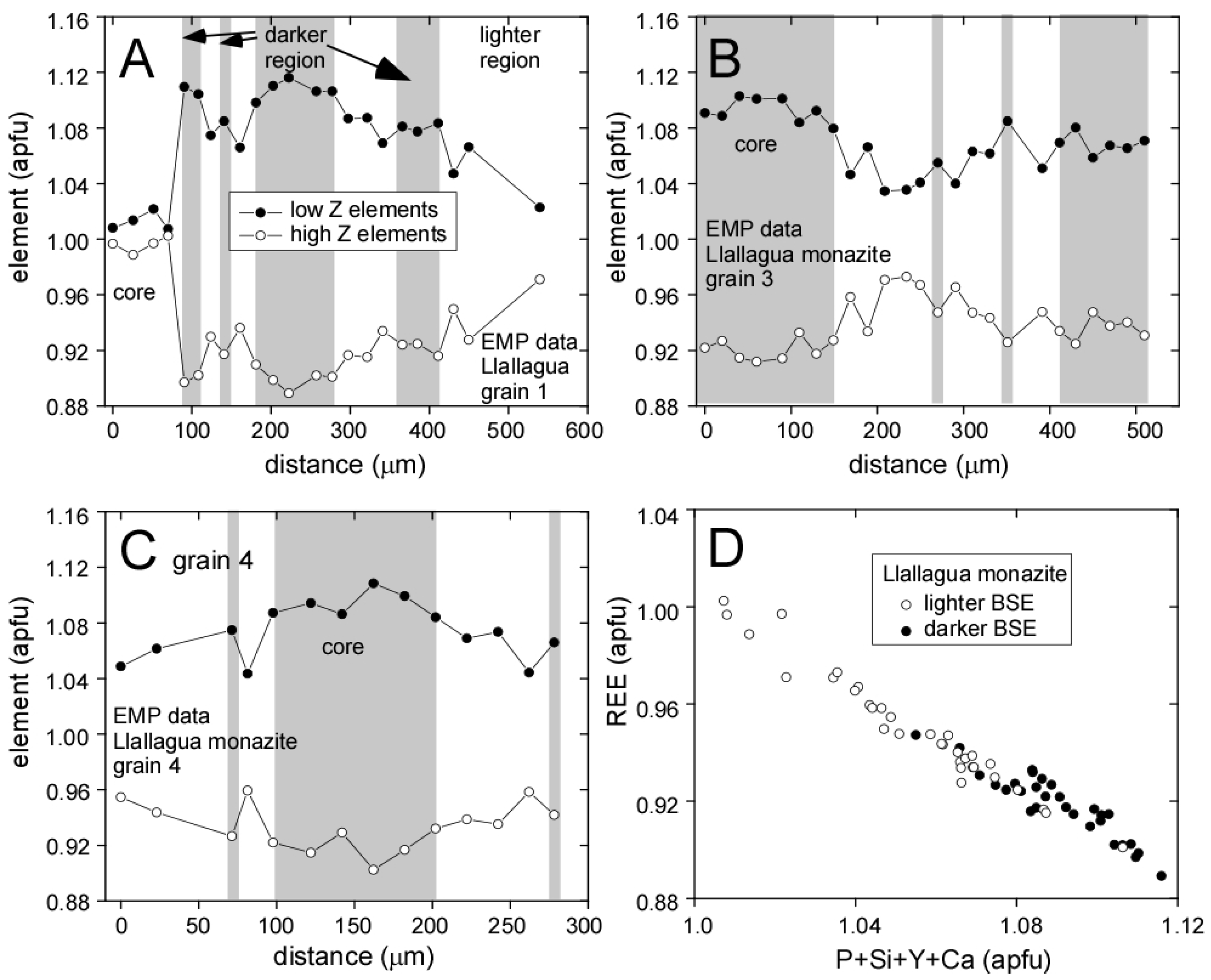
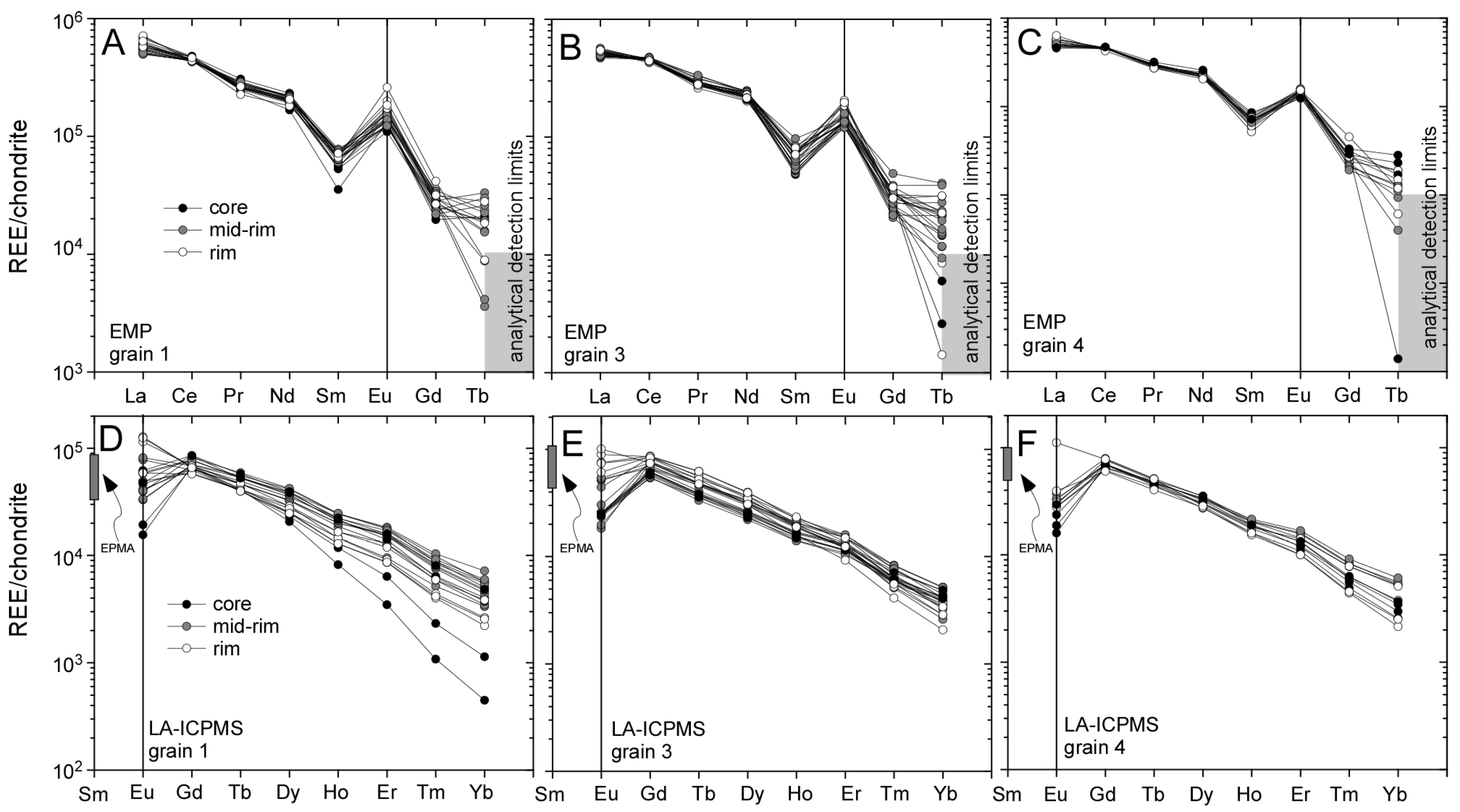
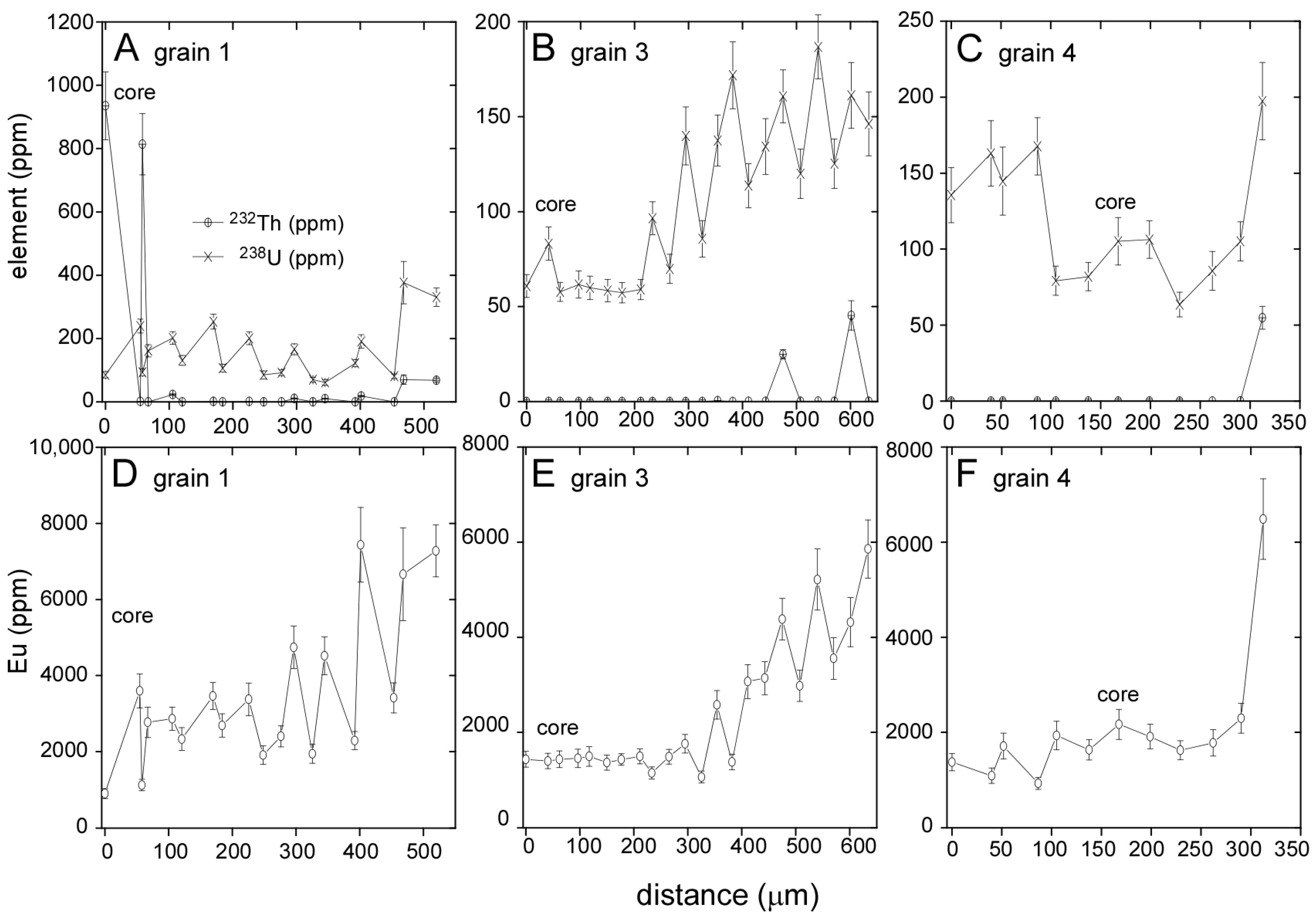
3.1. Llallagua Monazite EPMA
3.2. Llallagua Monazite LA-ICP-MS Analyses
4. Discussion and Conclusions
4.1. Evidence for Hydrothermal Origin
4.2. Insight from Compositional Data
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahlfeld, F. Zoning in the Bolivian Tin Belt. Econ. Geol. Bull. Soc. Econ. Geol. 1941, 36, 569–588. [Google Scholar] [CrossRef]
- Mlynarczyk, M.J.; Williams-Jones, A.E. The role of collisional tectonics in the metallogeny of the Central Andean tin belt. Earth Planet. Sci. Lett. 2005, 240, 656–667. [Google Scholar] [CrossRef]
- Ahfeld, F.; Schneider-Scherbina, A. Los Yacimientos Minerales y de Hidrocarburos de Bolivia; Ministry of Mines and Petroleum: La Paz, Bolivia, 1964; p. 388.
- Sillitoe, R.H.; Halls, C.C.; Grant, J.N. Porphyry tin deposits in Bolivia. Econ. Geol. Bull. Soc. Econ. Geol. 1975, 70, 913–927. [Google Scholar] [CrossRef]
- Bandy, M.C. Mineralogy of Llallagua, Bolivia. Tucson Gem and Mineral Society: Tucson, AZ, USA, 1976; p. 67. [Google Scholar]
- Hyrsl, J.; Petrov, A. Llallagua, Bolivia. Miner. Rec. 2006, 37, 117–162. [Google Scholar]
- Kempe, U.; Lehmann, B.; Wolf, D.; Rodionov, N.; Bombach, K.; Schwengfelder, U.; Dietrich, A. U/Pb SHRIMP geochronology of Th-poor, hydrothermal monazite: an example from the Llallagua tin porphyry deposit, Bolivia. Geochem. Cosmochim. Acta 2008, 72, 4352–4366. [Google Scholar] [CrossRef]
- Ahlfeld, F. The tin ores of Uncia-Llallagua, Bolivia. Econ. Geol. Bull. Soc. Econ. Geol. 1931, 26, 241–257. [Google Scholar] [CrossRef]
- Turneaure, F.S. The tin deposits of Llallagua, Bolivia. Econ. Geol. Bull. Soc. Econ. Geol. 1935, 30, 14–60. [Google Scholar] [CrossRef]
- Turneaure, F.S. A comparative study of major ore deposits of central Bolivia. Econ. Geol. Bull. Soc. Econ. Geol. 1960, 55, 217–254. [Google Scholar] [CrossRef]
- Kohn, M.J.; Vervoort, J.D. U-Th-Pb dating of monazite by single-collector ICP-MS: Pitfalls and potential. Geochem. Geophys. Geosys. 2008, 9, Q04031. [Google Scholar] [CrossRef]
- Hawkins, D.P.; Bowring, S.A. U-Pb systematics of monazite and xenotime: Case studies from the Paleoproterozoic of the Grand Canyon, Arizona. Contrib. Miner. Pet. 1997, 127, 87–103. [Google Scholar] [CrossRef]
- Crowley, J.L.; Brown, R.L.; Gervais, F.; Gibson, H.D. Assessing inheritance of zircon and monazite in granitic rocks from the Monashee complex Canadian cordillera. J. Pet. 2008, 49, 1915–1929. [Google Scholar] [CrossRef]
- Catlos, E.J. Generalizations about monazite: Implications for geochronologic studies. Am. Mineral. 2013, 98, 819–832. [Google Scholar] [CrossRef]
- Gordon, S.G. The mineralogy of the tin mines of Cerro de Llallagua, Bolivia. Proc. Acad. Nat. Sci. Phila. 1944, 96, 279–359. [Google Scholar]
- Long, K.; Ludington, S.; du Bray, E.; Andre-Ramos, O.; McKee, E.H. Geology and mineral deposits of the La Joya District, Bolivia. Soc. Econ. Geol. News 1992, 10, 13–16. [Google Scholar]
- Grant, J.N.; Halls, C.C.; Avila Salinas, W.W.; Snelling, N.J. K-Ar ages of igneous rocks and mineralization in part of the Bolivian tin belt. Econ. Geol. Bull. Soc. Econ. Geol. 1979, 74, 838–851. [Google Scholar] [CrossRef]
- Lehmann, B. Petrochemical factors governing the metallogeny of the Bolivian tin belt. In Tectonics of the Southern Central Andes, Structure and Evolution of an Active Continental Margin; Reutter, K.J., Scheuber, E., Wigger, P., Eds.; Springe: Berlin, Germany, 1994; pp. 317–326. [Google Scholar]
- Jimenez, N.; Lopez Velasquez, S. Magmatism in the Huarina Belt, Bolivia, and its geotectonic implications. Tectonophys 2008, 459, 85–106. [Google Scholar] [CrossRef]
- Gordon, S.G. Thorium-free monazite from Llallagua, Bolivia. In Academy of Natural Sciences, Philadelphia, Notulae Naturae; The Academy of Natural Sciences: Philadelphia, PA, USA, 1939; Volume 2, pp. 1–7. [Google Scholar]
- Corfu, F. Differential response of U-Pb systems in coexisting accessory minerals, Winnipeg River Subprovince, Canadian Shield: Implications for Archean crustal growth and stabilization. Contrib. Miner. Pet. 1988, 98, 312–325. [Google Scholar] [CrossRef]
- Parrish, R.R. U-Pb dating of monazite and its application to geological problems. Can. J. Earth Sci. 1990, 27, 1431–1450. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.; Paquette, J.; Wiedenbeck, M.; Montel, J.; Heinrich, W. Experimental resetting of the U-Th-Pb systems in monazite. Chem. Geol. 2002, 191, 165–181. [Google Scholar] [CrossRef]
- Rakovan, J.; McDaniel, D.K.; Reeder, R.J. Use of surface-controlled REE sectoral zoning in apatite from Llallagua, Bolivia, to determine a single-crystal Sm-Nd age. Earth Planet. Sci. Lett. 1997, 146, 329–336. [Google Scholar] [CrossRef]
- Wolf, D.; Schwengfelder, U.; Bombach, K.; Kempe, U.; Lehmann, B.; Dietrich, A. Pb/Pb single zircon evaporation dating at the Sn porphyry of Llallagua, Bolivia. Terra Nostra 2003, 2, 87. [Google Scholar]
- Dominy, S.C.; Annels, A.E.; Camm, G.; Wheeler, P.; Barr, S. Geology in the resource and reserve estimation of narrow vein deposits. Explor. Min. Geol. 1997, 6, 317–333. [Google Scholar]
- Dominy, S.C.; Camm, G.S. Tin mineralization in south-west England: Nature of veins and controls on ore localization. Proc. Ussher Soc. 1997, 9, 19. [Google Scholar]
- Catlos, E.J.; Miller, N.R. Ion microprobe 208Th-208Pb ages from high common Pb monazite, Morefield Mine, Amelia County, Virginia: Implications for Alleghanian tectonics. Am. J. Sci. 2016, 316, 470–503. [Google Scholar] [CrossRef]
- Montel, J.; Foret, S.; Veschambre, M.; Nicollet, C.; Provost, A. Electron microprobe dating of monazite. Chem. Geol. 1996, 131, 37–53. [Google Scholar] [CrossRef]
- Hetherington, C.J.; Jercinovic, M.J.; Williams, M.L.; Mahan, K. Understanding geologic processes with xenotime: composition, chronology, and a protocol for electron probe microanalysis. Chem. Geol. 2008, 254, 133–147. [Google Scholar] [CrossRef]
- Young, E.; Myers, A.; Munson, E.; Conklin, N. Mineralogy and geochemistry of fluorapatite from Cerro de Mercado, Durango, Mexico. US Geol. Surv. Prof. Pap. 1969, 650, D84–D93. [Google Scholar]
- Longerich, H.P.; Jackson, S.E.; Günther, D. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. Atom. Spectr. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stacke, A.; Birbaum, K.; Frick, D.A.; Gunther, D.; Enzweiler, J. Determination of reference values for NIST SRM 610—617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- D’Oriano, C.; Da Pelo, S.; Podda, F.; Cioni, R. Laser-ablation inductively coupled plasma mass spectrometry (LA-ICP-MS): setting operating conditions and instrumental performance. Period. Di Miner. 2008, 77, 65–74. [Google Scholar]
- Xu, P.; Guan, H.; Sun, M.; Malps, J. Methodology of trace element in situ analyses using laser ablation inductively coupled plasma mass spectrometry. Yanshi Xuebao Acta Petrol. Sin. 2000, 16, 291–304. [Google Scholar]
- Hellstrom, J.C.; Paton, C.; Woodhead, J.D.; Hergt, J. Iolite: Software for spatially resolved LA-(quad and MC) ICPMS analysis. Miner. Soc. Can. Short Course 2008, 40, 343–348. [Google Scholar]
- Jochum, K.P.; Stoll, B.; Weis, U.; Jacob, D.E.; Mertz Kraus, R.; Andreae, M.O. Non-matrix-matched calibration for the multi-element analysis of geological and environmental samples using 200 nm Femtosecond LA-ICP-MS: A comparison with nanosecond lasers. Geostand. Geoanal. Res. 2014, 38, 265–292. [Google Scholar] [CrossRef]
- Della Ventura, G.; Mottana, A.; Parodi, G.C.; Raudsepp, M.; Bellatreccia, F.; Caprilli, E.; Rossi, P.; Fiori, S. Monazite-huttonite solid-solutions from the Vico volcanic complex, Latium, Italy. Mineral. Mag. 1996, 60, 751–758. [Google Scholar] [CrossRef]
- Ronsbo, J.G. Coupled substitutions involving REEs and Na and Si in apatites in alkaline rocks from the Ilimaussaq intrusion, South Greenland, and the petrologic implications. Am. Mineral. 1989, 74, 896–901. [Google Scholar]
- Stormer, J.C.; Carmichael, I.E. Fluorine-hydroxyl exchange in apatite and biotite: A potential igneous geothermometer. Contrib. Miner. Petrol. 1971, 31, 121–131. [Google Scholar] [CrossRef]
- Smith, M.P.; Yardley, B.W.D. Fluid evolution during metamorphism of the Otago schist, New Zealand: (II) Influence of detrital apatite on fluid salinity. J. Metamorph. Geol. 1999, 17, 187–193. [Google Scholar] [CrossRef]
- Pyle, J.M.; Spear, F.S.; Wark, D.A. Electron microprobe analysis of REE in apatite, monazite and xenotime: Protocols and pitfalls. Rev. Miner. Geochem. 2002, 48, 337–362. [Google Scholar] [CrossRef]
- Roeder, P.L.; MacArthur, D.; Ma, X.; Palmer, G.R.; Mariano, A.N. Cathodoluminescence and microprobe study of rare-earth elements in apatite. Am. Mineral. 1987, 72, 801–811. [Google Scholar]
- Bowins, R.J.; Crocket, J.H. Monazite, xenotime and REE minerals in Archean banded iron-formation from the Sherman and Adams mines, Ontario, Canada. Can. Mineral. 2011, 49, 749–763. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and isotopic systematic of ocean basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Special Pub. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Mohr, D.W. Zoned porphyroblasts of metamorphic monazite in the Anakeesta Formation, Great Smoky Mountains, North Carolina. Am. Mineral. 1984, 69, 98–103. [Google Scholar]
- Spear, F.S.; Pyle, J.M. Apatite, monazite, and xenotime in metamorphic rocks. Rev. Miner. Geochem. 2002, 48, 293–335. [Google Scholar] [CrossRef]
- Uher, P.; Ondrejka, M.; Broska, I. S and As in accessory monazite: A role of “clinoanhydrite” and gasparite substitution. In Proceedings of the European Geosciences Union, Research Abstracts, Vienna, Austria, 15–20 April 2007; Volume 9, p. 09146. [Google Scholar]
- Betkowski, W.B.; Harlov, D.E.; Rakovan, J. Hydrothermal mineral replacement reactions for an apatite-monazite assemblage in alkali-rich fluids. In Proceedings of the Goldschmidt Conference, Sacramento, CA, USA, 8–13 June 2014; p. 193. [Google Scholar]
- Schandl, E.S.; Gorton, M. A textural and geochemical guide to the identification of hydrothermal monazite: Criteria for selection of samples for dating epigenetic hydrothermal ore deposits. Econ. Geol. Bull. Soc. Econ. Geol. 2004, 99, 1027–1035. [Google Scholar] [CrossRef]
- Cabella, R.; Lucchetti, G.; Marescotti, P. Authigenic monazite and xenotime from pelitic metacherts in pumpellyite-actinolite-facies conditions, Sestri-Voltaggio zone, central Liguria, Italy. Can. Mineral. 2001, 39, 717–727. [Google Scholar] [CrossRef]
- Čopjaková, R.; Novak, M.; Francu, E. Formation of authigenic monazite-(Ce) to monazite-(Nd) from upper carboniferous graywackes of the drahany upland: Roles of the chemical composition of host rock and burial temperature. Lithos 2011, 127, 373–385. [Google Scholar] [CrossRef]
- Alipour-Asll, M.M.; Mirnejad, H.H.; Milodowski, A.E. Occurrence and paragenesis of diagenetic monazite in the Upper Triassic black shales of the Marvast region, South Yazd, Iran. Miner. Petrol. 2012, 104, 197–210. [Google Scholar] [CrossRef]
- Dahl, P.S.; Terry, M.P.; Jercinovic, M.J.; Williams, M.L.; Hamilton, M.A.; Foland, K.A.; Clement, S.M.; Friberg, L.M. Electron probe (Ultrachron) microchronometry of metamorphic monazite: Unraveling the timing of polyphase thermotectonism in the easternmost Wyoming Craton (Black Hills, South Dakota). Am. Mineral. 2005, 90, 1712–1728. [Google Scholar] [CrossRef]
- Gasser, D.; Bruand, E.; Rubatto, D.; Stuewe, K. The behaviour of monazite from greenschist facies phyllites to anatectic gneisses: An example from the Chugach metamorphic complex, southern Alaska. Lithos 2012, 134–135, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.; Muhling, J.R. Reactions destroying detrital monazite in greenschist facies sandstones from the Witwatersrand Basin, South Africa. Chem. Geol. 2009, 264, 311–327. [Google Scholar] [CrossRef]
- Torab, F.M.; Lehmann, B.B. Magnetite-apatite deposits of the Bafq district, central Iran: Apatite geochemistry and monazite geochronology. Mineral. Mag. 2007, 71, 347–363. [Google Scholar] [CrossRef]
- Mercadier, J.; Skirrow, R.G.; Cross, A.J. Uranium and gold deposits in the Pine Creek Orogen (North Australian Craton): a link at 1.8 Ga? Precamb. Res. 2013, 238, 111–119. [Google Scholar] [CrossRef]
- Pandey, M.; Pant, N.C.; Kumar, S. Criteria to distinguish between regional and contact zone monazite: A case study from Proterozoic north Delhi fold belt (NDFB), India. Episodes 2013, 36, 275–289. [Google Scholar]
- Wall, F.; Mariano, A.N. Rare earth minerals in carbonatites: A discussion centered on the Kangankunde Carbonatite, Malawi. In Rare Earth Minerals: Chemistry, Origin and ore Deposits; Jones, A.P., Wall, F., Williams, T.C., Eds.; Chapman and Hall, Mineralogical Society Series: London, UK, 1996; pp. 193–225. [Google Scholar]
- Cressey, G.G.; Wall, F.; Cressey, B.A. Differential REE uptake by sector growth of monazite. Mineral. Mag. 1999, 63, 813–828. [Google Scholar] [CrossRef]
- Pilipiuk, A.N.; Ivanikov, V.V.; Bulakh, A.G. Unusual rocks and mineralisation in a new carbonatite complex at Kandaguba, Kola Peninsula, Russia. Lithos 2001, 56, 333–347. [Google Scholar] [CrossRef]
- Catlos, E.J.; Dubey, C.S. Sivasubramanian, Monazite ages from carbonatites and high-grade assemblages along the Kambam Fault Southern Granulite Terrain, South India. Am. Mineral. 2008, 93, 1230–1244. [Google Scholar] [CrossRef]
- Shore, M.; Fowler, A.D. Oscillatory zoning in minerals: A common phenomenon. Can. Mineral. 1996, 34, 1111–1126. [Google Scholar]
- Schaltegger, U.; Fanning, C.M.; Günther, D.; Maurin, J.C.; Schulmann, K.; Gebauer, D. Growth, annealing and recrystallization of zircon and preservation of monazite in high-grade metamorphism: Conventional and in situ U–Pb isotope, cathodoluminescence and microchemical evidence. Contrib. Mineral. Petrol. 1999, 134, 186–201. [Google Scholar] [CrossRef]
- Couëslan, C.G.; Pattison, D.M.; Dufrane, S. Paleoproterozoic metamorphic and deformation history of the Thompson nickel belt, Superior boundary zone, Canada, from in situ U-Pb analysis of monazite. Precamb. Res. 2013, 237, 13–35. [Google Scholar] [CrossRef]
- Hetherington, C.J.; Harlov, D.E. Metasomatic thorite and uraninite inclusions in xenotime and monazite from granitic pegmatites, Hidra anorthosite massif, southwestern Norway: Mechanics and fluid chemistry. Am. Mineral. 2008, 93, 806–820. [Google Scholar] [CrossRef]
- Sheard, E.R.; Williams-Jones, A.E.; Heiligmann, M.; Pederson, C.; Trueman, D.L. Controls on the concentration of zirconium, niobium, and the rare earth elements in the Thor Lake rare metal deposit, Northwest Territories, Canada. Econ. Geol. Bull. Soc. Econ. Geol. 2012, 107, 81–104. [Google Scholar] [CrossRef]
- Kucha, H. Continuity in the monazite-huttonite series. Mineral. Mag. 1980, 43, 1031–1034. [Google Scholar] [CrossRef]
- Watt, G.R. High-thorium monazite-(Ce) formed during disequilibrium melting of metapelites under granulite-facies conditions. Mineral. Mag. 1995, 59, 735–743. [Google Scholar] [CrossRef]
- Keppler, H. Influence of fluorine on the enrichment of high field strength trace elements in granitic rocks. Contrib. Mineral. Petrol. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Papoutsa, A.D.; Pe-Piper, G. The relationship between REE-Y-Nb-Th minerals and the evolution of an A-type granite, Wentworth Pluton, Nova Scotia. Am. Mineral. 2013, 98, 444–462. [Google Scholar] [CrossRef]
- Tropper, P.P.; Harlov, D.E.; Manning, C.E. Ce-monazite and Y-xenotime solubilities in H2O-NaF at 800 °C, 1 GPa: Implications for REE transport. Mineral. Mag. 2013, 77, 2358. [Google Scholar]
- Duc-Tin, Q.; Keppler, H. Monazite and xenotime solubility in granitic melts and the origin of the lanthanide tetrad effect. Contrib. Mineral. Petrol. 2015, 169. [Google Scholar] [CrossRef]
- Didier, A.A.; Bosse, V.V.; Boulvais, P.P.; Bouloton, J.J.; Paquette, J.L.; Montel, J.M.; Devidal, J.L. Disturbance versus preservation of U-Th-Pb ages in monazite during fluid-rock interaction: Textural, chemical and isotopic in situ study in microgranites (Velay Dome, France). Contrib. Mineral. Petrol. 2013, 165, 1051–1072. [Google Scholar] [CrossRef]
- Harlov, D.E.; Förster, H. Fluid-induced nucleation of (Y+REE)-phosphate minerals within apatite: Nature and experiment. Part II, Fluorapatite. Am. Mineral. 2003, 88, 1209–1229. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Fleet, M.E.; MacRae, N.D. Oriented monazite inclusions in apatite porphyroblasts from the Hemlo gold deposit, Ontario, Canada. Mineral. Mag. 1993, 57, 697–707. [Google Scholar] [CrossRef]
- Harlov, D.E.; Förster, H. High-grade fluid metasomatism on both a local and a regional scale: The Seward Peninsula, Alaska, and the Val Strona di Omegna, Ivrea-Verbano Zone, Northern Italy. Part II: Phosphate mineral chemistry. J. Petrol. 2002, 43, 801–824. [Google Scholar] [CrossRef]
- Harlov, D.E.; Andersson, U.B.; Förster, H.; Nystrom, J.; Dulski, P.; Broman, C. Apatite-monazite relations in the Kiirunavaara magnetite-apatite ore, northern Sweden. Chem. Geol. 2002, 191, 47–72. [Google Scholar] [CrossRef]
- Ziemann, M.A.; Förster, H.; Harlov, D.E.; Frei, D. Origin of fluorapatite-monazite assemblages in a metamorphosed, sillimanite-bearing pegmatoid, Reinbolt Hills, East Antarctica. Eur. J. Miner. 2005, 17, 567–579. [Google Scholar] [CrossRef]
- Finger, F.F.; Krenn, E.E. Three metamorphic monazite generations in a high-pressure rock from the Bohemian Massif and the potentially important role of apatite in stimulating polyphase monazite growth along a PT loop. Lithos 2007, 95, 103–115. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Davidson, G.J.; Mehrabi, B.; Meffre, S.; Ghazban, F. Significance of apatite REE depletion and monazite inclusions in the brecciated Se-Chahun iron oxide-apatite deposit, Bafq district, Iran: Insights from paragenesis and geochemistry. Chem. Geol. 2011, 281, 253–269. [Google Scholar] [CrossRef]
- Ayres, M.; Harris, N. REE fractionation and Nd-isotope disequilibrium during crustal anatexis: Constraints from Himalayan leucogranites. Chem. Geol. 1997, 139, 249–269. [Google Scholar] [CrossRef]
- Berger, M.; Braun, I. Pb-Pb dating of apatite by a stepwise dissolution technique. Chem. Geol. 1997, 142, 23–40. [Google Scholar] [CrossRef]
- Cawthorn, R.G. Rare earth element abundances in apatite in the Bushveld Complex: A consequence of the trapped liquid shift effect. Geology 2013, 41, 603–606. [Google Scholar] [CrossRef]
- Hyrsl, J.; Petrov, A. Pseudomorphs from Bolivia a review. Rocks Miner. 1998, 73, 410–414. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Yin, J.; Park, J. Chemistry and origin of monazites from carbonatite dikes in the Hongcheon-Jaeun district, Korea. J. Asian Earth Sci. 2005, 25, 57–67. [Google Scholar] [CrossRef]
- Dumond, G.; Williams, M.; Goncalves, P.; Jercinovic, M. Monazite as a monitor of melting, garnet growth, and feldspar recrystallization in continental lower crust: Athabasca granulite terrane, western Canadian Shield. In Proceedings of the Geological Society of America Annual Meeting, Houston, TX, USA, 5 October 2008; p. 206. [Google Scholar]
- Goncalves, P.; Trap, P.; Dumond, G.; Marquer, D.; Feybesse, J.; Paquette, J. Monazite as a monitor of melting in continental crust. In Proceedings of the Geological Society of America Annual Meeting, Minneapolis, MN, 9–12 October 2011; p. 330. [Google Scholar]
- Zhu, X.K.; O’Nions, R.K. Monazite chemical composition: Some implications for monazite geochronology. Contrib. Miner. Petrol. 1999, 137, 351–363. [Google Scholar] [CrossRef]
- Moeller, E. Eu anomalies in hydrothermal minerals: Kinetic versus thermodynamic interpretation. In Proceedings of the Ninth Quadrennial IAGOD Symposium, Beijing, China, 12–18 August 1994; Schweizerbart science publishers: Stuttgart, Germany, 1998; Volume 9, pp. 239–246. [Google Scholar]
- Wood, S.A. The geochemistry of rare earth elements and yttrium in geothermal waters. Special Pub. Soc. Econ. Geol. 2003, 10, 133–158. [Google Scholar]
- Bao, S.; Zhou, H.; Peng, X.; Ji, F.; Yao, H. Geochemistry of REE and yttrium in hydrothermal fluids from the Endeavour segment, Juan de Fuca Ridge. Geochem. J. 2008, 42, 359–370. [Google Scholar] [CrossRef]
- Schmidt, K.K.; Garbe-Schonberg, D.D.; Bau, M.M.; Koschinsky, A.A. Rare earth element distribution in >400 degrees C hot hydrothermal fluids from 5 degrees S, MAR: The role of anhydrite in controlling highly variable distribution patterns. Geochim. Cosmochim. Acta 2010, 74, 4058–4077. [Google Scholar] [CrossRef]
- Douville, E.; Charlou, J.L.; Oelkers, E.H.; Bienvenu, P.P.; Jove Colon, C.F.; Donval, J.P.; Fouquet, Y.; Prieur, D.; Appriou, P. The Rainbow Vent fluids (36°14′N, MAR): The influence of ultramafic rocks and phase separation on trace metal content in Mid-Atlantic Ridge hydrothermal fluids. Chem. Geol. 2002, 184, 37–48. [Google Scholar] [CrossRef]
- Xu, Y. Magnetically recoverable rare-metal-rich rutile and monazite in ore and tailings of the Climax and Henderson molybdenum mines. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 1992. [Google Scholar]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2004; p. 504. [Google Scholar]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States—A Summary of Domestic Deposits and a Global Perspective; USGS Scientific Investigations Report 2010-5220; USGS: Reston, VA, USA, 2010; p. 96.
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Minerals, mines, magnets (and more). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Lambert, I.; Miezitis, Y.; Mackowski, S.; McKay, A. Australia’s rare earth resources in global context. In International Geological Congress, Abstracts—Congres Geologique International, Resumes; International Geological Congress: Oslo, Norway, 2008; Volume 33, Abstract 1342645. [Google Scholar]
- Environmental Protection Agency. Rare Earth Elements: A Review of Production, Processing, Recycling, and Associated Environmental Issues; US Environmental Protection Agency: Washington, DC, USA, 2012; EPA600/R-12/572; p. 96.
- Gambogi, J. Rare earths. In U.S. Geological Survey Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2013; pp. 128–129. [Google Scholar]
- Palaparthi, J.; Chakrabarti, R.; Banerjee, D.; Guin, R.; Ghosal, S.; Agrahari, S.; Sengupta, D. Economically viable rare earth element deposits along beach placers of Andhra Pradesh, eastern coast of India. Arab J. Geosci. 2017, 10. [Google Scholar] [CrossRef]
- Pike, D.R. Thorium and rare earth bearing minerals in the Union of South Africa. In Proceedings of the United Nations International Conference on Peaceful Uses of Atomic Energy, Geneva, Switzerland, 1–13 September 1958; pp. 91–96. [Google Scholar]
- Young, E.J.; Sims, P.K. Petrography and Origin of Xenotime and Monazite Concentrations, Central City District, Colorado; U.S. Geological Survey Bulletin; USGS: Reston, VA, USA, 1961; pp. 273–299.
- Mariano, A.N.; Mariano, A.R. Rare earth mining and exploration in North America. Elements 2012, 8, 369–376. [Google Scholar] [CrossRef]
- LeBas, M.J.; Keller, J.J.; Kejie, T.; Wall, F.F.; Williams, C.T.; Zhang, P. Carbonatite dykes at bayan Obo, inner Mongolia, China. Miner. Pet. 1992, 46, 195–228. [Google Scholar] [CrossRef]
- Hutchinson, D.E.; Toussaint, L.F. Near-surface disposal of concentrated NORM wastes. Appl. Radiat. Isot. 1998, 49, 265–271. [Google Scholar] [CrossRef]
- Paschoa, A.S. Potential environmental and regulatory implications of naturally occurring radioactive materials (NORM). Appl. Radiat. Isot. 1998, 49, 189–196. [Google Scholar] [CrossRef]
- Padmanabhan, V.T. Radioactive minerals and private sector mining. Econ. Political Wkly. 2002, 37, 4365–4367. [Google Scholar]
- Paschoa, A.S.; Dias da Cunha, K. A critical look at NORM in the monazite cycle. In Proceedings of the American Institute of Physics Conference Proceedings, Rio de Janeiro, Brazil, 7–12 October 2007; pp. 119–123. [Google Scholar]

| Element(s) | Crystal | X-ray Line |
|---|---|---|
| P, S, Ca, Cl | PET | Kα |
| As, Ti, Fe, Mn | LIF | Kα |
| Al, Si, Mg, Na, F | TAP | Kα |
| Y | TAP | Lα |
| La, Ce, Nd, Eu, Tb | LIF | Lα |
| Pr, Sm, Gd | LIF | Lβ |
| Th | PET | Mα |
| U | PET | Mβ |
| Analysis | Grain 1 (n = 23) 2 | Grain 3 (n = 25) | Grain 4 (n = 16) | All Grains (n = 64) |
|---|---|---|---|---|
| P2O5 (wt %) 3 | 30.1 (0.7) 4 | 29.9 (0.4) | 29.9 (0.4) | 29.97 (0.51) |
| As2O5 | 0.02 (0.04) | 0.02 (0.03) | 0.04 (0.07) | 0.03 (0.05) |
| SiO2 | 0.151 (0.027) | 0.185 (0.024) | 0.182 (0.024) | 0.17 (0.03) |
| TiO2 | 0.010 (0.016) | 0.012 (0.018) | 0.006 (0.015) | 0.01 (0.02) |
| UO2 | 0.031 (0.043) | 0.033 (0.053) | 0.041 (0.035) | 0.03 (0.05) |
| Y2O3 | 2.1 (0.9) | 2.0 (0.3) | 2.1 (0.4) | 2.07 (0.59) |
| La2O3 | 16.1 (1.7) | 14.3 (0.7) | 14.5 (1.4) | 14.97 (1.54) |
| Ce2O3 | 32.2 (1.0) | 32.7 (0.8) | 32.7 (0.7) | 32.54 (0.87) |
| Pr2O3 | 2.97 (0.19) | 3.21 (0.20) | 3.23 (0.13) | 3.13 (0.22) |
| Nd2O3 | 11.2 (0.8) | 12.4 (0.7) | 12.3 (0.7) | 11.92 (0.91) |
| Sm2O3 | 1.15 (0.17) | 1.19 (0.22) | 1.22 (0.15) | 1.18 (0.19) |
| Eu2O3 | 1.01 (0.22) | 0.99 (0.16) | 0.97 (0.07) | 0.99 (0.17) |
| Gd2O3 | 0.67 (0.12) | 0.72 (0.15) | 0.64 (0.15) | 0.68 (0.14) |
| Tb2O3 | 0.06 (0.05) | 0.08 (0.05) | 0.05 (0.04) | 0.06 (0.05) |
| CaO | 0.53 (0.32) | 0.59 (0.45) | 0.63 (0.32) | 0.58 (0.37) |
| F | 0.87 (0.09) | 0.88 (0.11) | 0.92 (0.09) | 0.88 (0.10) |
| Cl | 0.06 (0.01) | 0.06 (0.01) | 0.06 (0.01) | 0.06 (0.01) |
| Total | 98.8 (0.9) | 98.9 (0.6) | 99.1 (0.5) | 98.90 (0.7) |
| P (apfu) 5 | 0.999 (0.009) | 0.995 (0.004) | 0.994 (0.005) | 0.996 (0.007) |
| Si | 0.006 (0.001) | 0.007 (0.001) | 0.007 (0.001) | 0.007 (0.001) |
| Y | 0.044 (0.018) | 0.042 (0.006) | 0.043 (0.007) | 0.043 (0.012) |
| La | 0.233 (0.026) | 0.207 (0.011) | 0.209 (0.021) | 0.217 (0.023) |
| Ce | 0.462 (0.019) | 0.471 (0.012) | 0.471 (0.009) | 0.468 (0.014) |
| Pr | 0.042 (0.003) | 0.046 (0.003) | 0.046 (0.002) | 0.045 (0.003) |
| Nd | 0.156 (0.011) | 0.174 (0.010) | 0.172 (0.010) | 0.167 (0.013) |
| Sm | 0.016 (0.002) | 0.016 (0.003) | 0.017 (0.002) | 0.016 (0.003) |
| Eu | 0.013 (0.003) | 0.013 (0.002) | 0.013 (0.001) | 0.013 (0.002) |
| Gd | 0.009 (0.001) | 0.009 (0.002) | 0.008 (0.002) | 0.009 (0.002) |
| Ca | 0.022 (0.013) | 0.025 (0.019) | 0.026 (0.013) | 0.024 (0.016) |
| F | 0.108 (0.011) | 0.109 (0.013) | 0.114 (0.011) | 0.110 (0.012) |
| Cl | 0.004 (0.001) | 0.004 (0.001) | 0.004 (0.001) | 0.004 (0.001) |
| Total | 2.01 (0.01) | 2.01 (0.01) | 2.01 (0.005) | 2.01 (0.005) |
| Isotope (ppm) 2 | Grain 1 (n = 19) 3 | Grain 3 (n = 22) | Grain 4 (n = 12) | All Grains (n = 56) |
|---|---|---|---|---|
| 75As | 173 (21) 4 | 207 (23) | 208 (27) | 195 (23) |
| 89Y | 24,119 (3008) | 22,946 (2596) | 23,808 (3348) | 23,562 (2929) |
| 153Eu | 3459 (507) | 2429 (318) | 2078 (339) | 2719 (400) |
| 157Gd | 14,344 (1774) | 14,445 (1601) | 14,291 (2012) | 14,374 (1763) |
| 159Tb | 1782 (210) | 1720 (191) | 1757 (248) | 1751 (212) |
| 163Dy | 8165 (982) | 7635 (826) | 8058 (1082) | 7903 (945) |
| 165Ho | 1038 (127) | 1030 (107) | 1057 (136) | 1037 (121) |
| 166Er | 2142 (261) | 2099 (213) | 2188 (289) | 2134 (249) |
| 169Tm | 167 (20) | 162 (16) | 169 (22) | 165 (19) |
| 172Yb | 682 (79) | 653 (65) | 686 (92) | 671 (77) |
| 175Lu | 59 (7) | 51 (5) | 55 (7) | 55 (6) |
| 204Pb | 5.2 (15.6) | 1.2 (12) | 3.0 (13) | 3.0 (14) |
| 208Pb | 0.5 (0.2) | 0.6 (0.2) | 0.3 (0.1) | 0.5 (0.2) |
| 232Th | 103 (33) | 3.3 (1.9) | 3.7 (1.9) | 39 (20) |
| 238U | 160 (22) | 107 (12) | 96 (13) | 123 (17) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catlos, E.J.; Miller, N.R. Speculations Linking Monazite Compositions to Origin: Llallagua Tin Ore Deposit (Bolivia). Resources 2017, 6, 36. https://doi.org/10.3390/resources6030036
Catlos EJ, Miller NR. Speculations Linking Monazite Compositions to Origin: Llallagua Tin Ore Deposit (Bolivia). Resources. 2017; 6(3):36. https://doi.org/10.3390/resources6030036
Chicago/Turabian StyleCatlos, Elizabeth J., and Nathan R. Miller. 2017. "Speculations Linking Monazite Compositions to Origin: Llallagua Tin Ore Deposit (Bolivia)" Resources 6, no. 3: 36. https://doi.org/10.3390/resources6030036