Liquid Crystal Gel Reduces Age Spots by Promoting Skin Turnover
Abstract
:1. Introduction

2. Experimental Section
2.1. Preparation of Creams
2.2. Animal Studies
2.2.1. Animals
2.2.2. Skin Treatments
2.2.3. Tissue Collection and Staining
2.3. Human Studies
2.3.1. Subjects
2.3.2. Skin Treatments
2.3.3. Skin Pigmentation Measurements
2.3.4. Trans-Epidermal Water Loss (TEWL)
2.3.5. Tape-Stripping and Staining
2.4. Statistical Analysis
3. Results and Discussion
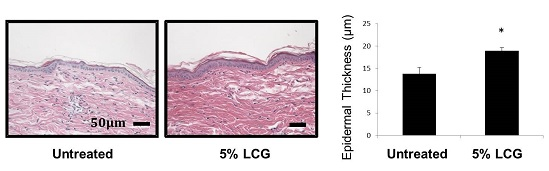
3.1. Low Concentration Liquid Crystal Gel (LCG) Thickens Epidermis in Mice


3.2. LCG Removes Spots on Human Skin

3.3. LCG Does Not Over-Stimulate Skin Turnover or Reduce Skin Barrier Function

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Madison, K.C. Barrier function of the skin: “La raison d'être” of the epidermis. J. Invest. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Nagasawa, T. New nanotechnology for the guided tissue regeneration of skin—Potential of lyotropic liquid crystals. Pharmazie 2006, 61, 112–116. [Google Scholar] [PubMed]
- Friberg, S.E. Micelles, microemulsions, liquid crystals, and the structure of stratum corneum lipids. J. Soc. Cosmet. Chem. 1990, 41, 155–171. [Google Scholar]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, H. Epidermal architecture that depends on turnover time. J. Dermatol. Sci. 1995, 10, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Nagasawa, T.; Nakamura, N.; Takenaga, M.; Mizoguchi, M.; Kawai, S.; Mizushima, Y.; Igarashi, R. Successful treatment of photo-damaged skin of nano-scale atRA particles using a novel transdermal delivery. J. Control. Release 2005, 104, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, I.L.; Coombs, B.D. Skin color measurements in terms of CIELAB color space values. J. Invest. Dermatol. 1992, 99, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Förster, M.; Bolzinger, M.A. Confocal Raman microspectroscopy for evaluating the stratum corneum removal by 3 standard methods. Skin Pharmacol. Physiol. 2011, 24, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Willis, C.M.; Stephens, C.J. Epidermal damage induced by irritants in man: A light and electron microscopic study. J. Invest. Dermatol. 1989, 93, 695–699. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Musashi, M.; Coler-Reilly, A.; Nagasawa, T.; Kubota, Y.; Kato, S.; Yamaguchi, Y. Liquid Crystal Gel Reduces Age Spots by Promoting Skin Turnover. Cosmetics 2014, 1, 202-210. https://doi.org/10.3390/cosmetics1030202
Musashi M, Coler-Reilly A, Nagasawa T, Kubota Y, Kato S, Yamaguchi Y. Liquid Crystal Gel Reduces Age Spots by Promoting Skin Turnover. Cosmetics. 2014; 1(3):202-210. https://doi.org/10.3390/cosmetics1030202
Chicago/Turabian StyleMusashi, Mina, Ariella Coler-Reilly, Teruaki Nagasawa, Yoshiki Kubota, Satomi Kato, and Yoko Yamaguchi. 2014. "Liquid Crystal Gel Reduces Age Spots by Promoting Skin Turnover" Cosmetics 1, no. 3: 202-210. https://doi.org/10.3390/cosmetics1030202