Methodology to Reach Full Spectral Photo-Protection by Selecting the Best Combination of Physical Filters and Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Epigallocatechin Gallate EGCG (TAIYO KAGAKU CO. LTD, Japan);
- Alpha-Tocoperol/Vitamin E (VE) (Koninklijke DSM N.V. (Royal DSM, The Nether-lands);
- Ferulic acid (FA) (Shanghai Lijing Industrial Co, LTD, China);
- Emblica (Merck KGaA, Germany);
- Rosmarinic acid (Chengdu Herbpurify Co., Ltd., China);
- Sodium metabisulfite (SM) (Sinopharm, China).
2.2. Methods
2.2.1. Pre-Selection of an Optimal Physical Filter/Antioxidant Ratio
- (1) Radical generation
- (2) Determination of the antioxidant capacities of six antioxidants
- (3) Ratio finding between physical filters and antioxidants
2.2.2. Measurements of Antioxidant Capacity of the Creams by Radical Protection Factor (RPF)
2.2.3. Evaluation of the Optical Properties of the Creams by UV-VIS Spectroscopy
3. Results and Discussion
3.1. Radical Generation by Physical Filters under UV Irradiation
3.2. Antioxidant Capacities of Various Antioxidants in Solution
3.3. Determination of Theoretical and Real Amount of Antioxidants to Counteract Radical Production by Physical Filters
- Low radical formation of physical filters during irradiation;
- Low amount of consumption of AO;
- Low costs of antioxidants;
- High availability and reproducibility of production of AO;
- Various chemical properties (hydrophilic/lipophilic/protein for optimal combinations).
3.4. Antioxidant Capacity in the Cream Formulation
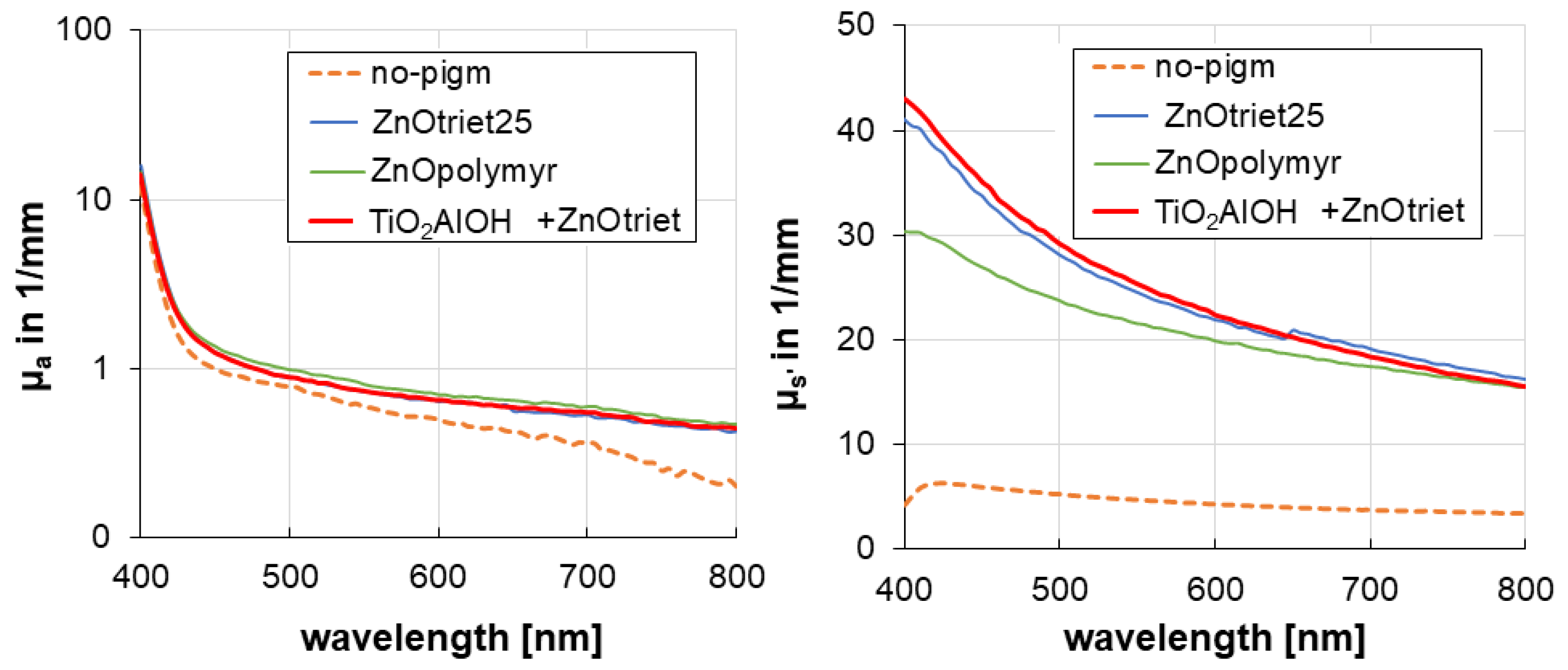
3.5. Optical Properties of the Creams
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Gabros, S.; Nessel, T.A.; Zito, P.M. Sunscreens and Photoprotection; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Latha, M.S.; Martis, J.; Shobha, V.; Sham Shinde, R.; Bangera, S.; Krishnankutty, B.; Bellary, S.; Varughese, S.; Rao, P.; Naveen Kumar, B.R. Sunscreening agents: A review. J. Clin. Aesthetic Dermatol. 2013, 6, 16–26. [Google Scholar]
- Lademann, J.; Schanzer, S.; Jacobi, U.; Schaefer, H.; Pflucker, F.; Driller, H.; Beck, J.; Meinke, M.; Roggan, A.; Sterry, W. Synergy effects between organic and inorganic UV filters in sunscreens. J. Biomed. Opt. 2005, 10, 014008. [Google Scholar] [CrossRef] [PubMed]
- Syring, F.; Weigmann, H.J.; Schanzer, S.; Meinke, M.C.; Knorr, F.; Lademann, J. Investigation of Model Sunscreen Formulations Comparing the Sun Protection Factor, the Universal Sun Protection Factor and the Radical Formation Ratio. Skin Pharmacol. Physiol. 2016, 29, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Lohan, S.B.; Muller, R.; Albrecht, S.; Mink, K.; Tscherch, K.; Ismaeel, F.; Lademann, J.; Rohn, S.; Meinke, M.C. Free radicals induced by sunlight in different spectral regions—In vivo versus ex vivo study. Exp. Dermatol. 2016, 25, 380–385. [Google Scholar] [CrossRef]
- Albrecht, S.; Jung, S.; Muller, R.; Lademann, J.; Zuberbier, T.; Zastrow, L.; Reble, C.; Beckers, I.; Meinke, M.C. Skin type differences in solar-simulated radiation-induced oxidative stress. Br. J. Dermatol. 2019, 180, 597–603. [Google Scholar] [CrossRef]
- Bellomo, G. Cell damage by oxygen free radicals. Cytotechnology 1991, 5, 71–73. [Google Scholar] [CrossRef]
- Silva, J.P.; Coutinho, O.P. Free radicals in the regulation of damage and cell death—Basic mechanisms and prevention. Drug Discov Ther 2010, 4, 144–167. [Google Scholar]
- Dreher, D.; Junod, A.F. Role of oxygen free radicals in cancer development. Eur. J. Cancer 1996, 32, 30–38. [Google Scholar] [CrossRef]
- Lyons, A.B.; Trullas, C.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Photoprotection beyond ultraviolet radiation: A review of tinted sunscreens. J. Am. Acad. Dermatol. 2021, 84, 1393–1397. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.S.; Hsia, A.; Miller, J.D.; Hanneman, K.; Scull, H.; Cooper, K.D.; Baron, E. Non-sunscreen photoprotection: Antioxidants add value to a sunscreen. J. Investig. Dermatol. Symp. Proc. 2009, 14, 56–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M.; Saeedi, M.; Charati, J.Y. Correlation between Sun Protection Factor and Antioxidant Activity, Phenol and Flavonoid Contents of some Medicinal Plants. Iran. J. Pharm. Res. 2014, 13, 1041–1047. [Google Scholar]
- Souza, C.; Maia Campos, P.; Schanzer, S.; Albrecht, S.; Lohan, S.B.; Lademann, J.; Darvin, M.E.; Meinke, M.C. Radical-Scavenging Activity of a Sunscreen Enriched by Antioxidants Providing Protection in the Whole Solar Spectral Range. Skin Pharmacol. Physiol. 2017, 30, 81–89. [Google Scholar] [CrossRef]
- Shimojo, Y.; Nishimura, T.; Hazama, H.; Ozawa, T.; Awazu, K. Measurement of absorption and reduced scattering coefficients in Asian human epidermis, dermis, and subcutaneous fat tissues in the 400- to 1100-nm wavelength range for optical penetration depth and energy deposition analysis (Errata). J. Biomed. Opt. 2021, 26, 019802. [Google Scholar] [CrossRef]
- Tseng, S.H.; Bargo, P.; Durkin, A.; Kollias, N. Chromophore concentrations, absorption and scattering properties of human skin in-vivo. Opt. Express 2009, 17, 14599–14617. [Google Scholar] [CrossRef] [Green Version]
- Meinke, M.C.; Syring, F.; Schanzer, S.; Haag, S.F.; Graf, R.; Loch, M.; Gersonde, I.; Groth, N.; Pflucker, F.; Lademann, J. Radical Protection by Differently Composed Creams in the UV/VIS and IR Spectral Ranges. Photochem. Photobiol. 2013, 89, 1079–1084. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Delfino, I.; Crucianelli, M.; Conte, N.; Avitabile, D.; Saladino, R. Green and Scalable Preparation of Colloidal Suspension of Lignin Nanoparticles and Its Application in Eco-friendly Sunscreen Formulations. ACS Omega 2021, 6, 21444–21456. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Lohan, S.B.; Schanzer, S.; Campos, P.; Lademann, J.; Meinke, M.C. Eco-friendly sunscreen formulation based on starches and PEG-75 lanolin increases the antioxidant capacity and the light scattering activity in the visible light. J. Photochem. Photobiol. B 2021, 222, 112264. [Google Scholar] [CrossRef] [PubMed]
- Friebel, M.; Roggan, A.; Muller, G.; Meinke, M. Determination of optical properties of human blood in the spectral range 250 to 1100 nm using Monte Carlo simulations with hematocrit-dependent effective scattering phase function. J. Biomed. Opt. 2006, 11, 034021. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Chen, B.; Lei, Y.; Keesuh, L.; Nan, L.; Dongfang, K.; Lademann, J.; Schanzer, S.; Lohan, S.B.; Meinke, M.C. New Innovative Sunscreen for Protection of Skin Types IV–V. IFSCC Mag. 2018, 21, 87–92. [Google Scholar]
- Wakefield, G.; Green, M.; Lipscomb, S.; Flutter, B. Modified titania nanomaterials for sunscreen applications—Reducing free radical generation and DNA damage. Mater. Sci. Technol. 2004, 20, 985–988. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Ugazio, E.; Sapino, S.; Fenoglio, I.; Greco, G.; Fubini, B. Role of particle coating in controlling skin damage photoinduced by titania nanoparticles. Free Radic. Res. 2009, 43, 312–322. [Google Scholar] [CrossRef]
- Elmets, C.A.; Singh, D.; Tubesing, K.; Matsui, M.; Katiyar, S.; Mukhtar, H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J. Am. Acad. Dermatol. 2001, 44, 425–432. [Google Scholar] [CrossRef]
- Fernando, P.M.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Hewage, S.R.; Chae, S.W.; Hyun, J.W. Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells. Biomol. Ther. 2016, 24, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.W.; Jiang, R.H.; Kim, K.D.; Lee, J.H.; Kim, C.D.; Yin, W.T.; Lee, J.H. Rosmarinic acid inhibits poly(I:C)-induced inflammatory reaction of epidermal keratinocytes. Life Sci. 2016, 155, 189–194. [Google Scholar] [CrossRef]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef]

| Cream Code/Physical filters | TiO2-AlOH Titanium Dioxide, Aluminum Hydroxide, Isostearic Acid | ZnOtriet25 Zinc Oxide, Triethoxycaprylylsilane | ZnOtriet45 Zinc Oxide, Triethoxycaprylylsilane | ZnOpolymyr Zinc Oxide, Polydimethyl-siloxane, Myristic Acid |
|---|---|---|---|---|
| Diameter in nm * | 10 | 25 | 25 | 35 |
| C1-No-pigm | 0 | 0 | 0 | 0 |
| C4-ZnOtriet | 10 | |||
| C5-ZnOpolymyr | 10 | |||
| P6-TiO2AlOH+ZnOtriet | 4 | 10 |
| Pigment | TiO2-AlOH Titanium Dioxide, Aluminum Hydroxide, Isostearic Acid | ZnOtriet25 Zinc Oxide, Triethoxycaprylylsilane | ZnOtriet45 Zinc Oxide, Triethoxycaprylylsilane | ZnOpolymyr Zinc Oxide, Polydimethyl-siloxane, Myristic Acid |
|---|---|---|---|---|
| Scavenged DPPH (%) | 45 | 53 | 42 | 23 |
| EGCG ICx% | 0.000047 | 0.000062 | 0.000043 | 0.000018 |
| EGCG used % | 0.000050 | 0.000010 | 0.000015 | 0.000250 |
| XEGCG | 0.90 | 6.20 | 2.90 | 0.10 |
| VE Icx % | 0.000792 | 0.001401 | 0.000681 | 0.000224 |
| VE used % | 0.00 | 0.00 | 0.00 | 0.001 |
| XVE | 1.0 | 1.4 | 0.9 | 0.2 |
| SM Icx % | 0.04068 | 0.05383 | 0.03669 | 0.01287 |
| SM used % | 0.0075 | 0.05 | 0.0025 | 0.025 |
| XSM | 5.4 | 1.1 | 14.7 | 0.5 |
| A.O. | DPPH IC50 (w.t. %) | Curve-Fitting | R2 |
|---|---|---|---|
| EGCG | 0.000057 | y = 0.03069 + 0.73277 [1 − [e (−18094.2851*x)]] | 0.99 |
| VE | 0.001107 | y = 0.0596 + 0.5012 [1 − [e (−1906.1098*x)]] | 0.96 |
| FA | 0.000227 | y = 0.02585 + 0.6568 [1 − [e (−6571.7067*x)]] | 0.99 |
| Emblica | 0.001597 | y = 0.09510 + 1.03919 [1 − [e (−309.1062*x)]] | 0.99 |
| Rosmarinic acid | 0.000461 | y = 0.09875 + 0.84752 [1 − [e (−1391.6724*x)]] | 0.98 |
| SM | 0.04905 | y = 0.1013 + 0.7953 [1 − [e (−14.1852*x)]] | 0.95 |
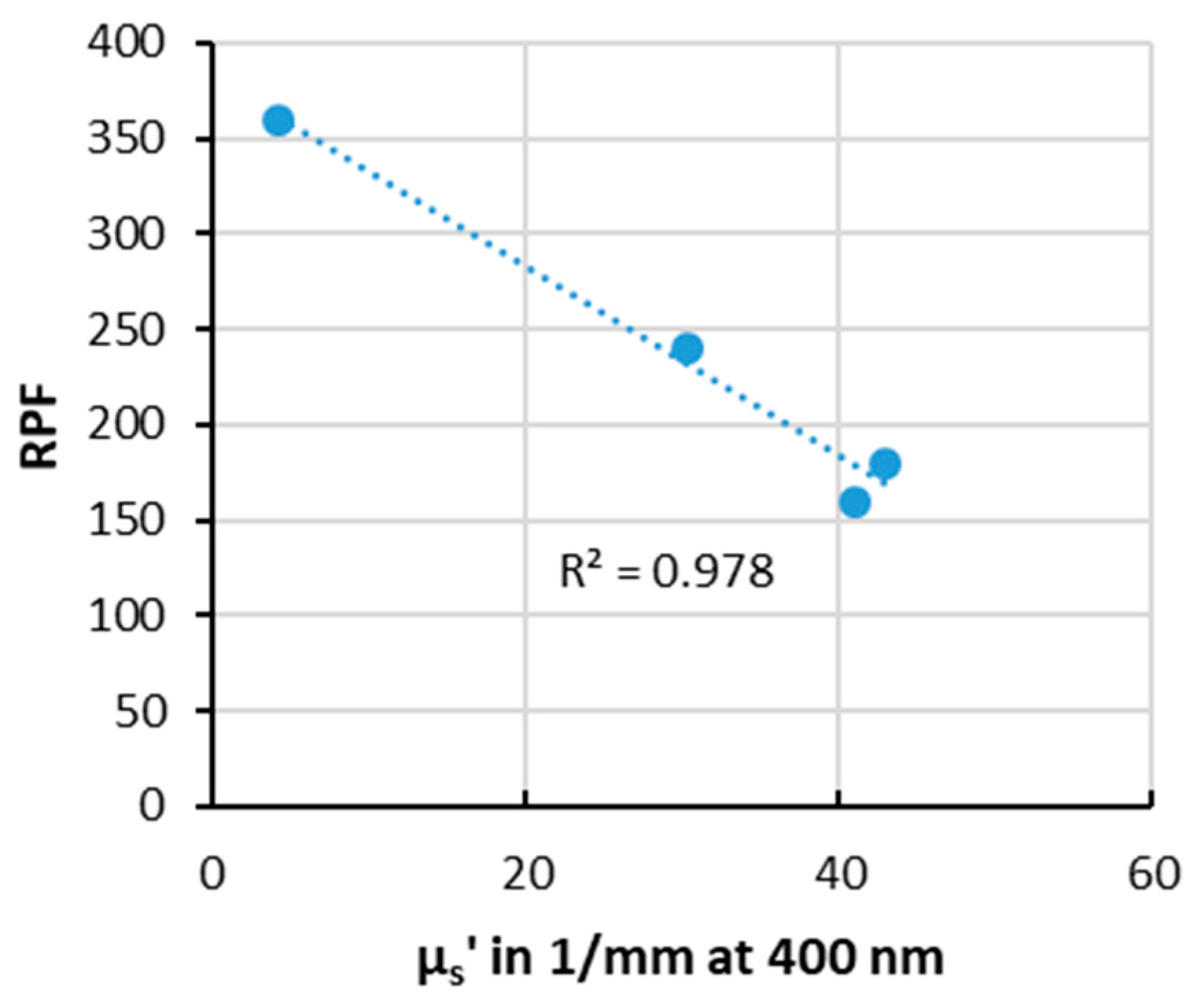
| Formulation | RPF in 1014 Radicals/mg | µs’ at 400 nm in 1/mm | µs’ at 800 nm in 1/mm |
|---|---|---|---|
| C1-No-pigm | 360 ± 14 | 4.2 | 3.4 |
| C4-ZnOtriet | 160 ± 9 | 41.1 | 16.3 |
| C5-ZnOpolymyr | 240 ± 2 | 30.4 | 15.5 |
| C6-TiO2 + AlOH + ZnOtriet | 180 ± 13 | 43.0 | 15.5 |
Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, A.; Lui, Y.; Zuo, J.; Lohan, S.B.; Schanzer, S.; Wiemann, S.; Keck, C.M.; Lademann, J.; Meinke, M.C. Methodology to Reach Full Spectral Photo-Protection by Selecting the Best Combination of Physical Filters and Antioxidants. Cosmetics 2023, 10, 1. https://doi.org/10.3390/cosmetics10010001
Lan A, Lui Y, Zuo J, Lohan SB, Schanzer S, Wiemann S, Keck CM, Lademann J, Meinke MC. Methodology to Reach Full Spectral Photo-Protection by Selecting the Best Combination of Physical Filters and Antioxidants. Cosmetics. 2023; 10(1):1. https://doi.org/10.3390/cosmetics10010001
Chicago/Turabian StyleLan, Alexandra, Yan Lui, Jinhui Zuo, Silke B. Lohan, Sabine Schanzer, Sabrina Wiemann, Cornelia M. Keck, Jürgen Lademann, and Martina C. Meinke. 2023. "Methodology to Reach Full Spectral Photo-Protection by Selecting the Best Combination of Physical Filters and Antioxidants" Cosmetics 10, no. 1: 1. https://doi.org/10.3390/cosmetics10010001






