Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization
Abstract
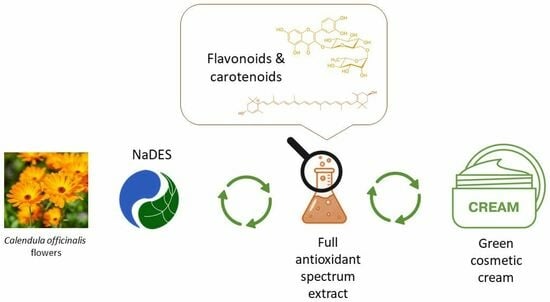
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biomass
2.3. NaDES Preparation
2.4. Calendula Extraction
2.4.1. NaDES Screening
2.4.2. Process Optimization Using the Design of Experiment Approach
2.4.3. Extraction Scale-Up
2.5. Extract Analysis
2.5.1. Total Carotenoid Content (TCC)
2.5.2. Total Flavonoid Content (TFC)
2.5.3. High-Performance Liquid Chromatography (HPLC)
2.6. Cream Formulation
2.7. Static Multiple Light Scattering-Based Stability Analysis
2.8. Rheology
2.9. Statistical Analysis
3. Results and Discussions
3.1. NaDES Screening
3.2. Extraction Optimization Using the Design of Experiment Approach
3.2.1. Ultrasound-Assisted Extraction (UAE)
Temperature − 0.680000 Time − 0.516241 solid/liquid ratio × Temperature +
0.010278 solid/liquid ratio × Time − 5.88146 solid/liquid ratio2
0.234605 Temperature − 0.118983 Time − 0.043623 Solid/liquid ratio ×
Temperature + 0.021898 Solid/liquid ratio × Time − 0.586024 Solid/liquid
ratio2
3.2.2. Dual Asymmetric Centrifugation (DAC)
+ 0.050855 − 0.003535 Speed solid/liquid ratio × Speed − 0.000865 Speed ×
Time − 7.81179 solid/liquid ratio2
0.524081 Time + 0.034061 Solid/liquid ratio × Time − 0.734255 Solid/liquid
ratio2 − 0.011086 Time2
3.3. Cosmetic Formulation of Bet:Gly Calendula Extract
3.3.1. Cream Preparation
3.3.2. Cream pH Measurements
3.3.3. Static Multiple Light Scattering-Based Stability Analysis
3.3.4. Rheology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef] [PubMed]
- Balázs, V.L.; Gulyás-Fekete, G.; Nagy, V.; Zubay, P.; Szabó, K.; Sándor, V.; Agócs, A.; Deli, J. Carotenoid Composition of Calendula officinalis Flowers with Identification of the Configuration of 5,8-Epoxy-carotenoids. ACS Agric. Sci. Technol. 2023, 3, 1092–1102. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Ko, J.; Yoo, Y.; Jin, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Afonso, J.; Mezzetta, A.; Marrucho, I.M.; Guazzelli, L. History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: The unbalanced turn of deep eutectic solvents. Green Chem. 2023, 25, 59–105. [Google Scholar] [CrossRef]
- Choi, H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Ver-Poorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules 2021, 26, 6556. [Google Scholar] [CrossRef] [PubMed]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; De Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Colombo Dugoni, G.; Ferro, M.; et al. Are natural deep eutectic solvents always a sustainable option? A bioassay-based study. Environ. Sci. Pollut. Res. 2023, 30, 17268–17279. [Google Scholar] [CrossRef] [PubMed]
- Thiery, E.; Delaye, P.-O.; Thibonnet, J.; Boudesocque-Delaye, L. Mechanochemical Suzuki-Miyaura Cross-Coupling with Natural Deep Eutectic Solvent as Liquid-Assisted Grinding Additive: Merging Two Fields for a Greener Strategy. Eur. J. Org. Chem. 2023, 26, e202300727. [Google Scholar] [CrossRef]
- Delaye, P.O.; Pénichon, M.; Boudesocque-Delaye, L.; Enguehard-Gueiffier, C.; Gueiffier, A. Natural Deep Eutectic Solvents as sustainable solvent for Suzuki-Miyaura cross-coupling reactions applied to imidazo-fused heterocycles. SynOpen 2018, 2, 306–311. [Google Scholar]
- Alonso, D.A.; Burlingham, S.J.; Chinchilla, R.; Guillena, G.; Ramón, D.J.; Tiecco, M. Asymmetric Organocatalysis in Deep Eutectic Solvents. Eur. J. Org. Chem. 2021, 2021, 4065. [Google Scholar] [CrossRef]
- Nolan, M.D.; Mezzetta, A.; Guazzelli, L.; Scanlan, E.M. Radical-mediated thiol–ene ‘click’ reactions in deep eutectic solvents for bioconjugation. Green Chem. 2022, 24, 1456–1462. [Google Scholar] [CrossRef]
- Hadi Nematollahi, M.; Carvalho, P.J. Green solvents for CO2 capture. Curr. Opin. Green Sustain. Chem. 2019, 18, 25–30. [Google Scholar] [CrossRef]
- He, W.; Zhan, T.; Han, H.; Xu, Y. Optimization of Deep Eutectic Solvents Enables Green and Efficient Cryopreservation. Langmuir 2024, 40, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Caprin, B.; Charton, V.; Vogelgesang, B. The use of NADES to support innovation in the cosmetic industry. In Advances in Botanical Research, 1st ed.; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Elsevier: London, UK, 2021; Volume 97, pp. 309–332. [Google Scholar] [CrossRef]
- Le Bras, C.; Maillet, G.O.; Lubrano, C. Base de Formulation Cosmétique D’origine Naturelle ou Végétale et Composition Cosmétique. Patent FR 3 017 292-A1, 14 August 2015. [Google Scholar]
- Lubrano, C.; Maillet, G.O.; Laperdrix, C. Utilisation Cosmétique d’un Solvant Eutectique pour Améliorer L’aspect de la Peau. Patent FR 3 046 352-A1, 7 July 2017. [Google Scholar]
- Laperdrix, C.; Sennelier, B. Cosmetic Use of Vegetable Ivory Extract (Phytelephas sp.). Patent WO 2019/081859-A1, 15 July 2021. [Google Scholar]
- Lubrano, C.; Sennelier, B.; Mervoyer, C. Cosmetic Use of Syringa vulgaris L. Meristematic Cells for a Soothing and/or Softening Action on the Skin. Patent WO 2019/063929-A1, 29 March 2019. [Google Scholar]
- Sennelier, B.; Laugier-Cassin, F.; Mervoyer, C. Cosmetic Use of Syringa vulgaris L. Meristematic Cells for Anti-Ageing Action on the Skin. Patent WO 2019/063927-A1, 4 April 2019. [Google Scholar]
- Laperdrix, C.; Lubrano, C.; Portet, B. Utilisation d’un Extrait de Réglisee (Glycyrrhiza glabra L.) pour une Action D’hydratation de la Peau, de ses Annexes ou des Muqueuses. Patent FR 3 042 415-A1, 21 April 2017. [Google Scholar]
- Caprin, B.; Charton, V.; Demargne, F. Extraits Végétaux Destinés à la Cosmétique, Solvants et Procédés pour les Obtenir. Patent FR 3036618-A1, 2 December 2016. [Google Scholar]
- Caprin, B.; Charton, V.; Demargne, F. Solvants Eutectiques pour la Dissolution de Stilbenoides ou leurs Dérivés. Patent FR 3068352-A1, 4 January 2019. [Google Scholar]
- Lavaud, A.; Laguerre, M.; Birtic, S.; Fabiano-Tixier, A.S.; Roller, M.; Chemat, F.; Bily, A.C. Solvant Eutectique D’extraction, Procédé D’extraction par Eutectigénèse Utilisant Ledit Solvant, et Extrait issu dudit Procédé. Patent FR 3034625-A1, 14 October 2016. [Google Scholar]
- Laguerre, M.; Harris, R.; Lavaud, A.; Tenon, M.; Birtic, S.; Bily, A.C.; Abbott, A.P. Eutectic Extract Formation and Purification. Patent WO 2019/219774-A2, 30 September 2021. [Google Scholar]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosiére, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural deep eutectic solvent formulations for spirulina: Preparation, intensification, and skin impact. Algal Res. 2021, 56, 102317. [Google Scholar] [CrossRef]
- Hilali, S.; Van Gheluwe, L.; Yagmur, M.; Wils, L.; Phelippe, M.; Clément-Larosière, B.; Montigny, B.; Jacquemin, J.; Thiery, T.; Boudesocque-Delaye, L. NaDES-based biorefinery of Spirulina (Arthrospira platensis): A new path for sustainable high value-added metabolites. Sep. Purif. Technol. 2024, 329, 125123. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, C.; Xu, C.; Zhang, P.; Chen, C.; Tang, L.; Zhang, J.; Zhang, H.; Jiang, S. Simultaneous extraction of crocin and geniposide from gardenia fruits (Gardenia jasminoides Ellis) by probe-type ultrasound-assisted natural deep eutectic solvents and their inhibition effects on low density lipoprotein oxidation. Ultrason. Sonochem. 2023, 101, 106658. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, S.; Zhou, H.; Wu, H.; Wang, S.; Yong, X.; Zhou, J. Separation and purification of glabridin from a deep eutectic solvent extract of Glycyrrhiza glabra residue by macroporous resin and its mechanism. Sep. Purif. Technol. 2023, 315, 123731. [Google Scholar] [CrossRef]
- Rente, D.; Cvjetko Bubalo, M.; Panić, M.; Paiva, A.; Caprin, B.; Radojčić Redovniković, I.; Duarte, A.R.C. Review of deep eutectic systems from laboratory to industry, taking the application in the cosmetics industry as an example. J. Clean. Prod. 2022, 380, 135147. [Google Scholar] [CrossRef]
- Jamaleddine, A.; Urrutigoïty, M.; Bouajila, J.; Merah, O.; Evon, P.; de Caro, P. Ecodesigned Formulations with Tomato Pomace Extracts. Cosmetics 2023, 10, 7. [Google Scholar] [CrossRef]
- Rocha, D.; Freitas, D.S.; Magalhães, J.; Fernandes, M.; Silva, S.; Noro, J.; Ribeiro, A.; Cavaco-Paulo, A.; Martins, M.; Silva, C. NADES-Based Cork Extractives as Green Ingredients for Cosmetics and Textiles. Processes 2023, 11, 309. [Google Scholar] [CrossRef]
- Barros Santos, M.C.; Barouh, N.; Baréa, B.; Villeneuve, P.; Bourlieu-Lacanal, C.; Simões Larraz Ferreira, M.; Durand, E. Sequential one-pot NaDES assisted extraction and biotransformation of rice bran: A new strategy to boost antioxidant activity of natural extracts. Process Biochem. 2022, 117, 110–116. [Google Scholar] [CrossRef]
- Wils, L.; Yagmur, M.; Phelippe, M.; Montigny, B.; Clément-Larosière, B.; Jacquemin, J.; Boudesocque-Delaye, L. Alternative Solvents for the Biorefinery of Spirulina: Impact of Pretreatment on Free Fatty Acids with High Added Value. Mar. Drugs 2022, 20, 600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ying, T.; Bao, B.; Huang, X. Characteristics of fruit ripening in tomato mutant epi. J. Zhejiang Univ. Sci. B 2005, 6, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Morais, E.S.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Extraction of High Value Triterpenic Acids from Eucalyptus globulus Biomass Using Hydrophobic Deep Eutectic Solvents. Molecules 2020, 25, 210. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Czaikoski, A.; Aparecida Almeida, N.; Fraga, S.; de Oliveira Rocha, L.; Lopes Cunha, R.; Maximo, G.J.; Caldas Batista, E.A. Extraction optimization, biological activities, and application in O/W emulsion of deep eutectic solvents-based phenolic extracts from olive pomace. Food Res. 2022, 161, 111753. [Google Scholar] [CrossRef] [PubMed]
- Tenambergen, F.; Maruiama, C.H.; Mäder, K. Dual asymmetric centrifugation as an alternative preparation method for parenteral fat emulsions in preformulation development. Int. J. Pharm. 2013, 447, 31–37. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Jafari, S.M.; Homayouni, A.; Hamishekar, H.; Mirzaei, H. Fabrication of double W1/O/W2 nano-emulsions loaded with oleuropein in the internal phase (W1) and evaluation of their release rate. Food Hydrocoll. 2019, 89, 44–55. [Google Scholar] [CrossRef]
- Kusmita, L.; Mutmainah, N.; Sabdono, A.; Trianto, A.; Radjasa, O.K.; Pangestuti, R. Characteristic Evaluation of Various Formulations of Anti-Aging Cream from Carotenoid Extract of Bacterial Symbiont Virgibacillus salarius Strain 19.PP.Sc1.6. Cosmetics 2021, 8, 120. [Google Scholar] [CrossRef]
- Franyoto, Y.D.; Kusmita, L.; Mutmainah, N.; Angrena, R.D. Total flavonoid content and formulation antioxidant cream stem of jatropha multifida. J. Phys. Conf. Ser. 2017, 1025, 012130. [Google Scholar] [CrossRef]
- Baldassarre, F.; Schiavi, D.; Di Lorenzo, V.; Biondo, F.; Vergaro, V.; Colangelo, G.; Balestra, G.M.; Ciccarella, G. Cellulose Nanocrystal-Based Emulsion of Thyme Essential Oil: Preparation and Characterisation as Sustainable Crop Protection Tool. Molecules 2023, 28, 7884. [Google Scholar] [CrossRef]
- Moravkova, T.; Stern, P. Rheological and textural properties of cosmetic emulsions. Appl. Rheol. 2011, 21, 35200. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, Y.; Zhu, L.; Liu, X.; Zhao, G.; Wang, S.; Yang, L.; Liu, H. Stability and rheological properties of water-in-oil (W/O) emulsions prepared with a soyasaponin-PGPR system. Future Food 2021, 4, 100096. [Google Scholar] [CrossRef]
- Wang, W.; Jia, R.; Hui, Y.; Liu, Y.; Song, Y.; Wang, B. Utilization of two plant polysaccharides to improve fresh goat milk cheese: Texture, rheological properties, and microstructure characterization. J. Dairy Sci. 2023, 106, 3900–3917. [Google Scholar] [CrossRef]
- de la Guardia, C.; Virno, A.; Musumeci, M.; Bernardin, A.; Silberberg, M.B. Rheologic and Physicochemical Characteristics of Hyaluronic Acid Fillers: Overview and Relationship to Product Performance. Facial Plast. Surg. 2022, 38, 116–123. [Google Scholar] [CrossRef]





| NaDES | Component 1 | Component 2 | Component 3 | Molar Ratio | Water Amount (% w/w) | |
|---|---|---|---|---|---|---|
| Nonpolar | C8:C10 | Octanoic acid | Decanoic acid | 3:1 | ||
| C9:C12 | Nonanoic acid | Lauric acid | 3:1 | |||
| C9:C10:C12 | Nonanoic acid | Decanoic acid | Lauric acid | 3:2:1 | ||
| C8:Lac | Octanoic acid | Lactic acid | 5:1 | 1 | ||
| Polar | Pro:Gly | Proline | Glycerol | 1:3 | ||
| Pro:Lev | Proline | Levulinic acid | 2:1: | 20 | ||
| Bet:Gly | Betaine | Glycerol | 1:8 | 20 | ||
| Bet:Lev | Betaine | Levulinic acid | 1:1 | |||
| Lev:Gly | Levulinic acid | Glycerol | 1:1 | |||
| Prop:Lev | 1,3-propanediol | Levulinic acid | 1:1 | |||
| Prop:Gly | 1,3-propanediol | Glycerol | 1:1 |
| %wt in the Analyzed Samples | |||
|---|---|---|---|
| Ingredients | Function | Control | Calendula Creams |
| BGCE (%wt) | Active | 0 | 1 or 10 |
| Xanthan gum (%wt) | Thickener | 0.25 | 0.25 |
| Sclerotium gum (%wt) | Thickener | 0.75 | 0.75 |
| Glycerol (%wt) | Humectant | 10 | 9 or 0 |
| Hydrogenated lecithin (%wt) | Emulsifier | 3 | 3 |
| Caprylic/capric triglycerides (%wt) | Emollient | 25 | 25 |
| Dehydroacetic acid/ benzyl alcohol mix (%wt) | Antimicrobial agent | 1 | 1 |
| Water (%wt) | Solvent | q.s 100 | |
| Run | Solid/Liquid Ratio (%) | Temperature (°C) | Time (min) | TCC (µg/g) | TFC (mg eq Rutin/g) |
|---|---|---|---|---|---|
| 1 | 6 | 60 | 60 | 211.9 | 21.5 |
| 2 | 6 | 45 | 37.5 | 255.3 | 20.7 |
| 3 | 6 | 30 | 60 | 265.0 | 22.3 |
| 4 | 6 | 30 | 15 | 281.5 | 23.0 |
| 5 | 10 | 45 | 15 | 223.7 | 12.9 |
| 6 | 6 | 60 | 15 | 281.2 | 24.4 |
| 7 | 6 | 45 | 37.5 | 282.5 | 20.7 |
| 8 | 2 | 60 | 37.5 | 135.8 | 12.5 |
| 9 | 10 | 30 | 37.5 | 255.9 | 18.8 |
| 10 | 2 | 45 | 15 | 141.0 | 12.8 |
| 11 | 2 | 30 | 37.5 | 89.9 | 9.2 |
| 12 | 10 | 60 | 37.5 | 177.9 | 11.6 |
| 13 | 6 | 45 | 37.5 | 274.3 | 24.7 |
| 14 | 2 | 45 | 60 | 126.4 | 11.8 |
| 15 | 6 | 45 | 37.5 | 414.8 | 25.1 |
| 16 | 6 | 45 | 37.5 | 367.9 | 24.9 |
| 17 | 10 | 45 | 60 | 212.8 | 19.7 |
| Run | Solid/Liquid Ratio (%) | Time (min) | Speed (rpm) | TCC (µg/g) | TFC (mg eq rutin/g) |
|---|---|---|---|---|---|
| 1 | 2 | 37.5 | 3500 | 238.6 | 18.6 |
| 2 | 6 | 37.5 | 2000 | 340.3 | 30.2 |
| 3 | 6 | 60 | 500 | 384.7 | 29.0 |
| 4 | 10 | 37.5 | 500 | 167.1 | 34.9 |
| 5 | 2 | 60 | 2000 | 240.5 | 24.9 |
| 6 | 10 | 60 | 2000 | 173.3 | 32.7 |
| 7 | 2 | 15 | 2000 | 240.3 | 18.8 |
| 8 | 10 | 15 | 2000 | 169.1 | 30.9 |
| 9 | 6 | 37.5 | 2000 | 387.3 | 38.5 |
| 10 | 6 | 37.5 | 2000 | 301.4 | 38.8 |
| 11 | 2 | 37.5 | 500 | 188.8 | 20.5 |
| 12 | 6 | 15 | 3500 | 316.3 | 37.7 |
| 13 | 6 | 37.5 | 2000 | 318.9 | 41.7 |
| 14 | 10 | 37.5 | 3500 | 132.1 | 34.7 |
| 15 | 6 | 37.5 | 2000 | 303.9 | 41.1 |
| 16 | 6 | 60 | 3500 | 302.1 | 32.6 |
| 17 | 6 | 15 | 500 | 192.0 | 33.9 |
| D1 | D90 | |
|---|---|---|
| Control | 5.4 ± 0.2 | 5.4 ± 0.1 |
| 1% BGCE Cream | 5.4 ± 0.1 | 5.0 ± 0.3 |
| 10% BGCE Cream | 5.3 ± 0.1 | 5.8 ± 0.1 |
| TSI | Droplet Diameter (µm) | |||
|---|---|---|---|---|
| D1 | D90 | D1 | D90 | |
| Control | 0.83 ± 0.21 | 0.90 ± 0.10 | 9.3 ± 0.2 | 9.7 ± 0.2 |
| 1% BGCE Cream | 1.23 ± 0.23 | 0.83 ± 0.15 | 9.2 ± 0.2 | 10.2 ± 0.2 |
| 10% BGCE Cream | 0.97 ± 0.35 | 1.00 ± 0.44 | 7.3 ± 0.3 | 8.6 ± 0.2 |
| Creams | η (0.01) | η (0.1) | η (1) | η (10) | η (100) | η (1000) |
|---|---|---|---|---|---|---|
| Control D1 | 1430.0 ± 37.8 | 221.1 ± 23.0 | 29.1 ± 0.7 | 4.1 ± 0.0 | 0.7 ± 0.0 | 0.1 ± 0.0 |
| Control D30 | 1072.5 ± 124.2 | 165.8 ± 19.8 | 21.9 ± 0.6 | 3.1 ± 0.1 | 0.5 ± 0.0 | 0.1 ± 0.0 |
| 1% BGCE D1 | 2236.3 ± 61.2 | 311.6 ± 7.3 | 40.6 ± 1.2 | 5.6 ± 0.1 | 0.9 ± 0.0 | 0.2 ± 0.0 |
| 1% BGCE D30 | 1945.6 ± 53.3 | 271.1 ± 6.3 | 35.3 ± 1.1 | 4.9 ± 0.1 | 0.8 ± 0.0 | 0.1 ± 0.0 |
| 10% BGCE D1 | 3084.0 ± 49.5 | 446.5 ± 19.9 | 55.8 ± 2.7 | 7.2 ± 0.6 | 1.2 ± 0.1 | 0.3 ± 0.0 |
| 10% BGCE D30 | 1694.7 ± 87.8 | 287.9 ± 10.8 | 37.2 ± 1.0 | 4.8 ± 0.1 | 0.7 ± 0.0 | 0.1 ± 0.0 |
| D1 | D30 | |||
|---|---|---|---|---|
| G′ (Pa) | G″ (Pa) | G′ (Pa) | G″ (Pa) | |
| Control | 188.1 ± 3.1 | 36.0 ± 3.2 | 215.7 ± 7.0 | 40.6 ± 5.6 |
| 1% BGCE Cream | 260.2 ± 7.9 | 47.1 ± 8.0 | 226.3 ± 4.3 | 41.0 ± 3.0 |
| 10% BGCE Cream | 389.6 ± 10.4 | 68.2 ± 3.8 | 228.7 ± 7.3 | 50.6 ± 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudesocque-Delaye, L.; Ardeza, I.M.; Verger, A.; Grard, R.; Théry-Koné, I.; Perse, X.; Munnier, E. Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization. Cosmetics 2024, 11, 17. https://doi.org/10.3390/cosmetics11010017
Boudesocque-Delaye L, Ardeza IM, Verger A, Grard R, Théry-Koné I, Perse X, Munnier E. Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization. Cosmetics. 2024; 11(1):17. https://doi.org/10.3390/cosmetics11010017
Chicago/Turabian StyleBoudesocque-Delaye, Leslie, Iron Mike Ardeza, Alexis Verger, Roxane Grard, Isabelle Théry-Koné, Xavier Perse, and Emilie Munnier. 2024. "Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization" Cosmetics 11, no. 1: 17. https://doi.org/10.3390/cosmetics11010017