Abstract
Vitamin D is a natural photoproduct that has many beneficial effects on different organs, including skin. Active forms of vitamin D and its derivatives exert biological effects on skin cells, thus maintaining skin homeostasis. In keratinocytes, they inhibit proliferation and stimulate differentiation, have anti-inflammatory properties, act as antioxidants, inhibit DNA damage and stimulate DNA repair after ultraviolet (UV) exposure. In melanocytes, they also inhibit cell proliferation, inhibit apoptosis and act as antioxidants. In fibroblasts, they inhibit cell proliferation, affect fibrotic processes and collagen production, and promote wound healing and regeneration. On the other hand, skin cells have the ability to activate vitamin D directly. These activities, along with the projected topical application of vitamin D derivatives, are promising for skin care and photo protection and can be used in the prevention or possible reversal of skin aging.
1. Introduction
1.1. Vitamin D
Vitamin D is a natural photoproduct found in plants, as vitamin D2 (D2), or in skin of humans and animals, as vitamin D3 (D3) [1,2,3,4,5,6,7,8,9]. It is formed by ultraviolet B irradiation (UVB) action from its precursors ergosterol or 7-dehydrocholesterol (7DHC), respectively, in fungi and plants or animal and human skin [1,6,7,10,11,12,13,14]. Other forms of vitamin D, D1, D4, and D5, are less important for humans. Chemically, it is a secosteroid with a broken B ring in its structure [1,7,10,11,12]. Since its discovery in 1922, vitamin D has found many applications in medicine and cosmetics [1,7,11,12,15,16]. Not only does it help to prevent rickets and osteomalacia by regulating calcium and bone metabolism, but it also helps to prevent or treat many other diseases and conditions through the regulation of cellular growth and immune and endocrine responses [8,10,17]. All these vitamin D-regulated biological processes are crucial in aging [18,19]. Many cells, especially skin cells, are capable of producing vitamin D in its active forms with specific biological actions on cells dependent on the target organs, thus making it categorized as a pro-hormone and its active forms such as hormones [1,3,11,12,20,21,22,23,24].
Vitamin D can be found in food in the form of D2 (ergocalciferol) or D3 (cholecaciferol) or is produced in the skin [25,26]. In the classical activation pathway, vitamin D pro-hormone is hydroxylated under the action of catalytic enzymes CYP2R1 and CYP27A1 to 25-hydroxyvitamin D3 (25(OH)D3), mainly in the liver [1,7,22,27,28,29] but also in skin cells including keratinocytes and fibroblasts [8,12,22,30,31,32]. This hormone precursor is a form of vitamin D found in the serum and is used to measure the vitamin D status in the body, since its levels in circulation persist longer, lasting 2–3 weeks [33]. The level of 25(OH)D3 varies among people across the globe [2,11,34]. A hormonally active calcitriol or 1,25-dihydroxy-vitamin D (1,25(OH)2D3) is produced from 25(OH)D3. The production takes place in many tissues or organs, primarily in kidneys, under the action of 1α-hydroxylase, an enzyme regulated by CYP27B1 [1,29,35,36]. The main active form of vitamin D is 1,25(OH)2D3, primarily regulating bone metabolism and calcium levels. Calcitriol upregulates the CYP24A1 gene, of which the enzymatic product degrades 1,25(OH)2D3 [37,38]. The levels of 1,25(OH)2D3 are typically short lasting, up to 15 h, and are closely regulated by parathyroid hormones, minerals calcium and phosphorus, and vitamin D levels.
Many metabolites of vitamin D have been discovered over the years [15,23,39,40]—some of them useful, others not [23,41]. Vitamin D and its analogs are most frequently used for the treatment of osteoporosis, since as suppressing parathyroid hormone (PTH) agents, they increase calcium absorption and bone mineral density [26,41,42]. In skin, these molecules find application in the treatment of psoriasis as potent inhibitors of keratinocytes proliferation and in alopecia as hair growth stimulators [2,3,41]. As an adjuvant therapy, vitamin D metabolites are used against cancer, such as leukemia, as inhibitors of tumor cell growth and metastasis [41,43,44,45], including skin cancer [46,47,48,49,50,51,52].
The transcriptional activity of hormonally active 1,25(OH)2D3 starts with its binding to the vitamin D receptor (VDR) that forms heterodimers with the retinoid receptor (RXR), a complex activating transcription of target genes through binding to the VDR binding element (VDRE) in the nucleus [1,53,54,55,56]. The activity of vitamin D goes beyond classical bone mineral metabolism. These activities include regulation of cell proliferation and differentiation, metabolism of lipids, apoptosis, immune functions, and protection against oxidative damage and aging, etc.
1.2. Vitamin D Metabolism in the Skin
Vitamin D metabolites are also activated in the skin cells [20,30], including fibroblasts [32,57] and keratinocytes [31]. Skin is also a target organ where vitamin D regulates many skin cells functions, thus making it a unique organ within the endocrine system [11]. The formation of vitamin D in the skin involves UVB-induced transformation of 7DHC to pre-vitamin D3, with further transformation to D3 configuration that is accelerated by an increased temperature [1,11,25,26]. Keratinocytes and fibroblasts produce 25(OH)D3 [12] and the hormonally active form of vitamin D3, 1,25(OH)2D3 [26], through the pathway shown in Figure 1.
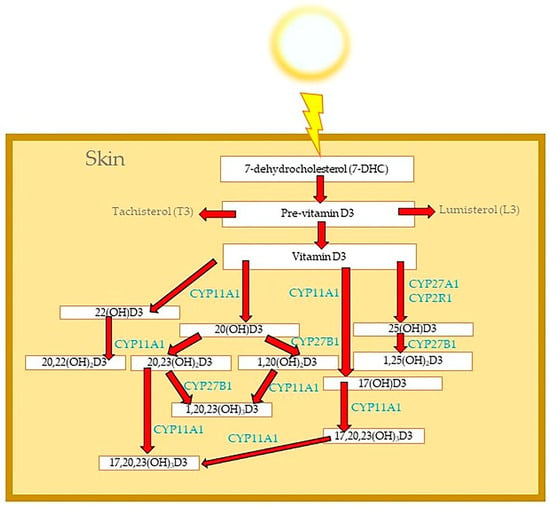
Figure 1.
Metabolism of vitamin D3 in the skin.
The alternative pathways of vitamin D activation in the skin include the metabolism of vitamin D by the CYP11A1 enzyme [23,24,32,58,59,60] and the production of many different vitamin D hydroxymetabolites (Figure 1). Hydroxylation of the side chain of vitamin D3 by CYP11A1 generates new vitamin D hydroxyderivatives, such as 20(OH)D3 [61] and 20,23(OH)2D3 [62]. Hydroxylation of these new metabolites of vitamin D3 under the CYP27B1 enzyme generates even more potent vitamin D3 hydroxyderivatives: 1,20(OH)2D3 [63] and 1,20,23(OH)3D3 as examples [23,62]. As tested on rats and mice, 20(OH)D3 is non-calcemic even at high doses, which is in contrast to 1,25(OH)2D3 inducing hypercalcemia and attendant toxicity [64,65,66]. These novel secosteroids are biologically active, similarly to 1,25(OH)2D3, yet they have lower toxicity and do not cause the calcemic effect, which makes them safer than 1,25(OH)2D3 for pharmacological use [40].
2. Skin and Photo-Aging
2.1. The Damaging Effects of UV Radiation on Skin Cells
Skin is the organ that protects the body from external factors, acting as a barrier between the environment and the internal milieu [67,68,69]. Skin consists of three layers; the epidermis is the outer layer, the dermis is the inner layer, and the hypodermis is composed of subdermal adipose tissue. Keratinocytes and melanocytes are predominant components of the epidermis, while fibroblasts are predominant cells in the dermis. Keratinocytes undergo a precise differentiation program forming a skin barrier [70,71,72]. Melanocytes are less abundant being found in the basal layer of the epidermis, hair follicles and partially in the dermis in nevi [73]. Melanocytes produce melanin pigment that absorbs UV energy, thus protecting the skin from its damaging effect [74,75]. Skin fibroblasts are located in the dermis and play an important role in wound healing, tissue fibrosis, inflammation, collagen turnover, etc. They produce extracellular matrix (ECM) components, such as collagen and elastin, as well as a variety of other important molecules, such as fibronectin and laminin [76]. The most abundant collagen in the skin is type I collagen. Matrix degrading enzymes, such as metalloproteinases (MMPs) and their tissue inhibitors (TIMPs), regulate collagen processing and are also produced by fibroblasts [77]. Collagen and elastin provide structure and support for the dermis.
The skin maintains its homeostasis via a cutaneous neuroendocrine system [78,79]. The loss of this homeostasis leads to many skin pathologies and to skin aging or cancer over time [69,80,81,82]. A meta-analysis of skin aging showed that the most common causes are solar exposure and smoking, while the most common signs of skin aging are wrinkling, followed by pathological pigmentation, sagging and telangiectasia [83,84,85]. The loss of the internal skin barrier is caused by internal factors, such as the metabolism of the cells and genetics [86,87,88,89]. The loss of the external skin barrier is caused by external factors, such as radiation, UVA (315–400 nm), UVB (280 to 315 nm), UVC (190–280 nm), infrared (IR) (750 nm–1000 μm), blue light (400–500 nm) [90,91] and chemicals [86,92,93]. An integral part of this process is the prolonged exposure of the skin to UVA, UVB or blue light causing photo-aging, characterized by loss of skin elasticity, wrinkling and skin hyperpigmentation, primarily on the face [69,86,94,95]. Harmful effects of UVC rays are usually blocked by the ozone layer and the upper layers of the epidermis [91,96,97]; therefore, UVC irradiation is not considered as a photo-aging factor [98].
Cellular changes occurring during intrinsic skin aging comprise the reduction in cell proliferation, especially in the epidermis that becomes thinner and the reduction in collagen production, which is notable in the dermis. Senescent keratinocytes, melanocytes and fibroblasts may accumulate in the skin as a part of the natural aging process [99,100,101]. Since their discovery [102,103], senescent cells have been considered as an important hallmark of skin aging [104,105,106]. They are characterized by the distinct morphological characteristics, such as enlarged cell size [107]. Under physiological conditions, senescence is a part of homeostasis related to aging, tissue remodeling and repair, wound healing, fibrosis, tumor suppression, etc. [108,109,110]. Under the influence of stress, cells stop dividing and enter so-called premature senescence, which is an irreversible arrested cell cycle [111], or face death [112,113,114]. The pathological conditions create an accumulation of senescent cells, which can further hinder tissue repair and regeneration [115,116,117], as seen in many age-related diseases, such as diabetes and atherosclerosis [118,119,120,121,122]. Cell senescence can cause inflammation, tissue fibrosis or induce tumors [123,124]. It is an important part of tumorigenesis [125,126]. Different types of stress can induce cell senescence, such as chemicals [127], therapeutic agents [128,129,130], UV radiation [131,132,133], pollution [134], or epigenetics factors, etc. [135]. Stress related inflammation and immunosuppression lead to tissue damage and accumulation of the senescent cells in the skin promoting skin aging [81,136,137,138,139,140,141]. Senescent cells can secrete pro-inflammatory cytokines, chemokines, proteases, ECM compounds, and growth factors [142,143,144,145,146]. This senescence-associated secretory phenotype (SASP) can further induce tissue damage [147,148,149]. Senescent cells are detected in all skin layers [132,150,151,152,153,154]. This process is mainly observed in fibroblasts and melanocytes [137,155]. Accumulations of the senescent fibroblasts in the skin leads to a loss of skin elasticity [156,157], hyper-pigmentation such as senile lentigo [158,159] or melasma [160]. Accumulations of the senescent melanocytes in the dermis are observed in melanocytic nevi [150,161] or facial wrinkles [162,163,164]. Accumulation of the senescent keratinocytes in the epidermis can affect actinic keratosis [132,165].
2.1.1. Oxidative Stress in the Skin
UV radiation can have deleterious effects on the skin causing oxidative stress, cancer or premature skin aging [166]. Oxidative stress results from the accumulation of reactive oxygen species (ROS) or reactive nitrogen species (RNS) in the skin cells in response to UV radiation (Figure 2). Under physiological conditions, ROS are produced in cells endogenously in a smaller amount as part of physiological signaling. Excessive ROS production can damage the skin and, over time, causes skin aging [167,168]. On a cellular level, this process leads to an impaired cell proliferation and differentiation, resulting in a thick epidermis [169,170,171]. Besides photo-aging, ROS can contribute to internal aging as well [172].

Figure 2.
Mechanism of action of novel vitamin D3 hydroxiderivatives in skin and the role in skin photo—aging.
Oxidative stress triggers the formation of ROS in fibroblasts, which can indirectly stimulate the production of MMP, collagenases that can cause the breakdown of the collagen in skin, thus contributing to the loss of skin structure [155,167] and subsequently resulting in the formation of wrinkles [173,174]. MMPs cause the degradation of ECM, such as collagen, fibronectin, elastin and other skin supportive proteins, thus inducing degenerative changes in skin manifesting as dryness, pigmentation, wrinkling, sagging, etc. (Figure 2). These changes induced by UV radiation are referred to as skin photo-aging changes [175].
Additionally, fibroblasts regulate inflammation. Inflammatory cytokines, such as IL-6, IL-8, IL-33 and TGFβ1, activate fibroblasts and promote differentiation and ECM production [76]. Similarly, so-called fibrotic genes, such as platelet-derived growth factor (PDGF), also stimulate fibroblasts [85].
2.1.2. DNA Damage in the Skin
Stress activates DNA damage response pathways [176]. Prolonged exposure to UVB causes DNA damage in skin cells in the form of cyclobutane pyrimidine dimers (CPD), 6-4 photoproducts (6-4PP) and 8-hydroxy-2′-deoxyguanosine (8-OH-DG) [97,177,178,179,180,181,182,183,184]. The exposure to UVA also causes a formation of so-called “dark CPD” (dCPD), mutations at non-pyrimidine sites, and 8-OH-DG [185,186] (Figure 2). This dCPT accounts for half of all CPDs produced and these are usually formed well after the UVA exposure depending on a pigment called melanin. Damaged DNA can be repaired by the skin nucleotide excision repair system (NER) [187,188], the base excision repair system (BER) [189] or nuclear mitotic apparatus (NuMA) [190]. If this system fails, the cells will undergo apoptosis and the skin will exhibit premature aging signs or pre-cancer changes culminating in cancerogenesis [166,191].
2.1.3. Wound Healing and Fibrotic Process in the Skin
Wound healing leads to the formation of a new tissue involving proliferation of fibroblasts, keratinocytes and endothelial cells in response to tissue injury [192]. These cells rebuild ECM. ECM and collagen are an integral part of tissue granulation and epithelization [193]. Scarring is a natural part of the wound healing localized to the injury site; however, excessive granulation tissue can lead to hypertrophic scars or even keloids [76,194]. A widely spread uncontrolled activity of fibroblasts, so-called fibrosis, can manifests as systemic sclerosis (SS) [195]. Once the wound healing process is finished, fibroblasts undergo senescence, thus limiting formation of excessive fibrosis [196]. However, factors that induce prolonged cell senescence, such as UV radiation, cause inflammation as a tissue response to the injury, which further induces fibroblasts to enter senescence [197,198,199,200,201]. Chronic inflammation and cell senescence decrease proliferation and migration of fibroblasts with subsequent inhibition of wound repair that leads to skin aging [202,203,204]. Accumulation of senescent cells delays wound healing and contributes to chronic wounds [153].
2.2. Endogenous Factors Protecting the Skin against UV Radiation and Photo-Aging
The skin has developed various mechanisms of protection against the UV-induced damage, including pigment production and synthesis of vitamin D and melatonin, as potent antioxidants [85,205,206]. Melanin is produced in melanocytes by the oxidation of L-tyrosine and subsequent conversion to L-dihydroxyphenylalanine (L-DOPA) under the action of the catalytic enzyme tyrosinase [73,75,206,207,208,209,210]. This process is called melanogenesis. Melanin absorbs UV radiation and protects nuclei of keratinocytes by stimulating cornification of keratinocytes [206,211,212,213,214]. As a form of protection against UV radiation, keratinocytes will enter cell-cycle arrest. In the case of prolonged UV exposure, the cell arrest will turn into an early senescence and apoptosis [85]. Although melanin can absorb UV radiation and protect skin, an excess of this pigment in skin forms hyperpigmentation and age spots [212,215]. These dark spots contain primarily eumelanin [212].
UVA is a large part of daily UV rays and is the main source of radiation used in tanning beds. It induces pigmentation that lasts longer than UVB-induced pigmentation, since it can go deeper through the skin beyond the epidermis. Unfortunately, UVA-induced melanin pigmentation does not have protective effects as UVB-induced pigmentation does [216]. DNA damage induced by UVA is primarily in cells located in the dermis, while the damage caused by UVB is more profound and primarily affects the epidermal cell layer [216]. The other, faster occurring mechanism is photo protection by vitamin D and its analogs that are activated in the skin under the influence of UVB [64,177]. According to some authors, vitamin D can induce re-pigmentation in damaged melanocytes (Figure 2). Levels of produced vitamin D correlate with melanin levels [217]. Activated vitamin D metabolites promote the above-described mechanism of skin protection and enhance DNA repair [177,205,218].
2.3. Mechanism of Action of Novel Vitamin D Hydroxyderivatives as Antioxidants and Their Potential in the Treatment of Skin Conditions Associated with Photo-Aging
Novel vitamin D derivatives exert their action in the skin through different molecular pathways. They are involved in DNA repair, oxidative stress and wound healing processes.
Nuclear factor erythroid 2-related factor 2 (Nrf2) and Klotho are important factors involved in the regulation of the anti-oxidative response. Klotho, or alpha-Klotho, is an antiaging gene partially regulated by vitamin D3 that plays a beneficial role in aging [219]. Nrf2 is located in the cytoplasm of the cells in its inactive state bound to Kelch-like ECH-associated protein 1 (Keap1) [220]. When cells are undergoing oxidative stress, Nrf2 translocates to the nucleus and binds to the antioxidant responsive element (ARE), thus activating target genes and the process of detoxification [220,221,222]. Nrf2 is involved in removing ROS, chemical detoxification, reducing inflammation in wound healing, skin protection, photo protection and pigmentation [221]. Nrf2 increases synthesis of detoxifying enzymes, such as glutathione peroxidase (GPx), superoxide dismutase (SOD), glutathione S-transferase (GST), gluthathione (GSH), catalase (CAT), heme oxygenase 1 (HO-1), NAD(PH):quinone oxidoreductase (NQO1,), etc. These enzymes further detoxify ROS products, such as peroxide (H2O2) and superoxide anion (O2) [221]. Nrf2 deficiency in mice leads to the development of precancerous and cancerous states [221]. In summary, Nrf2 protects skin from photo-aging by decreasing cytotoxicity and senescence of skin cells, keratinocytes, melanocytes and fibroblasts, and reducing oxidative stress [166,223,224]. Overexpression of Nrf2 in melanocytes leads to the inhibition of melanin production [225] and stimulation of epidermal differentiation [222]. Epidermal differentiation is stimulated by the Nrf2-AhR (aryl hydrocarbon receptor) complex [226]. The AhR controls the expression of the genes involved in epidermal differentiation, such as loricrin and involucrin, and the expression of CYP enzymes [226,227,228]. Stimulation of the Nrf2-AhR complex has been proposed for the treatment of atopic dermatitis, a skin disorder characterized by the dysfunctional skin barrier [226,229]. Of note, AhR has been identified as a receptor for vitamin D derivatives and melatonin [230,231,232,233], all having photo protective activities.
UV induces damage to melanocytes and inhibits Nrf2. Accordingly; products that act as antioxidants and stimulate production of Nrf2 protect melanocytes from UVA/B damage [234,235]. UV induced accumulation of ROS in cells activates nuclear factor kappa B (NF-kB), along with Nrf2 and AhR, crucial transcription factors involved in prevention of premature aging (Figure 2). NF-kB is an immune response regulator [236]. DNA damage can activate p53 and induce apoptosis [187]. Both, DNA damage and oxidative stress can activate p53, which can lead to cell senescence, aging or cancer [237,238,239,240,241,242]. NF-kB can inhibit p53, thus affecting DNA damage, cell metabolism and inflammatory and immune responses, through the process of cell-cycle arrest and apoptosis [236].
It was reported that 1,25(OH)2D3 and other vitamin D metabolites protect skin keratinocytes against UV-induced damage by modulating DNA damage or repair processes, reducing ROS, CPD, 6-4PPs, 8-OH-DG, and enhancing NER [49,177,243,244,245,246,247]. Similarly, novel secosteroids can protect human keratinocytes and melanocytes against UV-induced damage. For example, the topical application of 20(OH)D3 protects murine skin from UVR-induced damage [205,243,244,245]. Novel secosteroids can reduce levels of CPD and stimulate p53-phosphorylation in UV-exposed human keratinocytes and melanocytes [243]. Further, they increase the levels of inhibitory kappa-B protein (IκB) [248] to inhibit ROS-mediated NF-κB p65 translocation to the cell nucleus of keratinocytes and melanocytes [249,250,251]. The described signaling process controls the levels of p53. Novel secosteroids induce phosphorylation of p53 that activates p53 to induce DNA repair or inhibit ROS productions [243]. They also reduce the levels of H2O2 and NO and increase the levels of detoxifying enzymes, such as GSH, thus enhancing DNA repair similarly to melatonin [252]. Novel D3-hydroxyderivatives inhibit UVB-mediated DNA damage by the activation of the Nrf2 pathway and DNA repair as a response to UV-induced damage in skin cells [235] (Figure 2).
Vitamin D plays an important role in wound healing [253,254] and is shown to be effective in the prevention of hypertrophic scars [255,256] and systemic sclerosis [195]. Vitamin D regulates pro-inflammatory cytokines by increasing the levels of anti-inflammatory cytokines, such as IL-10, as part of the wound healing process [255]. An intact VDR is required for normal wound healing, since its ligand, 1,25(OH)2D3, regulates keratinocytes proliferation, differentiation and wound healing processes through VDR [257]. In addition, a lack of VDR induces skin fibrosis and scleroderma in mice [258].
CYP11A1-derived secosteroids can inhibit proliferation of human keratinocytes and melanocytes [61,62,63,64], stimulate cell differentiation [259,260], decrease inflammation [249,250,260] and prevent pathological skin fibrosis [261,262] similarly to 1,25(OH)2D3 (Figure 2). However, they can act through different receptors. Novel secosteroids exert their biological activity through VDR [259], the aryl hydrocarbon receptor (AhR) [230] and liver X receptors (LXRα and β) [231,263], yet at the same time, they acts as inverse agonists on retinoid-related orphan receptors (RORα and RORγ) in murine [262] and human skin cells [264]. Similarly to 1,25(OH)2D3 [253], they show inhibitory effects on induced fibrotic activities in fibroblasts [265]. They inhibited genes involved in fibrosis, such as fibronectin (FN1), a growth factor for fibroblasts, ACTA1 (smooth muscle actin protein), as well as inflammatory genes, such as IL-1, IL-8, IL-33 and TGFB1.
2.4. Current and Future Applications of Vitamin D Hydroxyderivatives in the Treatment of Skin Conditions and as Cosmetic Products
Vitamin D and its derivatives protect the skin against aging by counteracting oxidative stress [19]. In addition, novel D3 hydroxyderivatives stimulate the expression of so-called “anti-aging” genes in human keratinocytes [266], such as Nrf2. The main target for anti-aging skin products is an increase in collagen and elastin in skin to prevent formation of wrinkles. Vitamin D affects collagen turnover by repairing and replacing collagen. Vitamin D decreases collagenase, an enzyme that degrades collagen, and stimulates collagen production [19,253]. Hence, vitamin D can be classified as both an antioxidant and an anti-aging pharmacological agent. This dual feature makes vitamin D a promising target for skin care products or “anti-wrinkle” creams. In addition, Nrf2 activators can be used in treatment of vitiligo, a skin condition where melanocytes have impaired Nrf2-ARE signaling and are prone to oxidative damage [234]. Other skin conditions for which these activators are useful include the treatment of atopic dermatitis [226], UV-induced skin pigmentation [223], aging, wound healing, etc. [220]. Analogs of vitamin D are used for the treatment of vitiligo since they can restore pigment production in melanocytes and have anti-inflammatory effects [267,268]. Novel vitamin D metabolites can stimulate Nrf2 [205], and therefore, they could potentially be used in the treatment of vitiligo or atopic dermatitis [269]. Vitamin D analogs, calcipotriol (available as an ointment and cream) and tacalcitrol, are used for the topical treatment of plaque psoriasis [270]. These lesions are characterized by hyper-proliferation and impaired differentiation of keratinocytes in a response to inflammation. Novel secosteroids are also potent inhibitors of keratinocytes proliferation and stimulators of differentiation [64,250]. These molecules could be applied topically in the treatment of psoriasis in addition to UVB therapy. In addition, antifibrotic biological activities of vitamin D hydroxyderivatives make these molecules good candidates for the treatment of fibrosis and fibrogenesis in scleroderma [271,272,273]. The possible role of novel vitamins D in preventing or delaying the formation of senescent cells or reducing senescent cell viability, such as senolytic drugs [274,275,276,277,278], is not yet explored but isworth testing [279].
Compared to different anti-aging strategies, such as botox and fillers, vitamin D can be delivered into the skin topically [280,281]. Topical application, including transdermal delivery [282], is effective, safe and pain-free [283,284]. The main concern with the use of vitamin D is the potential for increasing calcium levels in blood [285]. These methods of delivery circumvent possible side effects and provide continuous drug delivery, as shown in a randomized controlled trial using vitamin D on the skin [284]. Topical application of vitamin D improves skin hydration and symptoms of dry skin [286]. Many clinical trials proved the efficacy and the safety of topical vitamin D analogs [287]. Topical oral vitamin gel has been described to alleviate oral mucositis in patients undergoing radiation [288]. The topical application of novel secosteroid derivatives is a promising skin anti-aging strategy and should be explored in more depth. It is also important to note that these molecules are present in natural products [289] and can be a safe ingredient for a growing global demand of halal cosmetics [290].
Vitamin D is a lipophilic molecule and requires an enhanced drug delivery in the skin, such as nanocarriers [291,292,293,294,295]. Vitamin D is sensitive to external factors, such as light, heat or moisture, which can affect its bioavailability [296]. To overcome this problem, biocompatible nanocarriers have been developed to protect the vitamin D drugs from the induced degradation and ensure appropriate delivery into the skin superior to existing spray or gel formulations [292,295,297,298].
Cosmetic products, such as sunscreens, can protect skin from damaging effects of UV [299]. Dermatologists recommend using sunscreen and avoiding the sun to protect the skin from UV [300]. Unfortunately, many chemicals found in sunscreens are not safe [301] and they can be toxic or absorbed systemically [302,303]. In addition, many sunscreens do not offer protection against UVA, IR, or blue light. On the other side, UVB is necessary for vitamin D production. Some sources show that limiting the exposure to UVB would block conversion of inactive pro-vitamin D (7DHC) to the active form of vitamin D potentially leading to an insufficient vitamin D production in the skin [42,304]. Some sunscreens can block the necessary vitamin D production in the skin [305,306]. This dual role makes exposure to UVB good and bad at the same time [307].
Currently, there are no optimal sun protection agents and no specific recommendations for optimal sunscreen use [308,309,310]. Therefore, there is a need for new active ingredients that are safe and stable when added to the cosmetic formulations [175,311,312] and possibly act as an anti-oxidant molecule [299], such as vitamin D [313]. Sunscreens with antioxidants can suppress ROS formation more efficiently [314] and prevent photo-aging, similar to vitamin D [279]. This natural product offers protection against UVB and UVA as well. Novel secosteroids decrease ROS formation in UVB-irradiated skin cells [205,243,260,315] and UVA-irradiated skin fibroblasts (our unpublished data). Thus, novel vitamin D hydoxyderivatives are potentially a great alternative to the existing sunscreens or other cream formulations designed to protect the skin since they can reduce UVB-caused effects in the skin [177]. Additionally, topical application of calcitrol protected mice exposed to UV radiation from non-melanoma skin cancer development [316]. It was reported that topical vitamin D in combination with other therapy can inhibit melanoma growth [317]. Our data show that novel secosteroids can also inhibit formation or progression of non-melanoma cancers [48] and melanomas as well [318].
Photo-aging, especially caused by UVA and UVB, accelerates the process of ECM degradation, which is the main culprit in UV-induced changes in the skin dermis [85,140].
3. Conclusions
Aging has negative social and emotional influence on people and contributes to an endless forage for the fountain of youth that will prevent or reverse signs of aging, especially in the skin. This review summarizes the influence of novel vitamin D hydroxyderivatives on skin aging via different mechanisms that offer protection against UV-induced cell damage, which causes all skin cells to enter senescence. The pleiotropic effects of vitamin D make this chemical an excellent candidate in the treatment of different skin diseases. Vitamin D and its metabolites regulate cell proliferation and differentiation in skin cells and enhance skin regeneration. They regulate cell signaling and the genes involved in aging, protect against oxidative damage, direct DNA damage and skin aging. Vitamin D, acting as an antioxidant, can prevent photo-aging by preventing or neutralizing ROS formations or inducing skin repair via inhibition of collagenase synthesis. It is also a natural product. Skin care products targeting aging have Nrf2 activity. Vitamin derivatives target both Nrf2 and AhR, and therefore, they could be used to strengthen the skin barrier function and to treat different skin conditions, such as vitiligo, atopic dermatitis, skin aging, and act as photo protective agents in the skin. Vitamin D regulates the interplay between the NF-kB and p53 that can contribute to the development of the novel cosmetics in skin protection. Therefore, new active ingredients that are safe and stable when added to the cosmetic formulations, such as novel vitamin D compounds, are a potential alternatives to existing sunscreen or other cream formulations designed to protect or rejuvenate the skin. They offer stimulation of natural protection through action on keratinocytes, melanocytes and fibroblasts. The prospects of cosmetic applications of vitamin D in the skin is highly promising, especially regarding their safety since they do not cause hypercalcemia. More research is warranted to explore the use of novel vitamin D derivatives in skin care.
Author Contributions
Conceptualization and methodology, Z.J.; methodology, Z.J.; resources, Z.J.; data curation, Z.J.; writing—original draft preparation, Z.J.; writing—review and editing, A.T.S.; visualization, Z.J.; supervision, A.T.S.; project administration, A.T.S.; funding acquisition, A.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NIH grants 1R01AR073004 and R01AR071189, VA merit 1I01BX004293-01A1 and DOD grant #W81XWH2210689 to ATS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hewison, M.; Bouillon, R.; Giovannucci, E.; Goltzman, D.; Meyer, B.M.; Welsh, J. Feldman and Pike’s Vitamin D, 5th ed.; Academic Press: Oxford, UK, 2024. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: An ancient hormone. Exp. Dermatol. 2011, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jäpelt, R.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Saternus, R.; Vogt, T. Endocrine actions of vitamin D in skin: Relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol. Cell. Endocrinol. 2017, 453, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kira, M.; Kobayashi, T.; Yoshikawa, K. Vitamin D and the skin. J. Dermatol. 2003, 30, 429–437. [Google Scholar] [CrossRef]
- Holick, M.F.; Slominski, A.T. Photobiology of vitamin D. In Feldman and Pike’s Vitamin D; Elsevier: Amsterdam, The Netherlands, 2024; pp. 27–45. [Google Scholar]
- Bikle, D.D. Vitamin D: Newer Concepts of Its Metabolism and Function at the Basic and Clinical Level. J. Endocr. Soc. 2020, 4, bvz038. [Google Scholar] [CrossRef]
- Oonincx, D.; van Keulen, P.; Finke, M.D.; Baines, F.M.; Vermeulen, M.; Bosch, G. Evidence of vitamin D synthesis in insects exposed to UVb light. Sci. Rep. 2018, 8, 10807. [Google Scholar] [CrossRef] [PubMed]
- Black, L.J.; Lucas, R.M.; Sherriff, J.L.; Björn, L.O.; Bornman, J.F. In Pursuit of Vitamin D in Plants. Nutrients 2017, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. 100 Years of Vitamin D: Historical aspects of vitamin D. Endocr. Connect. 2022, 11, e210594. [Google Scholar] [CrossRef]
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. Studies on experimental rockets: XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 53, 293–312. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Mitri, J.; Mathieu, C.; Badenhoop, K.; Tamer, G.; Orio, F.; Mezza, T.; Vieth, R.; Colao, A.; Pittas, A. Mechanisms in endocrinology: Vitamin D as a potential contributor in endocrine health and disease. Eur. J. Endocrinol. 2014, 171, R101–R110. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D deficiency accelerates ageing and age-related diseases: A novel hypothesis. J. Physiol. 2017, 595, 6825–6836. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef]
- Holick, M.F.; Smith, E.; Pincus, S. Skin as the site of vitamin D synthesis and target tissue for 1,25-dihydroxyvitamin D3. Use of calcitriol (1,25-dihydroxyvitamin D3) for treatment of psoriasis. Arch. Dermatol. 1987, 123, 1677–1683a. [Google Scholar] [CrossRef]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Tuckey, R.C.; Jenkinson, C.; Li, W.; Jetten, A.M. Alternative pathways for vitamin D metabolism. In Feldman and Pike’s Vitamin D; Elsevier: Amsterdam, The Netherlands, 2024; pp. 85–109. [Google Scholar]
- Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism and function in the skin. Mol. Cell. Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Jenkinson, C. The vitamin D metabolome: An update on analysis and function. Cell Biochem. Funct. 2019, 37, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Genehr, T.; Knuschke, P.; Pietzsch, J.; Meurer, M. UVB-induced conversion of 7-dehydrocholesterol to 1α,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J. Investig. Dermatol. 2001, 117, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Knuschke, P.; Meurer, M. UVB-induced conversion of 7-dehydrocholesterol to 1α,25-dihydroxyvitamin D3 (calcitriol) in the human keratinocyte line HaCaT. Photochem. Photobiol. 2000, 72, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Li, W.; Tuckey, R.C. Classical and non-classical metabolic transformation of vitamin D in dermal fibroblasts. Exp. Dermatol. 2016, 25, 231–232. [Google Scholar] [CrossRef]
- Gao, C.; Liao, M.Z.; Han, L.W.; Thummel, K.E.; Mao, Q. Hepatic Transport of 25-Hydroxyvitamin D(3) Conjugates: A Mechanism of 25-Hydroxyvitamin D(3) Delivery to the Intestinal Tract. Drug Metab. Dispos. 2018, 46, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.; Carlberg, C. Skin colour and vitamin D: An update. Exp. Dermatol. 2020, 29, 864–875. [Google Scholar] [CrossRef]
- Bikle, D.D.; Chang, S.; Crumrine, D.; Elalieh, H.; Man, M.Q.; Choi, E.H.; Dardenne, O.; Xie, Z.; Arnaud, R.S.; Feingold, K.; et al. 25 Hydroxyvitamin D 1α-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J. Investig. Dermatol. 2004, 122, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Genetic disorders of Vitamin D biosynthesis and degradation. J. Steroid Biochem. Mol. Biol. 2016, 165, 101–108. [Google Scholar] [CrossRef]
- Sakaki, T.; Sawada, N.; Komai, K.; Shiozawa, S.; Yamada, S.; Yamamoto, K.; Ohyama, Y.; Inouye, K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur. J. Biochem. 2000, 267, 6158–6165. [Google Scholar] [CrossRef] [PubMed]
- Tieu, E.W.; Tang, E.K.; Tuckey, R.C. Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. FEBS J. 2014, 281, 3280–3296. [Google Scholar] [CrossRef] [PubMed]
- Maestro, M.A.; Molnar, F.; Carlberg, C. Vitamin D and Its Synthetic Analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef]
- Slominski, A.T.; Tuckey, R.C.; Jetten, A.M.; Holick, M.F. Recent Advances in Vitamin D Biology: Something New under the Sun. J. Investig. Dermatol. 2023, 143, 2340–2342. [Google Scholar] [CrossRef]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A D-lightful story. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V28–V33. [Google Scholar] [CrossRef]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef]
- Grant, W.B. Cause of death for those with diabetes and/or cancer provides further support for an important role of vitamin D in reducing risk of many types of disease. Eur. J. Cancer Prev. 2012, 21, 307. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brozyna, A.A.; Kim, T.K.; Elsayed, M.M.; Janjetovic, Z.; Qayyum, S.; Slominski, R.M.; Oak, A.S.W.; Li, C.Z.; Podgorska, E.; et al. CYP11A1-derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas. Int. J. Oncol. 2022, 61, 96. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D receptor, a tumor suppressor in skin. Can. J. Physiol. Pharmacol. 2015, 93, 349–354. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brozyna, A.A.; Zmijewski, M.A.; Janjetovic, Z.; Kim, T.K.; Slominski, R.M.; Tuckey, R.C.; Mason, R.S.; Jetten, A.M.; Guroji, P.; et al. The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers. Adv. Exp. Med. Biol. 2020, 1268, 257–283. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Thomson, C.; Tongkao-on, W.; Song, E.J.; Carter, S.E.; Dixon, K.M.; Mason, R.S. Protection from ultraviolet damage and photocarcinogenesis by vitamin D compounds. Adv. Exp. Med. Biol. 2014, 810, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Rass, K. Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: An update. Adv. Exp. Med. Biol. 2014, 810, 208–233. [Google Scholar] [PubMed]
- Reichrath, J.; Reichrath, S. Sunlight, vitamin D and malignant melanoma: An update. Adv. Exp. Med. Biol. 2014, 810, 390–405. [Google Scholar]
- Reichrath, J.; Reichrath, S.; Heyne, K.; Vogt, T.; Roemer, K. Tumor suppression in skin and other tissues via cross-talk between vitamin D- and p53-signaling. Front. Physiol. 2014, 5, 166. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D in the Context of Evolution. Nutrients 2022, 14, 3018. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D Genomics: From In Vitro to In Vivo. Front. Endocrinol. 2018, 9, 250. [Google Scholar] [CrossRef]
- Bikle, D.D.; Oda, Y.; Tu, C.L.; Jiang, Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J. Steroid Biochem. Mol. Biol. 2015, 148, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Vantieghem, K.; De Haes, P.; Bouillon, R.; Segaert, S. Dermal fibroblasts pretreated with a sterol Δ7-reductase inhibitor produce 25-hydroxyvitamin D3 upon UVB irradiation. J. Photochem. Photobiol. B 2006, 85, 72–78. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids 2015, 103, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In Vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.J.; Janjetovic, Z.; Kim, T.K.; Sweatman, T.; Lu, Y.; Zjawiony, J.; Tuckey, R.C.; Miller, D.; Slominski, A. Chemical synthesis of 20-hydroxyvitamin D3, which shows antiproliferative activity. Steroids 2010, 75, 926–935. [Google Scholar] [CrossRef]
- Tang, E.K.Y.; Li, W.; Janjetovic, Z.; Nguyen, M.N.; Wang, Z.; Slominski, A.; Tuckey, R.C. Purified Mouse CYP27B1 Can Hydroxylate 20,23-Dihydroxyvitamin D, Producing 1α,20,23-Trihydroxyvitamin D, Which Has Altered Biological Activity. Drug Metab. Dispos. 2010, 38, 1553–1559. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Janjetovic, Z.; Li, W.; Nguyen, M.N.; Zmijewski, M.A.; Zjawiony, J.; Slominski, A. Metabolism of 1α-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1α,20-dihydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 2008, 112, 213–219. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Tuckey, R.C.; Bieniek, R.; Yue, J.M.; Li, W.; Chen, J.J.; Nguyen, M.N.; Tang, E.K.Y.; et al. 20-Hydroxyvitamin D is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol.-Cell Physiol. 2011, 300, C526–C541. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Kim, T.K.; Tieu, E.W.; Tang, E.K.; Lin, Z.; Kovacic, D.; Miller, D.D.; Postlethwaite, A.; Tuckey, R.C.; et al. Novel vitamin D analogs as potential therapeutics: Metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014, 34, 2153–2163. [Google Scholar] [PubMed]
- Slominski, A.T.; Janjetovic, Z.; Fuller, B.E.; Zmijewski, M.A.; Tuckey, R.C.; Nguyen, M.N.; Sweatman, T.; Li, W.; Zjawiony, J.; Miller, D.; et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS ONE 2010, 5, e9907. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Gay, D.L.; Treffeisen, E.; Wang, A.; Supapannachart, R.J.; Cotsarelis, G. Epithelial stem cells and implications for wound repair. Semin. Cell Dev. Biol. 2012, 23, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Honda, T.; Mostafa, A.; Kabashima, K. Stratum corneum as polymer sheet: Concept and cornification processes. Trends Mol. Med. 2022, 28, 350–359. [Google Scholar] [CrossRef]
- Choi, E.H. Aging of the skin barrier. Clin. Dermatol. 2019, 37, 336–345. [Google Scholar] [CrossRef]
- Lim, K.M. Skin Epidermis and Barrier Function. Int. J. Mol. Sci. 2021, 22, 3035. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier function of the skin: “la raison d’etre” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Tadokoro, R.; Shikaya, Y.; Takahashi, Y. Wide coverage of the body surface by melanocyte-mediated skin pigmentation. Dev. Biol. 2019, 449, 83–89. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Haydont, V.; Neiveyans, V.; Perez, P.; Busson, E.; Lataillade, J.; Asselineau, D.; Fortunel, N.O. Fibroblasts from the Human Skin Dermo-Hypodermal Junction are Distinct from Dermal Papillary and Reticular Fibroblasts and from Mesenchymal Stem Cells and Exhibit a Specific Molecular Profile Related to Extracellular Matrix Organization and Modeling. Cells 2020, 9, 368. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Bohm, M.; Paus, R. Translational Neuroendocrinology of Human Skin: Concepts and Perspectives. Trends Mol. Med. 2021, 27, 60–74. [Google Scholar] [CrossRef]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef]
- Agrawal, R.; Hu, A.; Bollag, W.B. The Skin and Inflamm-Aging. Biology 2023, 12, 1396. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato-Endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E.; Nikolakis, G. When the skin is in the center of interest: An aging issue. Clin. Dermatol. 2019, 37, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging: New Insights into an Old Dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef]
- Chamayou-Robert, C.; DiGiorgio, C.; Brack, O.; Doucet, O. Blue light induces DNA damage in normal human skin keratinocytes. Photodermatol. Photoimmunol. Photomed. 2022, 38, 69–75. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A.K.E.; Schiefer, J.L.; Fuchs, P.C.; Petzsch, P.; Kohrer, K.; Suschek, C.V.; Sturmer, E.K.; Oplander, C. Low-Dose Blue Light (420 nm) Reduces Metabolic Activity and Inhibits Proliferation of Human Dermal Fibroblasts. Life 2023, 13, 331. [Google Scholar] [CrossRef]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Aoki, A.; Takahashi, T.; Harano, F. Infrared-A Irradiation-induced Inhibition of Human Keratinocyte Proliferation and Potential Mechanisms. Photochem. Photobiol. 2020, 96, 1105–1115. [Google Scholar] [CrossRef]
- Gromkowska-Kepka, K.J.; Puscion-Jakubik, A.; Markiewicz-Zukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Niikura, T.; Oda, T.; Kumabe, Y.; Ohashi, H.; Sasaki, M.; Igarashi, T.; Kunisada, M.; Yamano, N.; Oe, K.; et al. Exploratory clinical trial on the safety and bactericidal effect of 222-nm ultraviolet C irradiation in healthy humans. PLoS ONE 2020, 15, e0235948. [Google Scholar] [CrossRef] [PubMed]
- Busch, L.; Kröger, M.; Zamudio Díaz, D.F.; Schleusener, J.; Lohan, S.B.; Ma, J.; Witzel, C.; Keck, C.M.; Meinke, M.C. Far-UVC- and UVB-induced DNA damage depending on skin type. Exp. Dermatol. 2023, 32, 1582–1587. [Google Scholar] [CrossRef]
- Pedic, L.; Pondeljak, N.; Situm, M. Recent information on photoaging mechanisms and the preventive role of topical sunscreen products. Acta Dermatovenerol. Alp. Panon. Adriat. 2020, 29, 201–207. [Google Scholar]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef]
- Low, E.; Alimohammadiha, G.; Smith, L.A.; Costello, L.F.; Przyborski, S.A.; von Zglinicki, T.; Miwa, S. How good is the evidence that cellular senescence causes skin ageing? Ageing Res. Rev. 2021, 71, 101456. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Itahana, K.; Campisi, J.; Dimri, G.P. Methods to detect biomarkers of cellular senescence: The senescence-associated β-galactosidase assay. Methods Mol. Biol. 2007, 371, 21–31. [Google Scholar] [CrossRef]
- Fuhrmann-Stroissnigg, H.; Santiago, F.E.; Grassi, D.; Ling, Y.; Niedernhofer, L.J.; Robbins, P.D. SA-β-Galactosidase-Based Screening Assay for the Identification of Senotherapeutic Drugs. J. Vis. Exp. 2019, 148, e58133. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Rhinn, M.; Ritschka, B.; Keyes, W.M. Cellular senescence in development, regeneration and disease. Development 2019, 146, dev151837. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Waaijer, M.E.C.; Slee-Valentijn, M.S.; Stijnen, T.; Westendorp, R.; Maier, A.B. Cellular senescence and chronological age in various human tissues: A systematic review and meta-analysis. Aging Cell 2020, 19, e13083. [Google Scholar] [CrossRef]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Mallette, F.A. Cellular Senescence in Postmitotic Cells: Beyond Growth Arrest. Trends Cell Biol. 2018, 28, 595–607. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, U.; Misra, A.; Tchkonia, T.; Kirkland, J.L. Impact of Senescent Cell Subtypes on Tissue Dysfunction and Repair: Importance and Research Questions. Mech. Ageing Dev. 2021, 198, 111548. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Bitar, M.S.; Abdel-Halim, S.M.; Al-Mulla, F. Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: Implications for evidence-based therapy of delayed wound healing in diabetes. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E951–E963. [Google Scholar] [CrossRef]
- Schosserer, M. The role and biology of senescent cells in ageing-related tissue damage and repair. Mech. Ageing Dev. 2022, 202, 111629. [Google Scholar] [CrossRef]
- Song, P.; An, J.; Zou, M.H. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 2020, 9, 671. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Zhou, D.; Borsa, M.; Simon, A.K. Hallmarks and detection techniques of cellular senescence and cellular ageing in immune cells. Aging Cell 2021, 20, e13316. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Small molecule compounds that induce cellular senescence. Aging Cell 2016, 15, 999–1017. [Google Scholar] [CrossRef]
- Saleh, T.; Tyutynuk-Massey, L.; Cudjoe, E.K., Jr.; Idowu, M.O.; Landry, J.W.; Gewirtz, D.A. Non-Cell Autonomous Effects of the Senescence-Associated Secretory Phenotype in Cancer Therapy. Front. Oncol. 2018, 8, 164. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Niklas, A.; Uruski, P.; Tykarski, A.; Ksiazek, K. Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells. Cell. Mol. Life Sci. 2020, 77, 213–229. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Dabritz, J.H.M.; Zhao, Z.; Yu, Y.; Dorr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Wang, A.S.; Ong, P.F.; Chojnowski, A.; Clavel, C.; Dreesen, O. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci. Rep. 2017, 7, 15678. [Google Scholar] [CrossRef]
- Valerio, H.P.; Ravagnani, F.G.; Ronsein, G.E.; Di Mascio, P. A single dose of Ultraviolet-A induces proteome remodeling and senescence in primary human keratinocytes. Sci. Rep. 2021, 11, 23355. [Google Scholar] [CrossRef]
- Martic, I.; Jansen-Durr, P.; Cavinato, M. Effects of Air Pollution on Cellular Senescence and Skin Aging. Cells 2022, 11, 2220. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, W.; Qu, J.; Liu, G.H. Emerging epigenetic insights into aging mechanisms and interventions. Trends Pharmacol. Sci. 2024, 45, 157–172. [Google Scholar] [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A.; et al. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular Senescence and the Senescence-Associated Secretory Phenotype as Drivers of Skin Photoaging. J. Investig. Dermatol. 2021, 141, 1119–1126. [Google Scholar] [CrossRef]
- Weinmullner, R.; Zbiral, B.; Becirovic, A.; Stelzer, E.M.; Nagelreiter, F.; Schosserer, M.; Lammermann, I.; Liendl, L.; Lang, M.; Terlecki-Zaniewicz, L.; et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. NPJ Aging Mech. Dis. 2020, 6, 4. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Hamsanathan, S.; Gurkar, A.U. Lipids as Regulators of Cellular Senescence. Front. Physiol. 2022, 13, 796850. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Senescence in Wound Repair: Emerging Strategies to Target Chronic Healing Wounds. Front. Cell Dev. Biol. 2020, 8, 773. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.; Linkova, N.; Dyatlova, A.; Kantemirova, R.; Kozlov, K. Senescence-Associated Secretory Phenotype of Cardiovascular System Cells and Inflammaging: Perspectives of Peptide Regulation. Cells 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.; Pawlikowski, J.; Manoharan, I.; van Tuyn, J.; Nelson, D.M.; Rai, T.S.; Shah, P.P.; Hewitt, G.; Korolchuk, V.I.; Passos, J.F.; et al. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 2013, 202, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, O.; Chojnowski, A.; Ong, P.F.; Zhao, T.Y.; Common, J.E.; Lunny, D.; Lane, E.B.; Lee, S.J.; Vardy, L.A.; Stewart, C.L.; et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 2013, 200, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.R.; Kawahara, M.; Malhotra, G.K.; Schaum, N.; Huang, J.; Ved, U.; Beausejour, C.M.; Coppe, J.P.; Rodier, F.; Campisi, J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013, 201, 613–629. [Google Scholar] [CrossRef]
- Wyles, S.P.; Carruthers, J.D.; Dashti, P.; Yu, G.; Yap, J.Q.; Gingery, A.; Tchkonia, T.; Kirkland, J. Cellular Senescence in Human Skin Aging: Leveraging Senotherapeutics. Gerontology 2024, 70, 7–14. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging. Int. J. Mol. Sci. 2022, 23, 6135. [Google Scholar] [CrossRef]
- Chen, W.; Kang, J.; Xia, J.; Li, Y.; Yang, B.; Chen, B.; Sun, W.; Song, X.; Xiang, W.; Wang, X.; et al. p53-related apoptosis resistance and tumor suppression activity in UVB-induced premature senescent human skin fibroblasts. Int. J. Mol. Med. 2008, 21, 645–653. [Google Scholar] [CrossRef]
- Langton, A.K.; Halai, P.; Griffiths, C.E.; Sherratt, M.J.; Watson, R.E. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016, 156, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Franzke, C.W.; Tasanen, K.; Schacke, H.; Zhou, Z.; Tryggvason, K.; Mauch, C.; Zigrino, P.; Sunnarborg, S.; Lee, D.C.; Fahrenholz, F.; et al. Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs. EMBO J. 2002, 21, 5026–5035. [Google Scholar] [CrossRef]
- Yoon, J.E.; Kim, Y.; Kwon, S.; Kim, M.; Kim, Y.H.; Kim, J.H.; Park, T.J.; Kang, H.Y. Senescent fibroblasts drive ageing pigmentation: A potential therapeutic target for senile lentigo. Theranostics 2018, 8, 4620–4632. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, J.E.; Kim, Y.H.; Kim, Y.; Park, T.J.; Kang, H.Y. The potential skin-lightening candidate, senolytic drug ABT263, for photoageing pigmentation. Br. J. Dermatol. 2022, 186, 740–742. [Google Scholar] [CrossRef]
- Shin, J.; Park, J.Y.; Kim, S.J.; Kang, H.Y. Characteristics of keratinocytes in facial solar lentigo with flattened rete ridges: Comparison with melasma. Clin. Exp. Dermatol. 2015, 40, 489–494. [Google Scholar] [CrossRef]
- Michaloglou, C.; Vredeveld, L.C.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; van der Horst, C.M.; Majoor, D.M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Waaijer, M.E.; Gunn, D.A.; Adams, P.D.; Pawlikowski, J.S.; Griffiths, C.E.; van Heemst, D.; Slagboom, P.E.; Westendorp, R.G.; Maier, A.B. P16INK4a Positive Cells in Human Skin Are Indicative of Local Elastic Fiber Morphology, Facial Wrinkling, and Perceived Age. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Waaijer, M.E.; Parish, W.E.; Strongitharm, B.H.; van Heemst, D.; Slagboom, P.E.; de Craen, A.J.; Sedivy, J.M.; Westendorp, R.G.; Gunn, D.A.; Maier, A.B. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 2012, 11, 722–725. [Google Scholar] [CrossRef]
- Jarrold, B.B.; Tan, C.Y.R.; Ho, C.Y.; Soon, A.L.; Lam, T.T.; Yang, X.; Nguyen, C.; Guo, W.; Chew, Y.C.; DeAngelis, Y.M.; et al. Early onset of senescence and imbalanced epidermal homeostasis across the decades in photoexposed human skin: Fingerprints of inflammaging. Exp. Dermatol. 2022, 31, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Nakamizo, S.; Ishida, Y.; Klassen, G.; Chong, P.; Wada, A.; Lim, J.S.Y.; Wright, G.D.; Kabashima, K.; Dreesen, O. Identification and quantification of senescent cell types by lamin B1 and HMGB1 in Actinic keratosis lesions. J. Dermatol. Sci. 2022, 105, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Gaze, D.C.; Tobin, D.J.; Marshall, H.S.; Panske, A.; Panzig, E.; Hibberts, N.A. In Vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J. Investig. Dermatol. Symp. Proc. 1999, 4, 91–96. [Google Scholar] [CrossRef]
- Kligman, L.H. Photoaging. Manifestations, prevention, and treatment. Clin. Geriatr. Med. 1989, 5, 235–251. [Google Scholar] [CrossRef]
- Meyskens, F.L., Jr.; Farmer, P.; Fruehauf, J.P. Redox regulation in human melanocytes and melanoma. Pigment. Cell Res. 2001, 14, 148–154. [Google Scholar] [CrossRef]
- Meyskens, F.L., Jr.; McNulty, S.E.; Buckmeier, J.A.; Tohidian, N.B.; Spillane, T.J.; Kahlon, R.S.; Gonzalez, R.I. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free Radic. Biol. Med. 2001, 31, 799–808. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Contet-Audonneau, J.L.; Jeanmaire, C.; Pauly, G. A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br. J. Dermatol. 1999, 140, 1038–1047. [Google Scholar] [CrossRef]
- Perez-Sanchez, A.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef]
- Hohn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; Konig, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef]
- Mason, R.S.; Sequeira, V.B.; Dixon, K.M.; Gordon-Thomson, C.; Pobre, K.; Dilley, A.; Mizwicki, M.T.; Norman, A.W.; Feldman, D.; Halliday, G.M.; et al. Photoprotection by 1α,25-dihydroxyvitamin D and analogs: Further studies on mechanisms and implications for UV-damage. J. Steroid Biochem. Mol. Biol. 2010, 121, 164–168. [Google Scholar] [CrossRef]
- Wondrak, G.T. Let the sun shine in: Mechanisms and potential for therapeutics in skin photodamage. Curr. Opin. Investig. Drugs 2007, 8, 390–400. [Google Scholar] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Premi, S.; Han, L.; Mehta, S.; Knight, J.; Zhao, D.; Palmatier, M.A.; Kornacker, K.; Brash, D.E. Genomic sites hypersensitive to ultraviolet radiation. Proc. Natl. Acad. Sci. USA 2019, 116, 24196–24205. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Takahashi, K.; Zmudzka, B.Z.; Kornhauser, A.; Miller, S.A.; Tadokoro, T.; Berens, W.; Beer, J.Z.; Hearing, V.J. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006, 20, 1486–1488. [Google Scholar] [CrossRef]
- Boros, G.; Miko, E.; Muramatsu, H.; Weissman, D.; Emri, E.; van der Horst, G.T.; Szegedi, A.; Horkay, I.; Emri, G.; Kariko, K.; et al. Identification of Cyclobutane Pyrimidine Dimer-Responsive Genes Using UVB-Irradiated Human Keratinocytes Transfected with In Vitro-Synthesized Photolyase mRNA. PLoS ONE 2015, 10, e0131141. [Google Scholar] [CrossRef] [PubMed]
- Kunisada, M.; Sakumi, K.; Tominaga, Y.; Budiyanto, A.; Ueda, M.; Ichihashi, M.; Nakabeppu, Y.; Nishigori, C. 8-Oxoguanine formation induced by chronic UVB exposure makes Ogg1 knockout mice susceptible to skin carcinogenesis. Cancer Res. 2005, 65, 6006–6010. [Google Scholar] [CrossRef]
- Sarasin, A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat. Res. 1999, 428, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.F.Z.; Kaina, B.; Meneghini, R.; Menck, C.F.M. How DNA lesions are turned into powerful killing structures: Insights from UV-induced apoptosis. Mutat. Res. 2009, 681, 197–208. [Google Scholar] [CrossRef]
- Mielko, Z.; Zhang, Y.; Sahay, H.; Liu, Y.; Schaich, M.A.; Schnable, B.; Morrison, A.M.; Burdinski, D.; Adar, S.; Pufall, M.; et al. UV irradiation remodels the specificity landscape of transcription factors. Proc. Natl. Acad. Sci. USA 2023, 120, e2217422120. [Google Scholar] [CrossRef]
- Javeri, A.; Lyons, J.G.; Huang, X.X.; Halliday, G.M. Downregulation of Cockayne syndrome B protein reduces human 8-oxoguanine DNA glycosylase-1 expression and repair of UV radiation-induced 8-oxo-7,8-dihydro-2′-deoxyguanine. Cancer Sci. 2011, 102, 1651–1658. [Google Scholar] [CrossRef]
- El-Khamisy, S.F. Oxidative DNA damage and repair at non-coding regulatory regions. Trends Cell Biol. 2023, 33, 939–949. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Gong, J.H.; Wong, J.P.; Wallace, J.L.; Clark-Lewis, I.; Overall, C.M. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 2002, 100, 1160–1167. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Diaconu, A.D.; Ostafie, I.; Ceasovschih, A.; Sorodoc, V.; Lionte, C.; Ancua, C.; Sorodoc, L. Role of Vitamin D in Systemic Sclerosis: A Systematic Literature Review. J. Immunol. Res. 2021, 2021, 9782994. [Google Scholar] [CrossRef]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.M.; Sun, M.; Gasek, N.S.; Hargis, G.R.; Sharafieh, R.; Xu, M. Role of Senescent Cells in Cutaneous Wound Healing. Biology 2022, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Herrling, T.; Jung, K.; Fuchs, J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef]
- Akbar, A.N.; Henson, S.M. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011, 11, 289–295. [Google Scholar] [CrossRef]
- Younis, L.T.; Abu Hassan, M.I.; Taiyeb Ali, T.B.; Bustami, T.J. 3D TECA hydrogel reduces cellular senescence and enhances fibroblasts migration in wound healing. Asian J. Pharm. Sci. 2018, 13, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Pulido, T.; Velarde, M.C.; Alimirah, F. The senescence-associated secretory phenotype: Fueling a wound that never heals. Mech. Ageing Dev. 2021, 199, 111561. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, J.J.; Boissy, R.E.; Hearing, V.J.; King, R.A.; Oetting, W.S.; Ortonne, J.P. The Pigmentary System: Physiology and Pathophysiology, 2nd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; 1310. [Google Scholar] [CrossRef]
- Logesh, R.; Prasad, S.R.; Chipurupalli, S.; Robinson, N.; Mohankumar, S.K. Natural tyrosinase enzyme inhibitors: A path from melanin to melanoma and its reported pharmacological activities. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188968. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Zippin, J.H.; Ito, S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res. 2021, 34, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Pavan, W.J.; Sturm, R.A. The Genetics of Human Skin and Hair Pigmentation. Annu. Rev. Genom. Hum. Genet. 2019, 20, 41–72. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Fisher, D.E. MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Short-term and long-term cellular and molecular events following UV irradiation of skin: Implications for molecular medicine. Expert. Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Fajuyigbe, D.; Lwin, S.M.; Diffey, B.L.; Baker, R.; Tobin, D.J.; Sarkany, R.P.E.; Young, A.R. Melanin distribution in human epidermis affords localized protection against DNA photodamage and concurs with skin cancer incidence difference in extreme phototypes. FASEB J. 2018, 32, 3700–3706. [Google Scholar] [CrossRef]
- Joly-Tonetti, N.; Wibawa, J.I.D.; Bell, M.; Tobin, D.J. An explanation for the mysterious distribution of melanin in human skin: A rare example of asymmetric (melanin) organelle distribution during mitosis of basal layer progenitor keratinocytes. Br. J. Dermatol. 2018, 179, 1115–1126. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, Y.; Coelho, S.G.; Schlenz, K.; Batzer, J.; Smuda, C.; Choi, W.; Brenner, M.; Passeron, T.; Zhang, G.; Kolbe, L.; et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res. 2011, 24, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Postlethwaite, A.E. Skin under the sun: When melanin pigment meets vitamin D. Endocrinology 2015, 156, 1–4. [Google Scholar] [CrossRef][Green Version]
- Nuszkiewicz, J.; Wozniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, M.J.; Shidfar, F.; Jazayeri, S.; Hosseini, A.F.; Ranjbaran, F. Effect of vitamin D supplementation on klotho protein, antioxidant status and nitric oxide in the elderly: A randomized, double-blinded, placebo-controlled clinical trial. Eur. J. Integr. Med. 2020, 35, 101089. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Frantz, M.C.; Rozot, R.; Marrot, L. NRF2 in dermo-cosmetic: From scientific knowledge to skin care products. Biofactors 2023, 49, 32–61. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Ogawa, T.; Roop, D. The KEAP1/NRF2 Signaling Pathway in Keratinization. Antioxidants 2020, 9, 751. [Google Scholar] [CrossRef]
- Kerns, M.L.; Miller, R.J.; Mazhar, M.; Byrd, A.S.; Archer, N.K.; Pinkser, B.L.; Lew, L.; Dillen, C.A.; Wang, R.; Miller, L.S.; et al. Pathogenic and therapeutic role for NRF2 signaling in ultraviolet light-induced skin pigmentation. JCI Insight 2020, 5, e139342. [Google Scholar] [CrossRef]
- Jeayeng, S.; Wongkajornsilp, A.; Slominski, A.T.; Jirawatnotai, S.; Sampattavanich, S.; Panich, U. Nrf2 in keratinocytes modulates UVB-induced DNA damage and apoptosis in melanocytes through MAPK signaling. Free Radic. Biol. Med. 2017, 108, 918–928. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, M.Y.; Sohn, K.C.; Jung, S.Y.; Lee, H.E.; Lim, J.W.; Kim, S.; Lee, Y.H.; Im, M.; Seo, Y.J.; et al. Nrf2 negatively regulates melanogenesis by modulating PI3K/Akt signaling. PLoS ONE 2014, 9, e96035. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef]
- Agostinis, P.; Garmyn, M.; Van Laethem, A. The Aryl hydrocarbon receptor: An illuminating effector of the UVB response. Sci. STKE 2007, 2007, pe49. [Google Scholar] [CrossRef]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of Ketoconazole as an AhR-Nrf2 Activator in Cultured Human Keratinocytes: The Basis of Its Anti-Inflammatory Effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Brozyna, A.A.; Zmijewski, M.A.; Xu, H.; Sutter, T.R.; Tuckey, R.C.; Jetten, A.M.; Crossman, D.K. Differential and Overlapping Effects of 20,23(OH)2D3 and 1,25(OH)2D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)2D3. Int. J. Mol. Sci. 2018, 19, 3072. [Google Scholar] [CrossRef]
- Brzeminski, P.; Fabisiak, A.; Slominski, R.M.; Kim, T.K.; Janjetovic, Z.; Podgorska, E.; Song, Y.; Saleem, M.; Reddy, S.B.; Qayyum, S.; et al. Chemical synthesis, biological activities and action on nuclear receptors of 20S(OH)D3, 20S,25(OH)2D3, 20S,23S(OH)2D3 and 20S,23R(OH)2D3. Bioorg. Chem. 2022, 121, 105660. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Slominski, R.M.; Qayyum, S.; Kim, T.K.; Janjetovic, Z.; Raman, C.; Tuckey, R.C.; Song, Y.; Slominski, A.T. Molecular and structural basis of interactions of vitamin D3 hydroxyderivatives with aryl hydrocarbon receptor (AhR): An integrated experimental and computational study. Int. J. Biol. Macromol. 2022, 209, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Slominski, R.M.; Song, Y.; Janjetovic, Z.; Podgorska, E.; Reddy, S.B.; Song, Y.; Raman, C.; Tang, E.K.Y.; et al. Metabolic activation of tachysterol3 to biologically active hydroxyderivatives that act on VDR, AhR, LXRs, and PPARgamma receptors. FASEB J. 2022, 36, e22451. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Meng, X.; Song, Z.; Lin, J. Nuclear factor erythroid 2-related factor 2 (Nrf2) as a potential therapeutic target for vitiligo. Arch. Biochem. Biophys. 2020, 696, 108670. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef]
- Carra, G.; Lingua, M.F.; Maffeo, B.; Taulli, R.; Morotti, A. P53 vs. NF-κB: The role of nuclear factor-kappa B in the regulation of p53 activity and vice versa. Cell. Mol. Life Sci. 2020, 77, 4449–4458. [Google Scholar] [CrossRef]
- Rossiello, F.; Herbig, U.; Longhese, M.P.; Fumagalli, M.; d’Adda di Fagagna, F. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr. Opin. Genet. Dev. 2014, 26, 89–95. [Google Scholar] [CrossRef]
- Songkiatisak, P.; Rahman, S.M.T.; Aqdas, M.; Sung, M.H. NF-κB, a culprit of both inflamm-ageing and declining immunity? Immun. Ageing 2022, 19, 20. [Google Scholar] [CrossRef]
- Fafian-Labora, J.A.; O’Loghlen, A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol. 2020, 30, 628–639. [Google Scholar] [CrossRef]
- Jiang, X.; Takahashi, N.; Matsui, N.; Tetsuka, T.; Okamoto, T. The NF-κB activation in lymphotoxin β receptor signaling depends on the phosphorylation of p65 at serine 536. J. Biol. Chem. 2003, 278, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, V.A.; Alameda, J.P.; Page, A.; Casanova, M.L. Role of NF-κB in Ageing and Age-Related Diseases: Lessons from Genetically Modified Mouse Models. Cells 2021, 10, 1906. [Google Scholar] [CrossRef] [PubMed]
- Tilstra, J.S.; Clauson, C.L.; Niedernhofer, L.J.; Robbins, P.D. NF-κB in Aging and Disease. Aging Dis. 2011, 2, 449–465. [Google Scholar]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.K.; Wasilewski, P.; Rosas, S.; Hanna, S.; Sayre, R.M.; Dowdy, J.C.; Li, W.; Tuckey, R.C. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J. Steroid Biochem. Mol. Biol. 2015, 148, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Thomson, C.; Gupta, R.; Tongkao-on, W.; Ryan, A.; Halliday, G.M.; Mason, R.S. 1α,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem. Photobiol. Sci. 2012, 11, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Tongkao-On, W.; Carter, S.; Reeve, V.E.; Dixon, K.M.; Gordon-Thomson, C.; Halliday, G.M.; Tuckey, R.C.; Mason, R.S. CYP11A1 in skin: An alternative route to photoprotection by vitamin D compounds. J. Steroid Biochem. Mol. Biol. 2015, 148, 72–78. [Google Scholar] [CrossRef]
- Song, E.J.; Gordon-Thomson, C.; Cole, L.; Stern, H.; Halliday, G.M.; Damian, D.L.; Reeve, V.E.; Mason, R.S. 1α,25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J. Steroid Biochem. Mol. Biol. 2013, 136, 131–138. [Google Scholar] [CrossRef]
- Zhang, C.; Tong, T.; Miao, D.C.; Wang, L.F. Vitamin D inhibits TNF-α induced apoptosis of human nucleus pulposus cells through regulation of NF-κB signaling pathway. J. Orthop. Surg. Res. 2021, 16, 411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Zmijewski, M.A.; Tuckey, R.C.; DeLeon, D.A.; Nguyen, M.N.; Pfeffer, L.M.; Slominski, A.T. 20-Hydroxycholecalciferol, Product of Vitamin D3 Hydroxylation by P450scc, Decreases NF-κB Activity by Increasing IκBα Levels in Human Keratinocytes. PLoS ONE 2009, 4, e5988. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Thorpe, E.M., Jr.; Slominski, A.T. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-κB activity in human keratinocytes. J. Cell. Physiol. 2010, 223, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Brozyna, A.A.; Tuckey, R.C.; Kim, T.K.; Nguyen, M.N.; Jozwicki, W.; Pfeffer, S.R.; Pfeffer, L.M.; Slominski, A.T. High basal NF-κB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br. J. Cancer 2011, 105, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Nahmias, Z.P.; Hanna, S.; Jarrett, S.G.; Kim, T.K.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J. Pineal Res. 2014, 57, 90–102. [Google Scholar] [CrossRef]
- Ding, J.; Kwan, P.; Ma, Z.; Iwashina, T.; Wang, J.; Shankowsky, H.A.; Tredget, E.E. Synergistic effect of vitamin D and low concentration of transforming growth factor beta 1, a potential role in dermal wound healing. Burns 2016, 42, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Role of vitamin D and calcium signaling in epidermal wound healing. J. Endocrinol. Investig. 2023, 46, 205–212. [Google Scholar] [CrossRef]
- Akoh, C.C.; Orlow, S.J. A Review of Vitamin D and Scarring: The Potential for New Therapeutics. J. Drugs Dermatol. 2020, 19, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Moravvej, H.; Memariani, H.; Memariani, M. Vitamin D Deficiency and Keloids: Causal Factor or Bystander? Dermatology 2022, 238, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Hu, L.Z.; Nguyen, T.; Fong, C.; Zhang, J.; Guo, P.; Bikle, D.D. Vitamin D Receptor Is Required for Proliferation, Migration, and Differentiation of Epidermal Stem Cells and Progeny during Cutaneous Wound Repair. J. Investig. Dermatol. 2018, 138, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Luo, J.; Li, D.; Li, C.; Huang, J.; Yu, H.; Lin, X.; Li, Y.; Man, M.; Zhang, J.; et al. Deficiency of vitamin D receptor in keratinocytes augments dermal fibrosis and inflammation in a mouse model of HOCl-induced scleroderma. Biochem. Biophys. Res. Commun. 2022, 591, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zbytek, B.; Janjetovic, Z.; Tuckey, R.C.; Zmijewski, M.A.; Sweatman, T.W.; Jones, E.; Nguyen, M.N.; Slominski, A.T. 20-hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J. Investig. Dermatol. 2008, 128, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Tuckey, R.C.; Li, W.; Raman, C.; Panich, U.; Slominski, A.T. CYP11A1-derived vitamin D 3 products protect against UVB-induced inflammation and promote keratinocytes differentiation. Free. Radic. Biol. Med. 2020, 155, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Brown Lobbins, M.L.; Scott, I.O.; Slominski, A.T.; Hasty, K.A.; Zhang, S.; Miller, D.D.; Li, W.; Kim, T.K.; Janjetovic, Z.; Patel, T.S.; et al. 17,20S(OH)2pD Can Prevent the Development of Skin Fibrosis in the Bleomycin-Induced Scleroderma Mouse Model. Int. J. Mol. Sci. 2021, 22, 8926. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Postlethwaite, A.; Kang, H.S.; Kim, T.K.; Tuckey, R.C.; Crossman, D.K.; Qayyum, S.; Jetten, A.M.; Slominski, A.T. Antifibrogenic Activities of CYP11A1-derived Vitamin D3-hydroxyderivatives Are Dependent on RORgamma. Endocrinology 2021, 162, bqaa198. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Qayyum, S.; Song, Y.; Janjetovic, Z.; Oak, A.S.W.; Slominski, R.M.; Raman, C.; Stefan, J.; Mier-Aguilar, C.A.; et al. Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs). Sci. Rep. 2021, 11, 8002. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Reddy, S.B.; Podgorska, E.; Scott, S.G.; Szpotan, J.; Mobley, A.A.; Li, W.; Boda, V.K.; Ravichandran, S.; Tucley, R.C.; et al. Novel Vitamin D3 Hydroxymetabolites Require Involvement of the Vitamin D Receptor or Retinoic Acid-Related Orphan Receptors for Their Antifibrogenic Activities in Human Fibroblasts. Cells 2024, 13, 239. [Google Scholar] [CrossRef]
- Stefan, J.; Kim, T.K.; Schedel, F.; Janjetovic, Z.; Crossman, D.K.; Steinbrink, K.; Slominski, R.M.; Zmijewski, J.; Tulic, M.K.; Reiter, R.J.; et al. Differential and Overlapping Effects of Melatonin and Its Metabolites on Keratinocyte Function: Bioinformatics and Metabolic Analyses. Antioxidants 2021, 10, 618. [Google Scholar] [CrossRef]
- Abdel Latif, A.A.; Ibrahim, S.M. Monochromatic excimer light versus combination of topical steroid with vitamin D3 analogue in the treatment of nonsegmental vitiligo: A randomized blinded comparative study. Dermatol. Ther. 2015, 28, 383–389. [Google Scholar] [CrossRef]
- Al-Smadi, K.; Ali, M.; Alavi, S.E.; Jin, X.; Imran, M.; Leite-Silva, V.R.; Mohammed, Y. Using a Topical Formulation of Vitamin D for the Treatment of Vitiligo: A Systematic Review. Cells 2023, 12, 2387. [Google Scholar] [CrossRef]
- Wat, H.; Dytoc, M. Off-label uses of topical vitamin D in dermatology: A systematic review. J. Cutan. Med. Surg. 2014, 18, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Galvan, J.; Crutchley, N.; Praestegaard, M.; Iversen, L.; Gisondi, P.; Carrascosa, J.M.; Halioua, B.; Bewley, A.; Pinter, A. Calcipotriol and Betamethasone Dipropionate Cream Based on PAD Technology for the Treatment of Plaque Psoriasis: A Narrative Review. Dermatol. Ther. 2023, 13, 2153–2169. [Google Scholar] [CrossRef]
- Usategui, A.; Criado, G.; Del Rey, M.J.; Fare, R.; Pablos, J.L. Topical vitamin D analogue calcipotriol reduces skin fibrosis in experimental scleroderma. Arch. Dermatol. Res. 2014, 306, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Terao, M.; Yang, L.; Matsumura, S.; Yutani, M.; Murota, H.; Katayama, I. A vitamin D analog inhibits Th2 cytokine- and TGFβ-induced periostin production in fibroblasts: A potential role for vitamin D in skin sclerosis. Dermato-Endocrinology 2015, 7, e1010983. [Google Scholar] [CrossRef]
- Wadhwa, B.; Relhan, V.; Goel, K.; Kochhar, A.M.; Garg, V.K. Vitamin D and skin diseases: A review. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 344–355. [Google Scholar] [CrossRef]
- Robbins, P.D.; Jurk, D.; Khosla, S.; Kirkland, J.L.; LeBrasseur, N.K.; Miller, J.D.; Passos, J.F.; Pignolo, R.J.; Tchkonia, T.; Niedernhofer, L.J. Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 779–803. [Google Scholar] [CrossRef] [PubMed]
- Pils, V.; Ring, N.; Valdivieso, K.; Lammermann, I.; Gruber, F.; Schosserer, M.; Grillari, J.; Ogrodnik, M. Promises and challenges of senolytics in skin regeneration, pathology and ageing. Mech. Ageing Dev. 2021, 200, 111588. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.L.; Pitcher, L.E.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Development of New Approaches for Skin Care. Plast. Reconstr. Surgery 2022, 150, 12S–19S. [Google Scholar] [CrossRef]
- Danczak-Pazdrowska, A.; Gornowicz-Porowska, J.; Polanska, A.; Krajka-Kuzniak, V.; Stawny, M.; Gostynska, A.; Rubis, B.; Nourredine, S.; Ashiqueali, S.; Schneider, A.; et al. Cellular senescence in skin-related research: Targeted signaling pathways and naturally occurring therapeutic agents. Aging Cell 2023, 22, e13845. [Google Scholar] [CrossRef]
- Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a Shield against Aging. Int. J. Mol. Sci. 2023, 24, 4546. [Google Scholar] [CrossRef] [PubMed]
- Pozos-Nonato, S.; Dominguez-Delgado, C.L.; Campos-Santander, K.A.; Benavides, A.A.; Pacheco-Ortin, S.M.; Higuera-Piedrahita, R.I.; Resendiz-Gonzalez, G.; Molina-Trinidad, E.M. Novel Nanotechnological Strategies for Skin Anti-aging. Curr. Pharm. Biotechnol. 2023, 24, 1397–1419. [Google Scholar] [CrossRef]
- Griffiths, T.W.; Watson, R.E.B.; Langton, A.K. Skin ageing and topical rejuvenation strategies. Br. J. Dermatol. 2023, 189, i17–i23. [Google Scholar] [CrossRef] [PubMed]
- Alsaqr, A.; Rasoully, M.; Musteata, F.M. Investigating transdermal delivery of vitamin D3. AAPS PharmSciTech 2015, 16, 963–972. [Google Scholar] [CrossRef]
- Sadat-Ali, M.; Bubshait, D.A.; Al-Turki, H.A.; Al-Dakheel, D.A.; Al-Olayani, W.S. Topical delivery of vitamin D3: A randomized controlled pilot study. Int. J. Biomed. Sci. 2014, 10, 21–24. [Google Scholar] [CrossRef]
- Bubshait, D.A.; Al-Dakheel, D.A.; Alanii, F.M. Topical vitamin D3: A randomized controlled trial (RCT). Clin. Nutr. ESPEN 2018, 27, 16–19. [Google Scholar] [CrossRef]
- Chauhan, K.; Shahrokhi, M.; Huecker, M.R. Vitamin D. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Russell, M. Assessing the relationship between vitamin D3 and stratum corneum hydration for the treatment of xerotic skin. Nutrients 2012, 4, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.K. The rationale behind topical vitamin d analogs in the treatment of psoriasis: Where does topical calcitriol fit in? J. Clin. Aesthetic Dermatol. 2010, 3, 46–53. [Google Scholar]
- Bakr, I.S.; Zaki, A.M.; El-Moslemany, R.M.; Elsaka, R.O. Vitamin D oral gel for prevention of radiation-induced oral mucositis: A randomized clinical trial. Oral Dis. 2021, 27, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Atigadda, V.; Brzeminski, P.; Fabisi6ak, A.; Tang, E.K.Y.; Tuckey, R.C.; Slominski, A.T. Detection of 7-Dehydrocholesterol and Vitamin D3 Derivatives in Honey. Molecules 2020, 25, 2583. [Google Scholar] [CrossRef] [PubMed]
- Isa, R.M.; Man, S.; Rahman, N.N.A.; Aziz, A. Determinants of consumer adoption of halal cosmetics: A systematic literature review. J. Cosmet. Dermatol. 2023, 22, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tsai, P.C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ramezanli, T.; Kilfoyle, B.E.; Zhang, Z.; Michniak-Kohn, B.B. Polymeric nanospheres for topical delivery of vitamin D3. Int. J. Pharm. 2017, 516, 196–203. [Google Scholar] [CrossRef]
- Guttoff, M.; Saberi, A.H.; McClements, D.J. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: Factors affecting particle size and stability. Food Chem. 2015, 171, 117–122. [Google Scholar] [CrossRef]
- Almouazen, E.; Bourgeois, S.; Jordheim, L.P.; Fessi, H.; Briancon, S. Nano-encapsulation of vitamin D3 active metabolites for application in chemotherapy: Formulation study and in vitro evaluation. Pharm. Res. 2013, 30, 1137–1146. [Google Scholar] [CrossRef]
- Ramezanli, T.; Zhang, Z.; Michniak-Kohn, B.B. Development and characterization of polymeric nanoparticle-based formulation of adapalene for topical acne therapy. Nanomedicine 2017, 13, 143–152. [Google Scholar] [CrossRef]
- Ballard, J.M.; Zhu, L.; Nelson, E.D.; Seburg, R.A. Degradation of vitamin D3 in a stressed formulation: The identification of esters of vitamin D3 formed by a transesterification with triglycerides. J. Pharm. Biomed. Anal. 2007, 43, 142–150. [Google Scholar] [CrossRef]
- Tapfumaneyi, P.; Imran, M.; Alavi, S.E.; Mohammed, Y. Science of, and insights into, thermodynamic principles for dermal formulations. Drug Discov. Today 2023, 28, 103521. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Gkiouras, K.; Nigdelis, M.P.; Bogdanos, D.P.; Goulis, D.G. Efficacy of Vitamin D3 Buccal Spray Supplementation Compared to Other Delivery Methods: A Systematic Review of Superiority Randomized Controlled Trials. Nutrients 2020, 12, 691. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Arellano-Mendoza, M.I.; Stengel, F. Current challenges in photoprotection. J. Am. Acad. Dermatol. 2017, 76, S91–S99. [Google Scholar] [CrossRef] [PubMed]
- Stechschulte, S.A.; Kirsner, R.S.; Federman, D.G. Sunscreens for non-dermatologists: What you should know when counseling patients. Postgrad. Med. 2011, 123, 160–167. [Google Scholar] [CrossRef]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; De Giorgi, M.; Guido, M.; Congedo, M.; De Donno, A. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, E50–E57. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Reply to “the five paradoxes of vitamin D and the importance of sunscreen protection”. Clin. Pediatr. 2013, 52, 994. [Google Scholar] [CrossRef]
- Abdi, S.A.H.; Ali, A.; Sayed, S.F.; Nagarajan, S.; Abutahir; Alam, P.; Ali, A. Sunscreen Ingredient Octocrylene’s Potency to Disrupt Vitamin D Synthesis. Int. J. Mol. Sci. 2022, 23, 154. [Google Scholar] [CrossRef]
- Grigalavicius, M.; Iani, V.; Juzeniene, A. Layer Thickness of SPF 30 Sunscreen and Formation of Pre-vitamin D. Anticancer. Res. 2016, 36, 1409–1415. [Google Scholar]
- Neale, R.E.; Lucas, R.M.; Byrne, S.N.; Hollestein, L.; Rhodes, L.E.; Yazar, S.; Young, A.R.; Berwick, M.; Ireland, R.A.; Olsen, C.M. The effects of exposure to solar radiation on human health. Photochem. Photobiol. Sci. 2023, 22, 1011–1047. [Google Scholar] [CrossRef]
- Lucas, R.M.; Neale, R.E.; Madronich, S.; McKenzie, R.L. Are current guidelines for sun protection optimal for health? Exploring the evidence. Photochem. Photobiol. Sci. 2018, 17, 1956–1963. [Google Scholar] [CrossRef]
- Berwick, M. The good, the bad, and the ugly of sunscreens. Clin. Pharmacol. Ther. 2011, 89, 31–33. [Google Scholar] [CrossRef]
- Young, A.R.; Narbutt, J.; Harrison, G.I.; Lawrence, K.P.; Bell, M.; O’Connor, C.; Olsen, P.; Grys, K.; Baczynska, K.A.; Rogowski-Tylman, M.; et al. Optimal sunscreen use, during a sun holiday with a very high ultraviolet index, allows vitamin D synthesis without sunburn. Br. J. Dermatol. 2019, 181, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Manela-Azulay, M.; Bagatin, E. Cosmeceuticals vitamins. Clin. Dermatol. 2009, 27, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N. Approaches in line with human physiology to prevent skin aging. Front. Physiol. 2023, 14, 1279371. [Google Scholar] [CrossRef]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.Y.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef]
- Kim, J.S.; Jung, M.; Yoo, J.; Choi, E.H.; Park, B.C.; Kim, M.H.; Hong, S.P. Protective Effect of Topical Vitamin D3 against Photocarcinogenesis in a Murine Model. Ann. Dermatol. 2016, 28, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Azin, M.; Ngo, K.H.; Hojanazarova, J.; Demehri, S. Topical Calcipotriol Plus Imiquimod Immunotherapy for Nonkeratinocyte Skin Cancers. JID Innov. 2023, 3, 100221. [Google Scholar] [CrossRef] [PubMed]
- Podgorska, E.; Kim, T.K.; Janjetovic, Z.; Urbanska, K.; Tuckey, R.C.; Bae, S.; Slominski, A.T. Knocking out the Vitamin D Receptor Enhances Malignancy and Decreases Responsiveness to Vitamin D3 Hydroxyderivatives in Human Melanoma Cells. Cancers 2021, 13, 3111. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).