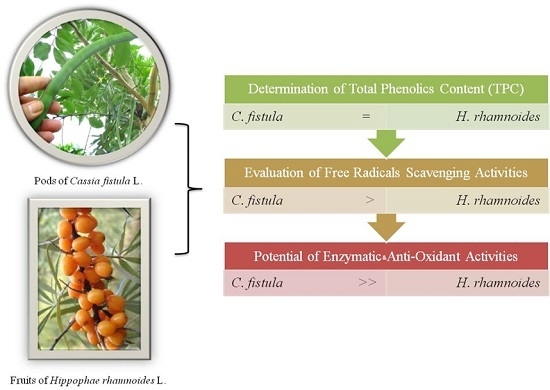
Relative Free Radicals Scavenging and Enzymatic Activities of Hippophae rhamnoides and Cassia fistula Extracts: Importance for Cosmetic, Food and Medicinal Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Key Chemicals, Reagents and Enzymes
2.2. Plants
2.3. Analytical Apparatus
2.4. Plant Extraction
2.5. Total Phenolic Content (TPC) Determination
2.6. DPPH Free Radical Scavenging Activity
2.7. Nitric Oxide Radical [NO•] Scavenging Assay
2.8. Hydroxyl Ion Radical [OH•] Scavenging Assay
2.9. Superoxide Anion Radical [O2−•] Scavenging Assay
2.10. β-Glucuronidase Inhibition Assay
2.11. α-Glucosidase Inhibition Assay
2.12. α-Tyrosinase Inhibition Assay
2.13. Statistical Analysis
3. Results and Discussion
4. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| C. fistula | Cassia fistula |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DMSO | Dimethylsulfoxide |
| EDTA | Ethyldiaminetetraacetic |
| FAs:FCR | Fatty acids Folin-Ciocalteu reagent |
| GAE | Gallic acid equivalent |
| H. rhamnoides | Hippophae rhamnoides |
| IC50 | Concentration requested to obtain 50% inhibition |
| Na2CO3 | Sodium bicarbonate |
| TCA | Trichloroacetic acid |
| TPC | Total phenolic content |
| TYR | Tyrosinase |
| UFAs | Unsaturated fatty acids |
References
- Menaa, F.; Badole, S.L.; Menaa, B.; Menaa, A. Promising plant extracts with in vivo anti-melanoma potential. In Bioactive Dietary Factors and Plant Extracts in Dermatology, 1st ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Humana Press Inc., Springer: New York, NY, USA, 2013; pp. 283–290. [Google Scholar]
- Menaa, F.; Menaa, A.; Tréton, J. Polyphenols against skin aging. In Polyphenols in Human Health and Disease, 1st ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press, Elsevier Publisher: Cambridge, MA, USA, 2013; pp. 819–829. [Google Scholar]
- Menaa, F.; Menaa, A. Skin photoprotection by polyphenols in animal models and humans. In Polyphenols in Human Health and Disease, 1st ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press, Elsevier Publisher: Cambridge, MA, USA, 2013; pp. 831–838. [Google Scholar]
- Zia-Ul-Haq, M.; Shakeel, A.; Mughal, Q.; Sezai, E. Compositional studies and anti-oxidant potential of Albizia lebbeck (L.) Benth. pods and seeds. Turk. J. Biol. 2013, 37, 25–32. [Google Scholar]
- Khan, B.A.; Akhtar, N.; Braga, V.A. Anti-Aging Effects of Hippophae rhamnoides Emulsion on Human Skin. Trop. J. Pharm. Res. 2012, 11, 955–962. [Google Scholar] [CrossRef]
- Zenser, T.V.; Lakshmi, V.M.; Davis, B.B. Human and Escherichia Coli β-glucuronidase hydrolysis of glucuronide conjugates of benzidine and 4-aminobiphenyl, and their hydroxy metabolites. Drug Metab. Dispos. 1999, 27, 1064–1067. [Google Scholar] [PubMed]
- Ho, K.J.; Ho, L.H. Determination of urinary beta-glucuronidase activity. Single-point versus enzyme kinetic measuring system. Enzyme 1980, 25, 361–370. [Google Scholar]
- Sanjeev, J.; William, B.D.; Zhi-wel, C.F.; Sly, W.S.; Jeffery, H.G. Structure of human β-glucuronidase reveals candidate lysosomal targeting and active site motifs. Nat. Struct. Biol. 1996, 3, 375–381. [Google Scholar]
- Marsh, C.A. A glucuronide-decomposing enzyme from rumen micro-organisms. 2. Purification and kinetics. Biochem. J. 1954, 58, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Takasuna, K.; Kasai, Y.; Kitano, Y.; Mori, K.; Kobayashi, R.; Hagiwara, T.; Kakihata, K.; Hirohashi, M.; Nomura, M.; Nagai, E. Protective effects of kampo medicines and baicalin against intestinal toxicity of a new anticancer camptothecin derivative, irinotecan hydrochloride (CPT-11), in rats. Jpn. J. Cancer Res. 1995, 86, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Sperker, B.; Backman, J.T.; Kroemer, H.K. The role of beta-glucuronidase in drug disposition and drug targeting in humans. Clin. Pharmacokinet. 1997, 33, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Takasuna, K.; Hagiwara, T.; Hirohashi, M.; Kato, M.; Nomura, M.; Nagai, E.; Yokoi, T.; Kamataki, T. Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats. Cancer Chemother. Pharmacol. 1998, 42, 280–286. [Google Scholar] [CrossRef]
- Schmiegelow, P.; Nüssgen, A.; Grasedyck, K.; Lindner, J. Skin changes in advanced age Biochemical findings corresponding to morphology? Z. Gerontol. 1986, 19, 179–189. [Google Scholar] [PubMed]
- Koehler, V. Topical Compositions e.g., Ointment Contg. Beta-Glucuronidase—For Treatment of Non-Infectious Skin Disorders e.g., Psoriasis. German Patent DE 2819129 A1, 15 November 1979. [Google Scholar]
- Holtzinger, G. Cosmetic Composition and Method for Suppressing Human Body Malodor Arise from Sweat. European Patent EP0714655 A1, 5 June 1996. [Google Scholar]
- Lembcke, B.; Löser, C.; Fölsch, U.R.; Wöhler, J.; Creutzfeldt, W. Adaptive responses to pharmacological inhibition of small intestinal alpha-glucosidases in the rat. Gut 1987, 28, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S. Molecular mechanism in α-glucosidase and glucoamylase. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Alpha glucosidase inhibitors. Endocrinol. Metab. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Hirsh, A.J.; Yao, S.Y.; Young, J.D.; Cheeseman, C.I. Inhibition of glucose absorption in the rat jejunum: A novel action of alpha-d-glucosidase inhibitors. Gastroenterology 1997, 113, 205–211. [Google Scholar] [CrossRef]
- Walaszek, Z.M.; Hanausek, W. Inhibition of 7,12 dimethylbenzanthracene-induced rat mammary tumorigenesis by 2,5-di-o-acetyl-d-glucaro-1,4:6,3-dilactone, an in vivo ß-glucuronidase inhibitor. Carcinogenesis 1984, 5, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Declercq, L.; Maes, D.H.; Corstjens, H.A. Cosmetic Compositions Containing Alpha Glucosidase Inhibitors and Methods of Use. U.S. Patent 9072717 B2, 7 July 2007. [Google Scholar]
- Declercq, L.; Maes, D.H.; Corstjens, H.A. Cosmetic Compositions Containing Alpha Glucosidase Inhibitors and Methods of Use. U.S. Patent 20090074822 A1, 19 March 2009. [Google Scholar]
- Menaa, F.; Menaa, A. Progressive genetic architecture of human skin pigmentation. J. Primatol. 2013, 3, 116. [Google Scholar] [CrossRef]
- Kumar, C.M.; Sathisha, U.V.; Dharmesh, S.; Rao, A.G.; Singh, S.A. Interaction of sesamol (3,4-methylenedioxyphenol) with tyrosinase and its effect on melanin synthesis. Biochimie 2011, 93, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.E.; Kwon, B.S.; Francke, U. Human tyrosinase gene, mapped to chromosome 11 (q14 → q21), defines second region of homology with mouse chromosome 7. Genomics 1988, 3, 17–24. [Google Scholar] [CrossRef]
- Theos, A.C.; Tenza, D.; Martina, J.A.; Hurbain, I.; Peden, A.A.; Sviderskaya, E.V.; Stewart, A.; Robinson, M.S.; Bennett, D.C.; Cutler, D.F.; et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005, 16, 5356–5372. [Google Scholar] [CrossRef] [PubMed]
- Witkop, C.J. Albinism: Hematologic-storage disease, susceptibility to skin cancer, and optic neuronal defects shared in all types of oculocutaneous and ocular albinism. Ala. J. Med. Sci. 1979, 16, 327–330. [Google Scholar] [CrossRef]
- Menaa, F. Latest Approved Therapies for Metastatic Melanoma: What Comes Next? J. Skin Cancer 2013, 2013, 735282. [Google Scholar] [CrossRef] [PubMed]
- Ubeid, A.A. Short-sequence oligopeptides with inhibitory activity against mushroom and human tyrosinase. J. Investig. Dermatol. 2009, 129, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Fais, A. Tyrosinase inhibitor activity of coumarin-resveratrol hybrids. Molecules 2009, 14, 2514–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.S. An Updated Review of Tyrosinase Inhibitor. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Choi, S. Aloesin inhibits the hyperpigmentation induced by UV radiation. Clin. Exp. Dermatol. 2002, 27, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of Highly Potent Tyrosinase Inhibitor, T1, with Significant Anti-Melanogenesis Ability by zebrafish in vivo Assay and Computational Molecular Modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Khan, L.; Marwat, G.A.; Ahmed, N.; Saleem, M. Comparative study of Vitamin C contents in fruits and medicinal plants. J. Chem. Soc. Pak. 2008, 30, 406–409. [Google Scholar]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 49, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Akhtar, N.; Hussain, I.; Abbas, K.A.; Rasul, A. Whitening efficacy of plant extracts including Hippophae rhamnoides and Cassia fistula extracts on the skin of Asian patients with melasma. Postep. Dermatol. Alergol. 2013, 30, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Neergheen, V.S.; Aruoma, O.I. Phytochemical constituents of Cassia fistula. Afr. J. Biotechnol. 2005, 4, 1530–1540. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Menaa, A.; Menaa, F. A Novel Cassia fistula (L)-Based Emulsion Elicits Skin Anti-Aging Benefits in Humans. Cosmetics 2015, 2, 368–383. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Nariya, P.B.; Acharya, R.N.; Shukla, V.J. In vitro anti-oxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. Ayu 2013, 34, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Manonmani, G.; Bhavapriya, V.; Kalpana, S.; Govindasamy, S.; Apparanantham, T. Anti-oxidant activity of Cassia fistula (Linn.) flowers in alloxan induced diabetic rats. J. Ethnopharmacol. 2005, 97, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Universal Protein Resource ß-glucuronidase. Available online: http://www.uniprot.org/uniprot/A3KMY8 (accessed on 2 February 2014).
- Universal Protein Resource α-glucosidase. Available online: http://www.uniprot.org/uniprot/P38138 (accessed on 2 February 2014).
- Universal Protein Resource Tyrosinase. Available online: http://www.uniprot.org/uniprot/K9HSW6 (accessed on 2 February 2014).
- The Plant List, C. fistula L. Available online: http://www.theplantlist.org/1.1/browse/A/Leguminosae/Cassia/ (accessed on 2 February 2014).
- The plant list H. rhamnoides L. Available online: http://www.theplantlist.org/1.1/browse/A/Elaeagnaceae/Hippophae/ (accessed on 2 February 2014).
- Khan, B.A.; Akhtar, N. Phytochemical analysis and acute toxicity tests of two medicinal plant extracts. J. Med. Plant Res. 2012, 6, 3545–3548. [Google Scholar]
- Elmastas, M.; Gulcin, I.; Isildak, O.; Kufrevioglu, O.I.; Ibaoglu, K.; Aboul-Enein, H.Y. Radical scavenging activity and anti-oxidant capacity of Bay leaf extracts. J. Iran. Chem. Soc. 2006, 3, 258–266. [Google Scholar] [CrossRef]
- Ramakrishna, H.; Sushma, S.; Murthy, D.R.; Mamatharani, D.R.; Panduranga, M.G. Hydroxy radical and DPPH scavenging activity of crude protein extract of Leucas linifolia: A folk medicinal plant. Asian J. Plant Sci. Res. 2012, 2, 30–35. [Google Scholar]
- Collins, R.A.; Ng, T.B.; Fong, W.P.; Wan, C.C.; Yeung, H.W. Inhibition of glycohydrolase enzyme by aqueous extracts of Chines medicinal herbs in microplate format. Biochem. Mol. Biol. Int. 1997, 42, 1163–1169. [Google Scholar] [PubMed]
- Tiwari, A.K.; Swapna, M.; Ayesha, S.B.; Zehra, A.; Agawane, S.B.; Madhusudana, K. Identification of proglycemic and antihyperglycemic activity in anti-oxidant rich fraction of some common food grains. Int. Food Res. J. 2011, 18, 915–923. [Google Scholar]
- Lee, K.H.; Ab Aziz, F.H.; Syahida, A.; Abas, F.; Shaari, K.; Israf, D.A.; Lajis, N.H. Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and antityrosinase activities. Eur. J. Med. Chem. 2009, 44, 3195–3200. [Google Scholar] [CrossRef] [PubMed]
- Korekar, G.; Stobdan, T.S.; Chaurasia, O.P.; Singh, S.B. Phenolic content and anti-oxidant capacity of various solvent extracts from Seabuckthorn (Hippophae rhamnoides L.) fruit pulp, seeds, leaves and stem bark. Acta Aliment. 2011, 40, 449–458. [Google Scholar] [CrossRef]
- Alwen, A.; Benito Moreno, R.M.; Vicente, O.; Heberle-Bors, E. Plant endogenous beta glucuronidase activity: How to avoid interference with the use of the E. coli beta glucuronidase as a reporter gene in transgenic plants. Transgenic Res. 1992, 1, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cho, S. N-benzylbenzamides a new class of potent tyrosinase inhibitors. Bioorganic Med. Chem. 2006, 16, 2682–2684. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef] [PubMed]

| Extracts: Scavenging Assay | H. rhamnoides (IC50 in µg/mL) | C. fistula (IC50 in µg/mL) | Ascorbic Acid (IC50 in µg/mL) |
|---|---|---|---|
| Nitric oxide radical [NO•] | 139.38 ± 1.14 a | 116.82 ± 0.89 c | 66.04 ± 1.01 b |
| DPPH radical | 107.26 ± 1.34 a | 89.07 ± 1.31 c | 31.34 ± 2.018 b |
| Hydroxyl radical [OH•] | 91.04 ± 1.04 a | 86.45 ± 0.93 a | 74.91 ± 1.22 b |
| Superoxide radical [O2−•] | 122.15 ± 1.09 a | 103.25 ± 1.37 c | 47.21 ± 1.14 b |
| Extract | β-Glucuronidase | α-Glucosidase | α-Tyrosinase | |||
|---|---|---|---|---|---|---|
| % Inhibition at 0.05 mg | IC50 (µg) | % Inhibition at 0.05 mg | IC50 (µg) | % Inhibition at 0.05 mg | IC50 (µg) | |
| H. rhamnoides | 65.55 ± 0.1 a | 61.21 ± 0.5 a | 41.90 ± 0.25 d | 51.09 ± 0.14 f | 72.57 ± 0.3 g | 45.9 ± 0.2 h |
| C. fistula | 61.23 ± 0.2 a | 33.80 ± 0.2 b | 10.52±0.53 e | 55.31 ± 0.31 f | 77.64 ± 0.6 g | 39.2 ± 0.12 i |
| Control | - | 33.80 ± 0.1 b l-asp acid | - | 38.61 ± 0.12 Acarbose | - | 6.00 ± 0.01 Kojic acid |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.A.; Akhtar, N.; Menaa, B.; Menaa, A.; Braga, V.A.; Menaa, F. Relative Free Radicals Scavenging and Enzymatic Activities of Hippophae rhamnoides and Cassia fistula Extracts: Importance for Cosmetic, Food and Medicinal Applications. Cosmetics 2017, 4, 3. https://doi.org/10.3390/cosmetics4010003
Khan BA, Akhtar N, Menaa B, Menaa A, Braga VA, Menaa F. Relative Free Radicals Scavenging and Enzymatic Activities of Hippophae rhamnoides and Cassia fistula Extracts: Importance for Cosmetic, Food and Medicinal Applications. Cosmetics. 2017; 4(1):3. https://doi.org/10.3390/cosmetics4010003
Chicago/Turabian StyleKhan, Barkat Ali, Naveed Akhtar, Bouzid Menaa, Abder Menaa, Valdir A. Braga, and Farid Menaa. 2017. "Relative Free Radicals Scavenging and Enzymatic Activities of Hippophae rhamnoides and Cassia fistula Extracts: Importance for Cosmetic, Food and Medicinal Applications" Cosmetics 4, no. 1: 3. https://doi.org/10.3390/cosmetics4010003
APA StyleKhan, B. A., Akhtar, N., Menaa, B., Menaa, A., Braga, V. A., & Menaa, F. (2017). Relative Free Radicals Scavenging and Enzymatic Activities of Hippophae rhamnoides and Cassia fistula Extracts: Importance for Cosmetic, Food and Medicinal Applications. Cosmetics, 4(1), 3. https://doi.org/10.3390/cosmetics4010003