Comparative Analysis of Four Facial Foundation Lotions with Reference to Its Antioxidant Richness and Bio-Safety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cosmetic Ethanol–Water Extract
2.3. Determination of Total Protein
2.4. Determination of Phenol
2.5. Total Antioxidants Capacity
2.6. Free Radical Scavenging Activity Using DPPH
2.7. Anti-Lipid Peroxidation Effect
2.8. Ferric Reducing Antioxidant Power (FRAP)
2.9. Metal Chelating Effect
2.10. Anti-Hemolytic Activity
2.11. Statistical Analysis
3. Results and Discussions
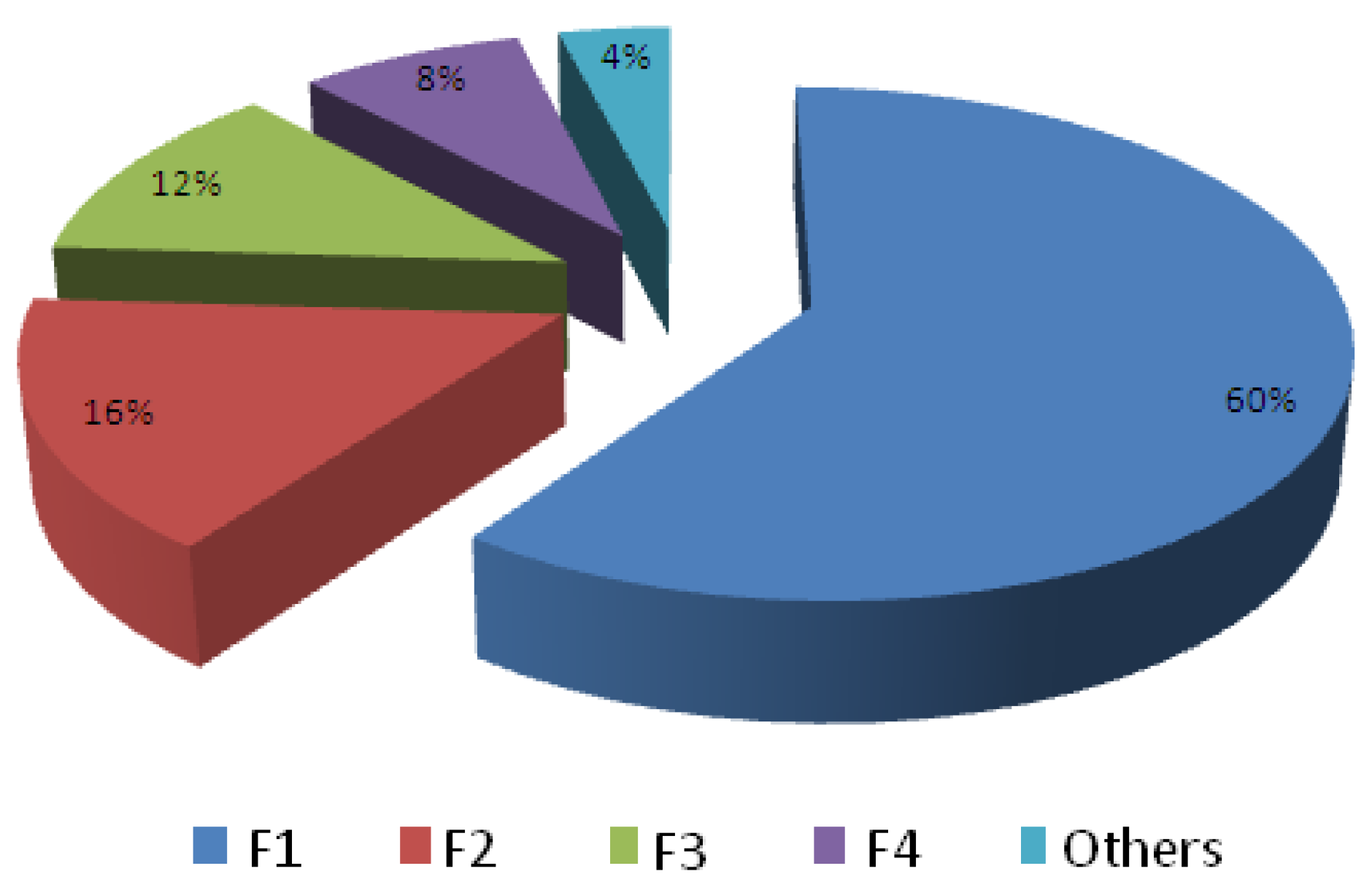
3.1. Survey Reports
3.2. Bioactive Components in the Foundation Lotion
3.2.1. Antioxidant Study
3.2.2. Anti-Hemolytic Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benedetto, A.D.; Agnihothri, R.; McGirt, L.Y.; Bankova, L.G.; Beck, L.A. Atopic dermatitis: A disease caused by innate immune defects? J. Investig. Dermatol. 2009, 129, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, N.; Balakrishnan, K.P. Evaluation of some medicinal plants for their antioxidant properties. Int. J. Pharm Tech. Res. 2011, 3, 381–385. [Google Scholar]
- Benzie, I.F.F. Evolution of dietary antioxidants. Comp. Biochem. Physiol A Mol. Integr. Physiol. 2003, 136, 113–126. [Google Scholar] [CrossRef]
- Epstein, H. Cosmeceuticals and polyphenols. Clin. Dermatol. 2009, 275, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Orth, D.S. Preservative-Free and Self-Preserving Cosmetics and Drugs: Principles and Practices; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Wang, K.H.; Lin, R.D.; Hsu, F.L.; Huang, Y.H.; Chang, H.C.; Huang, C.Y.; Lee, M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Sane, K.L.; Ammar, W.B.; Azeiz, S.; Brahim, A.H.; et al. Chemical composition and antioxidant and antimicrobial activities of wormwood (Artemisia absinthium L.) essential oils and phenolics. J. Chem. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Akhtar, N.; Menaa, B.; Menaa, B.; Braga, B.A.; Menaa, F. Relative free radicals scavenging and enzymatic activities of Hippophae rhamnoides and Cassia fistula extracts: Importance for cosmetic, food and medicinal applications. Cosmetics 2017, 4, 3. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar]
- Cao, G.; Prior, R.L. Measurement of total antioxidant capacity in nutritional and clinical studies. In Handbook of Antioxidants; Cadenas, E., Packer, L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 47–55. [Google Scholar]
- Sanchez-Moreno, C.; Jimenez-Escrig, A.; Saura-Calixto, F. Study of low-density lipoprotein oxidizability indexes to measure the antioxidant activity of dietary polyphenols. Nutr. Res. 2000, 20, 941–953. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 7, 307–315. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, M.L.M. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.R.; Sahareen, T.; Singh, M.; Kumar, S. Role of biogenic silver nanoparticles in disruption of cell–cell adhesion in Staphylococcus aureus and Escherichia coli biofilm. J. Ind. Eng. Chem. 2015, 26, 73–80. [Google Scholar] [CrossRef]
- Draelos, Z.D. The Multifunctional Value of Sunscreen containing Cosmetics. Skin Ther. Lett. 2011, 16, 1–3. [Google Scholar]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Menaa, A.; Menaa, F. A novel Cassia fistula (L.)-based emulsion elicits skin anti-aging benefits in humans. Cosmetics 2015, 2, 368–383. [Google Scholar] [CrossRef]
- Sharif, A.; Akhtar, N.; Khan, M.S.; Menaa, A.; Menaa, B.; Khan, B.A.; Menaa, F. Formulation and evaluation on human skin of a water-in-oil emulsion containing Muscat hamburg black grape seed extract. Int. J. Cosmet. Sci. 2015, 37, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bouftira, I.; Abdelly, C.; Sfar, S. Characterization of cosmetic cream with Mesembryanthemum crystallinum plant extract: Influence of formulation composition on physical stability and antioxidant activity. Int. J. Cosmet. Sci. 2008, 30, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.K.; Kumar, A.; Kumar, V. Antimicrobial, antioxidant and phyto-chemicals from fruit and vegetable wastes: A review. Int. J. Food Ferment. Technol. 2012, 2, 123–136. [Google Scholar]
- Corsini, E.; Papale, A.; Galbiati, G.; Roggen, E.L. Safety evaluation of cosmetic ingredients: In vitro opportunities for the identification of contact allergens. Cosmetics 2014, 1, 61–74. [Google Scholar] [CrossRef]
- Hans, R.K.; Agrawal, N.; Verma, K.; Misra, R.B.; Ray, R.S.; Farooq, M. Assessment of the phototoxic potential of cosmetic products. Food Chem. Toxicol. 2008, 46, 1653–1658. [Google Scholar] [CrossRef] [PubMed]

| Items | Foundation Lotions | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| Protein (µg/mL eq BSA) | 0.023 ± 0.001 | 0.484 ± 0.07 | 0.531 ± 0.02 | 0.598 ± 0.3 |
| Polyphenol (µg/mL eq GA) | 82.500 ± 3.53 | 158.500 ± 12.02 | 500 ± 12.02 | 256.500 ± 9.19 |
| Total Antioxidant Capacity (µg/mL) | 6.250 ± 0.35 | 12.150 ± 0.91 | 13.000 ± 0.70 | 12.200 ± 0.99 |
| Items | Foundation Lotions | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| DPPH | 12.5 ± 0.70 | 10.75 ± 1.00 | 8.40 ± 0.78 | 9.45 ± 1.50 |
| Reducing Property | 111.5 ± 4.94 | 0 | 130 ± 7.07 | 78.75 ± 3.89 |
| Lipid Peroxidation | 253.5 ± 4.94 | 256 ± 8.48 | 257.85 ± 11.1 | 260.1 ± 14.28 |
| Metal Chelating | 36.85 ± 2.47 | 41.9 ± 2.68 | 25.45 ± 0.63 | 36.85 ± 2.61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Seth, P.; Poddar, S. Comparative Analysis of Four Facial Foundation Lotions with Reference to Its Antioxidant Richness and Bio-Safety. Cosmetics 2017, 4, 12. https://doi.org/10.3390/cosmetics4020012
Singh M, Seth P, Poddar S. Comparative Analysis of Four Facial Foundation Lotions with Reference to Its Antioxidant Richness and Bio-Safety. Cosmetics. 2017; 4(2):12. https://doi.org/10.3390/cosmetics4020012
Chicago/Turabian StyleSingh, Mukesh, Pallavi Seth, and Shamayita Poddar. 2017. "Comparative Analysis of Four Facial Foundation Lotions with Reference to Its Antioxidant Richness and Bio-Safety" Cosmetics 4, no. 2: 12. https://doi.org/10.3390/cosmetics4020012






